Effects of Storage Temperature, Packaging Material and Wash Treatment on Quality and Shelf Life of Tartary Buckwheat Microgreens
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Packaging
2.2. Temperature, Packaging and Wash Treatments
2.3. Analysis of Packaging Headspace Atmosphere Composition
2.4. Overall Quality and Off-Odor Analysis
2.5. Weight Loss and Electrolyte Leakage Analysis
2.6. Microbial Counts
2.7. Determination of Total Phenolics, Total Flavonoids and Main Phenolic Compounds
2.8. Determination of Antioxidant Capacity
2.9. Statistical Analysis
3. Results and Discussion
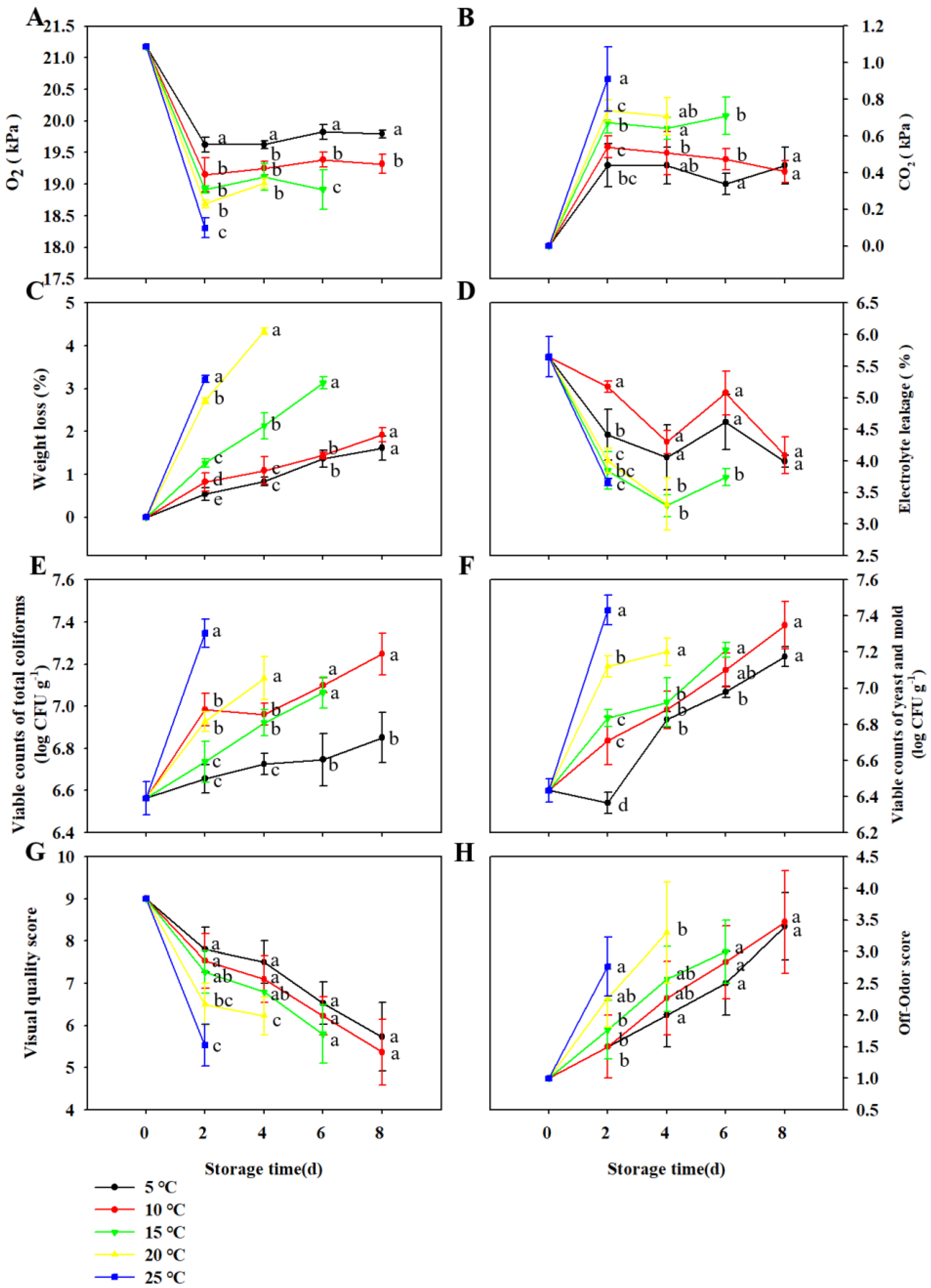
3.1. Effect of Storage Temperatures on Quality and Shelf Life of TBM
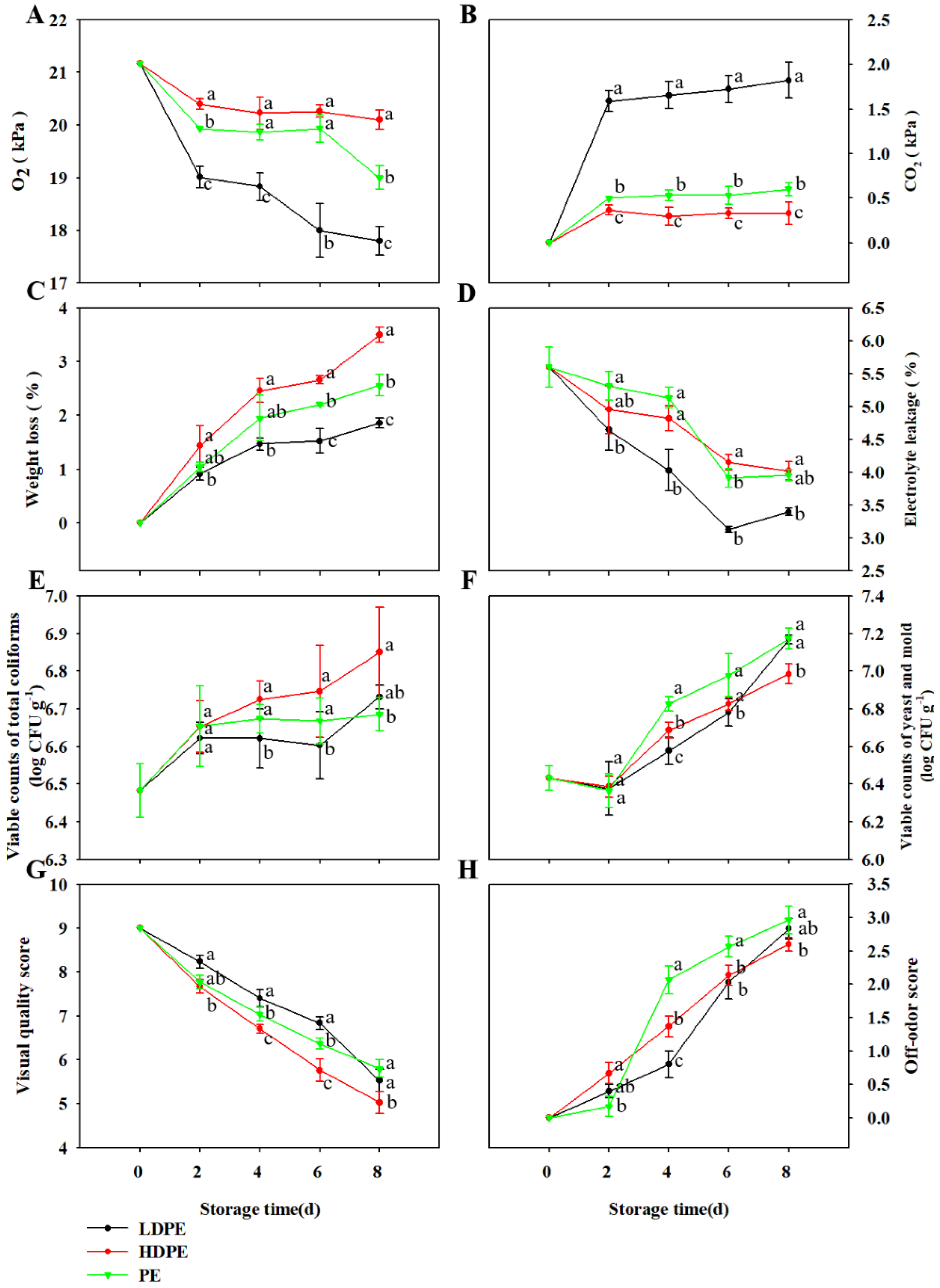
3.2. Effect of Packaging Materials on the Quality of TBM Stored at 5 °C
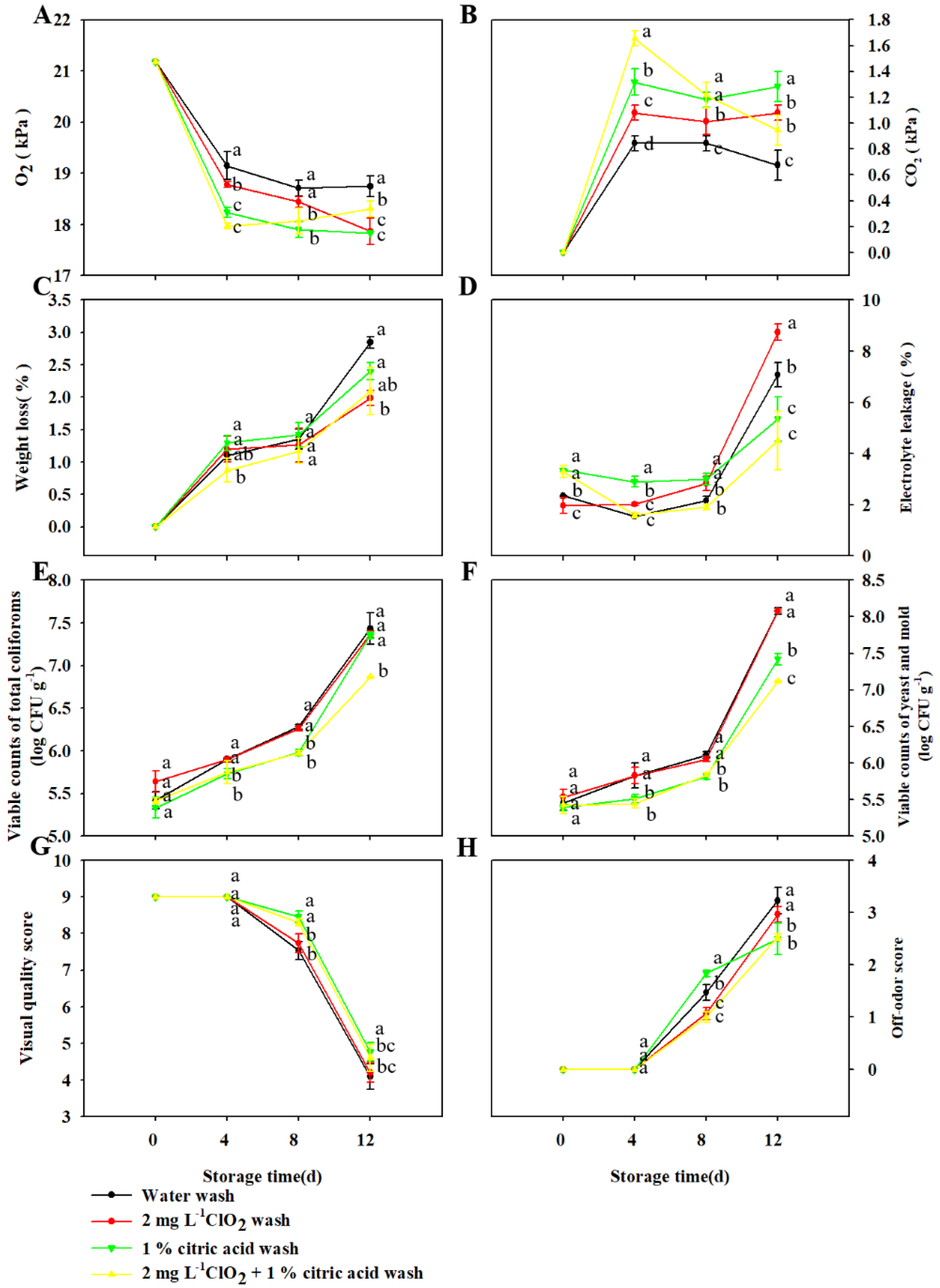
3.3. Effect of Wash Treatment on the Quality of TBM Packed in LDPE Bags Stored at 5 °C
3.4. Changes in Bioactive Compounds and Antioxidant Activity of TBM during Postharvest Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.M.; Santamaria, P. Microgreens production with low potassium content for patients with impaired kidney function. Nutrients 2018, 10, 675. [Google Scholar] [CrossRef]
- Liu, H.K.; Li, Z.H.; Zhang, X.W.; Liu, Y.P.; JianGuo, H.; Yang, C.W.; Zhao, X.Y. The effects of ultrasound on the growth, nutritional quality and microbiological quality of sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- Chandra, D.; Kim, J.G.; Kim, Y.P. Changes in microbial population and quality of microgreens treated with different sanitizers and packaging films. Hortic. Environ. Biotechnol. 2012, 53, 32–40. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, Y.Y.; Zhao, Z.Y.; Liu, W.F.; Chen, Y.Q.; Yang, G.J.; Xia, X.D.; Cao, Y.F. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout. Food Control 2019, 104, 83–90. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Luo, Y.G.; Lester, G.E.; Kou, L.P.; Yang, T.B.; Wang, Q. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. LWT Food Sci. Technol. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Sun, B.; Lin, P.X.; Xia, P.X.; Di, H.M.; Zhang, J.Q.; Zhang, C.L.; Zhang, F. Low-temperature storage after harvest retards the deterioration in the sensory quality, health-promoting compounds, and antioxidant capacity of baby mustard. RSC Adv. 2020, 10, 36495–36503. [Google Scholar] [CrossRef]
- Kou, L.P.; Luo, Y.G.; Yang, T.B.; Xiao, Z.L.; Turner, E.R.; Lester, G.E.; Wang, Q.; Camp, M.J. Postharvest biology, quality and shelf life of buckwheat microgreens. LWT Food Sci. Technol. 2013, 51, 73–78. [Google Scholar] [CrossRef]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Nei, D.; Latiful, B.M.; Enomoto, K.; Inatsu, Y.; Kawamoto, S. Disinfection of radish and alfalfa seeds inoculated with Escherichia coli O157:H7 and Salmonella by a gaseous acetic acid treatment. Foodborne Pathog. Dis. 2011, 8, 1089–1094. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.T.; Ren, G.X.; Hu, Y.C.; Peng, L.X.; Zhao, J.L.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.S.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Ruan, J.J.; Zhou, Y.X.; Yan, J.; Zhou, M.L.; Woo, S.H.; Weng, W.F.; Cheng, J.P.; Zhang, K.X. Tartary buckwheat: An under-utilized edible and medicinal herb for food and nutritional security. Food Rev. Int. 2020, 38, 440–454. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Xie, Z.K.; Yu, L.L.; Wang, Q. Effect of light exposure on sensorial quality, concentrations of bioactive compounds and antioxidant capacity of radish microgreens during low temperature storage. Food Chem. 2014, 151, 472–479. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Carr, B.T. Sensory Evaluation Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar] [CrossRef]
- Chun, H.H.; Song, K.B. The combined effects of aqueous chlorine dioxide, fumaric acid, and ultraviolet-C with modified atmosphere packaging enriched in CO2 for inactivating preexisting microorganisms and Escherichia coli O157:H7 and Salmonella typhimurium inoculated on buckwheat sprouts. Postharvest Biol. Technol. 2013, 86, 118–124. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- Lee, S.W.; Seo, J.M.; Lee, M.K.; Chun, J.H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1999, 76, 270–276. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, T.T.; Liu, H.K.; Chen, Y.Y.; Pang, X.J.; Zheng, L.L.; Chang, S.M.; Kang, Y.F. Moderate vacuum packing and low temperature effects on qualities of harvested mung bean (Vigna radiata L.) sprouts. Postharvest Biol. Technol. 2018, 145, 83–92. [Google Scholar] [CrossRef]
- Derossi, A.; De Pilli, T.; Severini, C.; McCarthy, M.J. Mass transfer during osmotic dehydration of apples. J. Food Eng. 2008, 86, 519–528. [Google Scholar] [CrossRef]
- Jiang, Y.; Shiina, T.; Nakamura, N.; Nakahara, A. Electrical conductivity evaluation of postharvest strawberry damage. J. Food Sci. 2001, 66, 1392–1395. [Google Scholar] [CrossRef]
- Diaz-Mula, H.M.; Marin, A.; Jordan, M.J.; Gil, M.I. Off-odor compounds responsible for quality loss of minimally processed baby spinach stored under MA of low O2 and high CO2 using GC-MS and olfactometry techniques. Postharvest Biol. Technol. 2017, 129, 129–135. [Google Scholar] [CrossRef]
- Luo, Y.; McEvoy, J.L.; Wachtel, M.R.; Kim, J.G.; Huang, Y. Package atmosphere affects postharvest biology and quality of fresh-cut cilantro leaves. HortScience 2004, 39, 567–570. [Google Scholar] [CrossRef]
- Yuan, G.F.; Wang, X.P.; Guo, R.F.; Wang, Q.M. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]

| Storage Time (d) | Chlorogenic Acid (g kg−1) | Orientin (g kg−1) | Isoorientin (g kg−1) | Vitexin (g kg−1) | Isovitexin (g kg−1) | Rutin (g kg−1) | Quercetin (g kg−1) |
|---|---|---|---|---|---|---|---|
| Water/0 | 1.29 b ± 0.05 | 0.18 a ± 0.02 | 0.42 b ± 0.01 | 1.19 a ± 0.02 | 1.70 b ± 0.04 | 78.53 a ± 0.85 | 0.61 a ± 0.16 |
| ClO2 + ctric acid/0 | 1.38 a ± 0.09 | 0.20 a ± 0.03 | 0.48 a ± 0.02 | 1.24 a ± 0.08 | 1.78 a ± 0.10 | 79.34 a ± 2.09 | 0.63 a ± 0.21 |
| Water/4 | 1.26 b ± 0.01 | 0.17 a ± 0.01 | 0.44 b ± 0.01 | 1.18 b ± 0.01 | 1.68 b ± 0.02 | 78.36 a ± 0.65 | 0.61 a ± 0.09 |
| ClO2 + ctric acid/4 | 1.61 a ± 0.00 | 0.19 a ± 0.00 | 0.49 a ± 0.00 | 1.25 a ± 0.01 | 1.79 a ± 0.01 | 79.61 a ± 0.40 | 0.60 a ± 0.11 |
| Water/8 | 1.19 b ± 0.02 | 0.22 a ± 0.01 | 0.43 b ± 0.01 | 1.20 b ± 0.01 | 1.69 b ± 0.02 | 74.89 a ± 1.07 | 0.41 a ± 0.13 |
| ClO2 + ctric acid/8 | 1.50 a ± 0.01 | 0.23 a ± 0.00 | 0.49 a ± 0.00 | 1.29 a ± 0.01 | 1.83 a ± 0.02 | 76.53 a ± 0.79 | 0.40 a ± 0.09 |
| Water/12 | 1.06 b ± 0.02 | 0.17 b ± 0.03 | 0.41 b ± 0.00 | 1.16 a ± 0.00 | 1.61 b ± 0.01 | 64.15 b ± 0.64 | 0.52 a ± 0.12 |
| ClO2 + ctric acid/12 | 1.34 a ± 0.01 | 0.21 a ± 0.02 | 0.47 a ± 0.01 | 1.22 a ± 0.02 | 1.76 a ± 0.03 | 66.76 a ± 1.32 | 0.55 a ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Li, W.; Chen, H.; Liao, Q.; Xia, M.; Wu, D.; Liu, C.; Chen, J.; Zou, L.; Peng, L.; et al. Effects of Storage Temperature, Packaging Material and Wash Treatment on Quality and Shelf Life of Tartary Buckwheat Microgreens. Foods 2022, 11, 3630. https://doi.org/10.3390/foods11223630
Yan H, Li W, Chen H, Liao Q, Xia M, Wu D, Liu C, Chen J, Zou L, Peng L, et al. Effects of Storage Temperature, Packaging Material and Wash Treatment on Quality and Shelf Life of Tartary Buckwheat Microgreens. Foods. 2022; 11(22):3630. https://doi.org/10.3390/foods11223630
Chicago/Turabian StyleYan, Huiling, Wenfei Li, Hongxu Chen, Qingxia Liao, Mengying Xia, Dingtao Wu, Changying Liu, Jianxiong Chen, Liang Zou, Lianxin Peng, and et al. 2022. "Effects of Storage Temperature, Packaging Material and Wash Treatment on Quality and Shelf Life of Tartary Buckwheat Microgreens" Foods 11, no. 22: 3630. https://doi.org/10.3390/foods11223630
APA StyleYan, H., Li, W., Chen, H., Liao, Q., Xia, M., Wu, D., Liu, C., Chen, J., Zou, L., Peng, L., Zhao, G., & Zhao, J. (2022). Effects of Storage Temperature, Packaging Material and Wash Treatment on Quality and Shelf Life of Tartary Buckwheat Microgreens. Foods, 11(22), 3630. https://doi.org/10.3390/foods11223630







