Characteristics and Microbiome Profiling of Korean Gochang Bokbunja Vinegar by the Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation of Gochang Bokbunja Vinegar
2.2. Temperature, Sugar Concentration, Alcohol Concentration, Specific Gravity, pH, and Total Acidity Measurement
2.3. Organic Acid Analysis
2.4. 16S rRNA Gene-Based Metagenomic Analysis Method
2.5. Electronic Tongue Analysis
3. Results
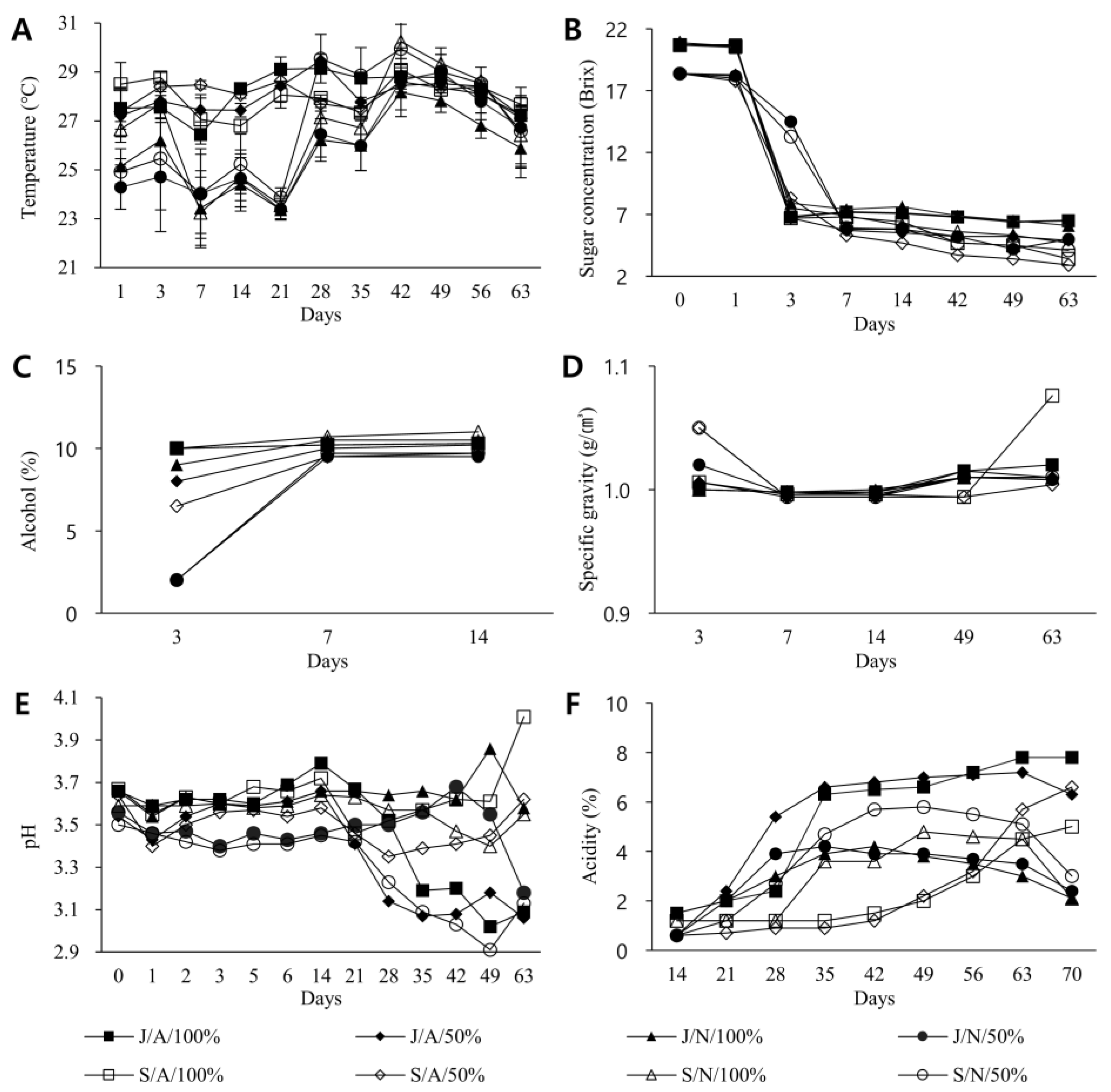
3.1. Exploration of Basic Physicochemical Properties of Vinegar by Fermentation Condition and Period
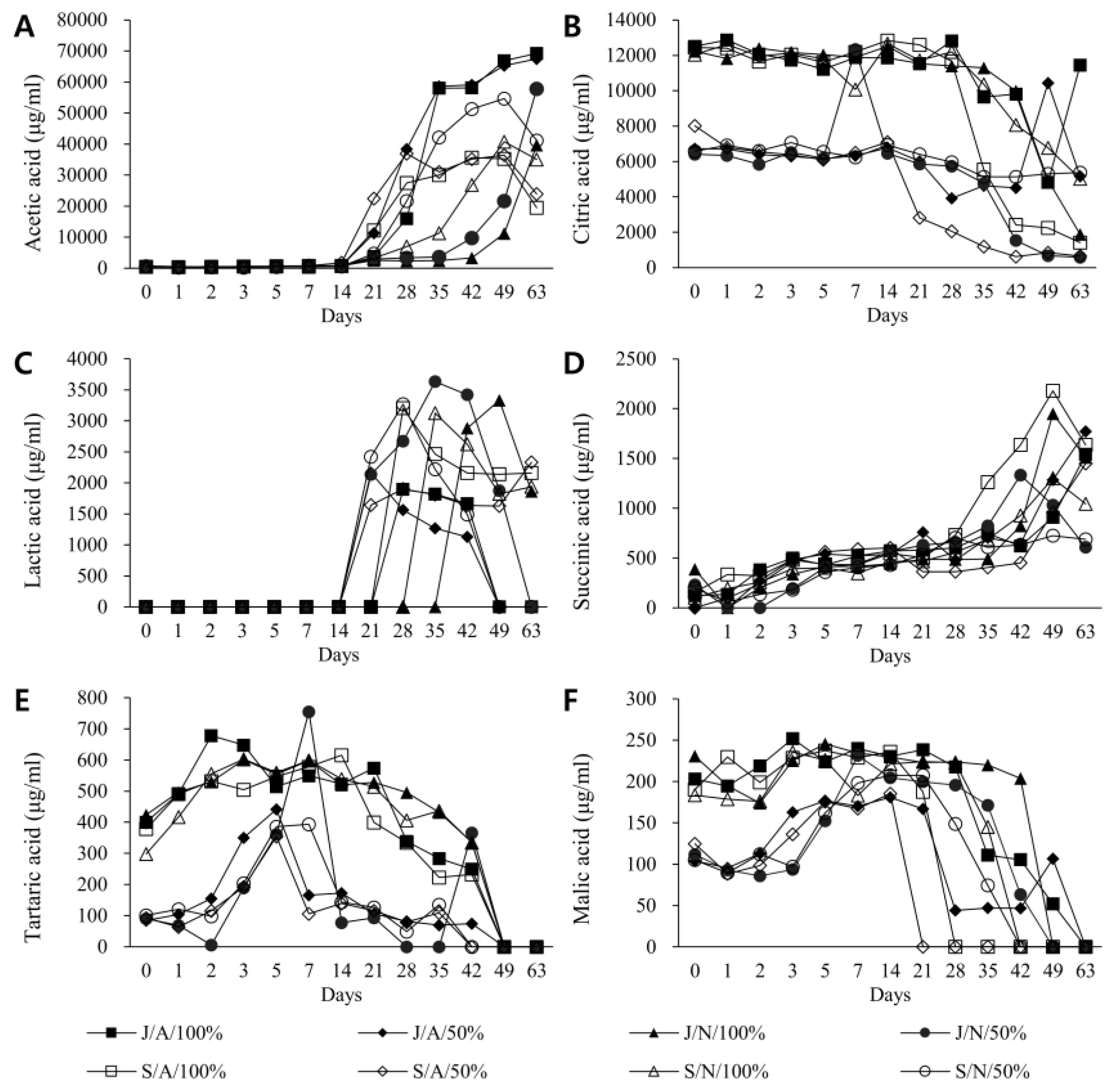
3.2. Changes in Major Organic Acids by Fermentation Condition and Duration
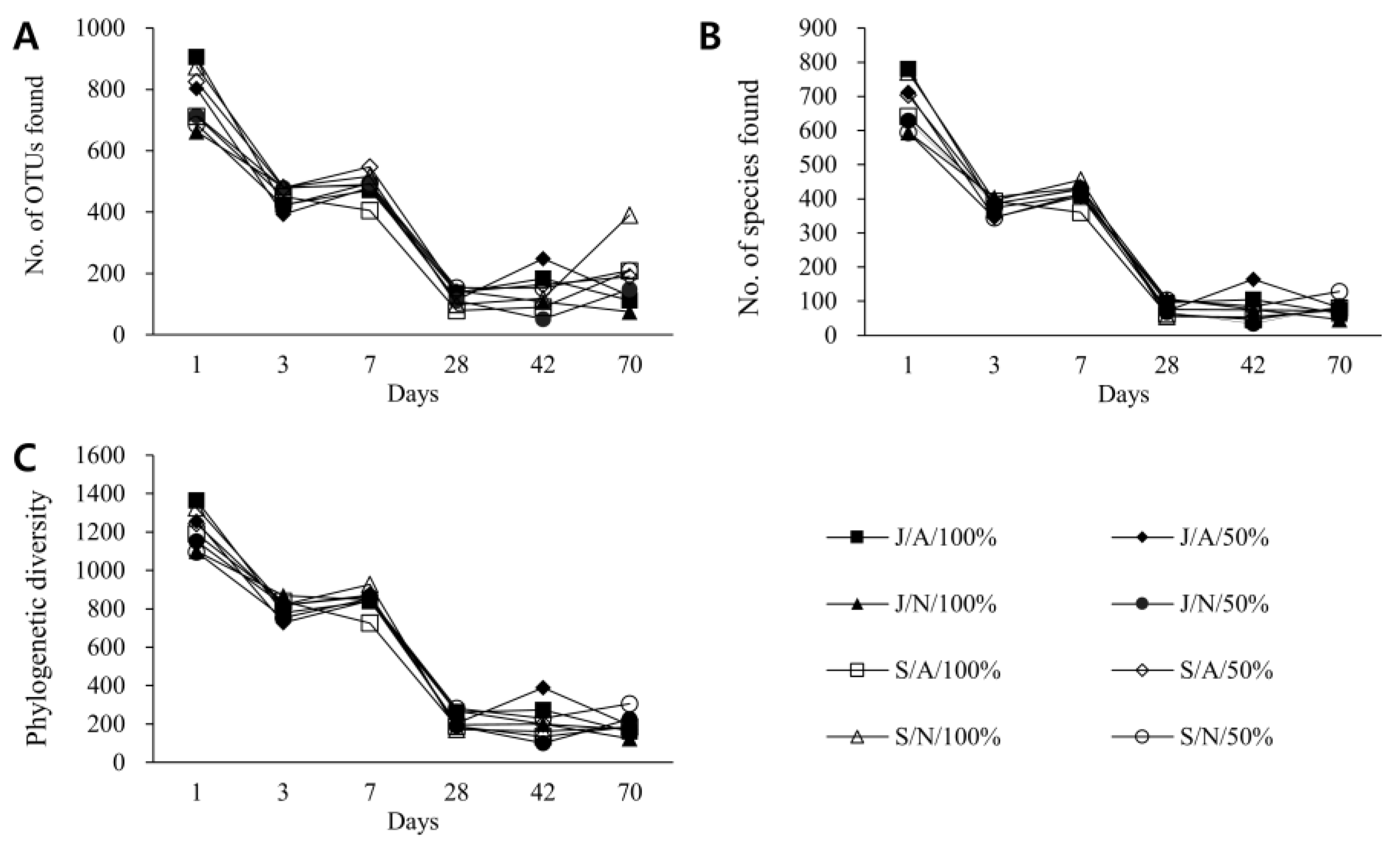
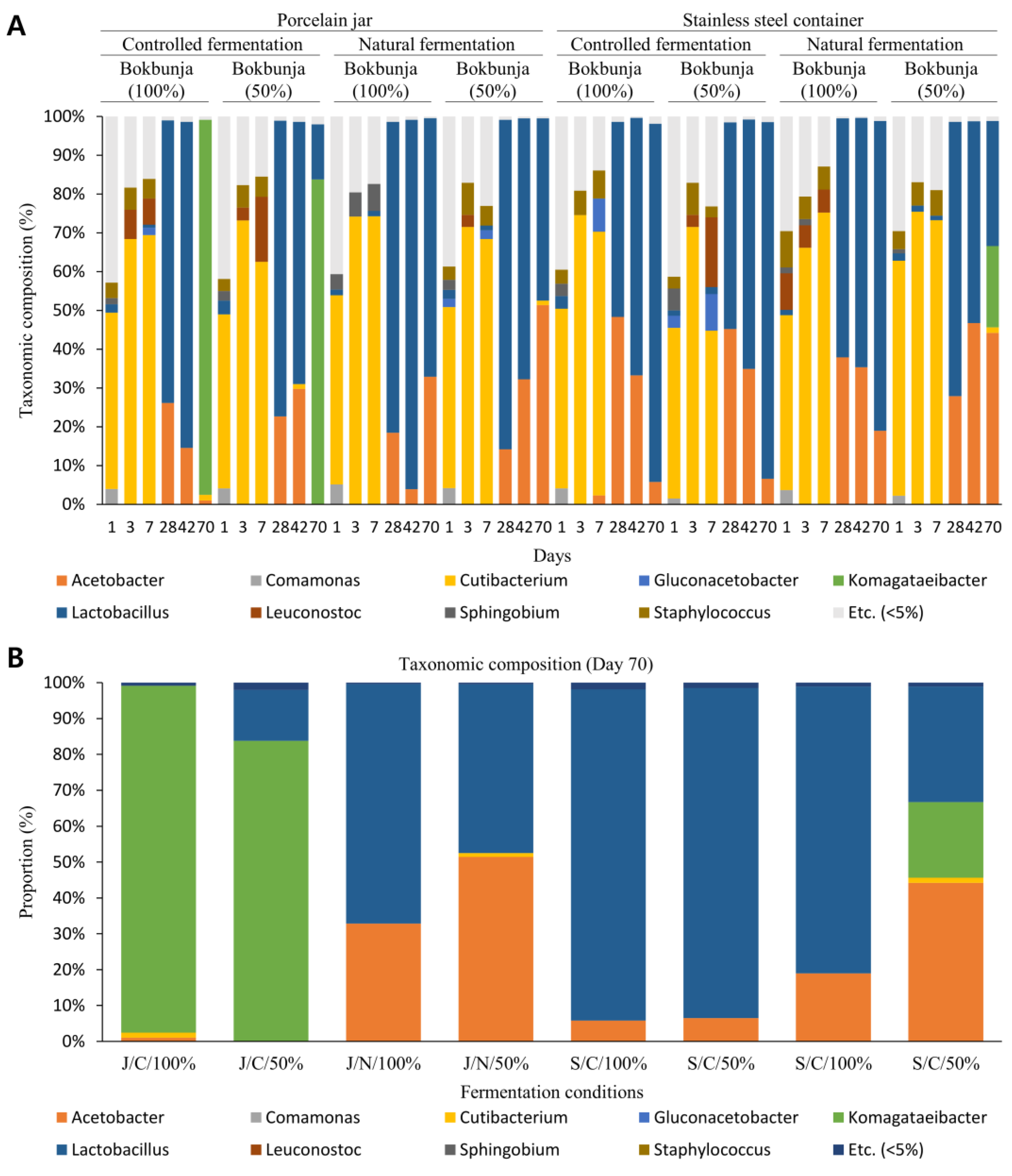
3.3. Changes in Composition and Diversity of Microbiome of Vinegar According to Fermentation Conditions over Time
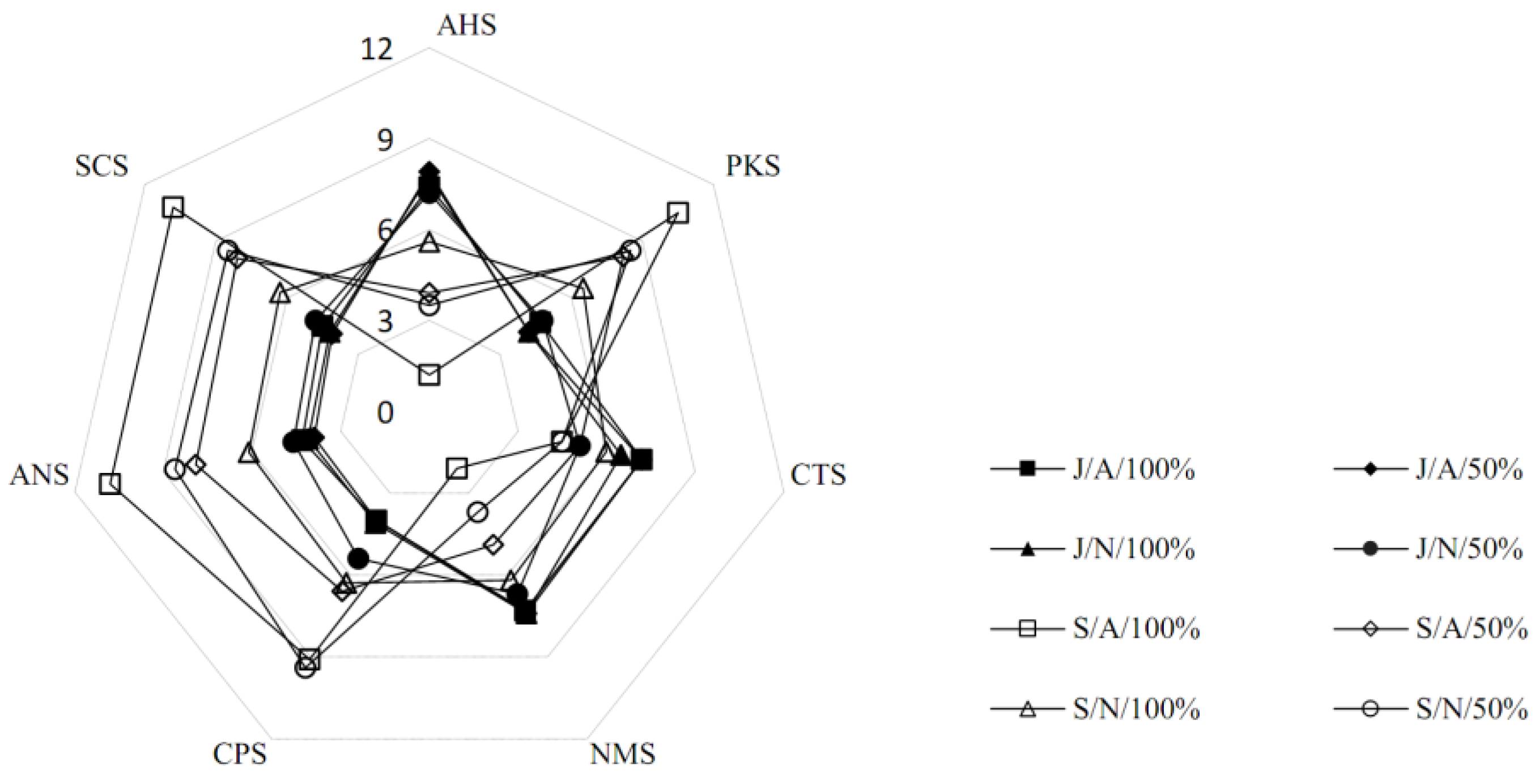
3.4. Electronic Tongue-Based Taste Pattern Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A.T. ‘Ome sweet’ omics—A genealogical treasury of words. Scientist 2001, 15, 8. [Google Scholar]
- The Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Li, R.Q.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef]
- Zhao, L.P. GENOMICS The tale of our other genome. Nature 2010, 465, 879–880. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Kergourlay, G.; Taminiau, B.; Daube, G.; Verges, M.C.C. Metagenomic insights into the dynamics of microbial communities in food. Int. J. Food Microbiol. 2015, 213, 31–39. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Recent Past, Present, and Future of the Food Microbiome. Annu. Rev. Food Sci. Technol. 2018, 9, 589–608. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Ali, Z.; Wang, Z.B.; Amir, R.M.; Younas, S.; Wali, A.; Adowa, N.; Ayim, I. Potential Uses of Vinegar as a Medicine and Related in vivo Mechanisms. Int. J. Vitam. Nutr. Res. 2016, 86, 140–151. [Google Scholar] [CrossRef]
- Luzon-Quintana, L.M.; Castro, R.; Duran-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.M.; Yu, Y.J.; Li, G.Q.; Shi, J.S.; Xu, Z.H. Batch-to-batch uniformity of bacterial community succession and flavor formation in the fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2015, 50, 64–69. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Sci. Rep. 2016, 6, 26818. [Google Scholar] [CrossRef]
- Nie, Z.Q.; Zheng, Y.; Wang, M.; Han, Y.; Wang, Y.N.; Luo, J.M.; Niu, D.D. Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour. Technol. 2013, 148, 325–333. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, Y.P.; Guo, Y.Y.; Han, Y. Analysis of Bacterial Diversity During Acetic Acid Fermentation of Tianjin Duliu Aged Vinegar by 454 Pyrosequencing. Curr. Microbiol. 2015, 71, 195–203. [Google Scholar] [CrossRef]
- Trcek, J.; Mahnic, A.; Rupnik, M. Diversity of the microbiota involved in wine and organic apple cider submerged vinegar production as revealed by DHPLC analysis and next-generation sequencing. Int. J. Food Microbiol. 2016, 223, 57–62. [Google Scholar] [CrossRef]
- Lee, J. Analysis of bokbunja products show they contain Rubus occidentalis L. fruit. J. Funct. Foods 2015, 12, 144–149. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Lee, I. Analysis of distillation process adoptability for Rubus coreanus balsamic vinegar production. J. Korea Converg. Soc. 2021, 12, 185–191. [Google Scholar]
- Ji-Wung, K.; Songyie, P.; Kyu-Seo, C.; Rak-Ho, S.; Jihye, J.; Young-rae, I. Quality Characteristics and Antioxidant Activity of Bokbunja(Black Raspberry) Vinegars. Food Eng. Prog. 2012, 16, 340–346. [Google Scholar]
- An, J.H.; Kim, D.L.; Lee, T.B.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Effect of Rubus Occidentalis Extract on Metabolic Parameters in Subjects with Prediabetes: A Proof-of-concept, Randomized, Double-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2016, 30, 1634–1640. [Google Scholar] [CrossRef]
- Woo, S.-M.; Shin, J.-S.; Seong, J.-H.; Yeo, S.-H.; Choi, J.-H.; Kim, T.-Y.; Jeong, Y.-J. Quality Characteristics of Brown Rice Takju by Different Nuruks. Journal of the Korean Society of Food Science and Nutrition. J. Korean Soc. Food Sci. Nutr. 2010, 39, 301–307. [Google Scholar] [CrossRef]
- Ryu, E.-H.; Chae, K.-S.; Gim, S.-W.; Kim, Y.-S.; Kim, K.-D.; Kwon, J.-W. Physicochemical properties and antioxidant activities of vinegar using black raspberry pomace. Korean J. Food Sci. Technol. 2021, 53, 104–110. [Google Scholar]
- Kim, J.K.; Lee, M.J.; Lee, Y.J.; Kim, H.J.; Jeong, M.H.; Kim, H.J. Development of HPLC-UV method for detection and quantification of seven organic acids in animal feed. Anal. Sci.Technol. 2016, 29, 202–208. [Google Scholar] [CrossRef]
- Choi, G.-I.; Kim, H.-J.; Kim, H.-J.; Kim, H.-R.; Kim, D.-H.; Ahn, J.-S.; Ahn, J.-S.; Son, Y.-G.; Song, I.-H. Changes of Organic Acids in Takju During Storage Conditions. Journal of Food Hygiene and Safety. J. Food Hyg. Saf. 2012, 27, 127–132. [Google Scholar] [CrossRef]
- Kim, S.; Seo, H.; Rahim, M.A.; Lee, S.; Kim, Y.S.; Song, H.Y. Changes in the Microbiome of Vaginal Fluid after Menopause in Korean Women. J. Microbiol. Biotechnol. 2021, 31, 1490–1500. [Google Scholar] [CrossRef]
- Kim, S.; Seo, H.; Rahim, M.A.; Tajdozian, H.; Kim, Y.S.; Song, H.Y. Characteristics of Vaginal Microbiome in Women with Pelvic Inflammatory Disease in Korea. Pol. J. Microbiol. 2021, 70, 345–357. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhu, Y.; Ben, A.; Qi, J. A novel strategy for discriminating different cultivation and screening odor and taste flavor compounds in Xinhui tangerine peel using E-nose, E-tongue, and chemometrics. Food Chem. 2022, 384, 132519. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Feng, F.; Luo, L.X. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biot. 2015, 99, 4997–5024. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R. Vinegar. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Springer: Boston, MA, USA, 1998; pp. 1–44. [Google Scholar]
- Nakano, S.; Fukaya, M. Analysis of proteins responsive to acetic acid in Acetobacter: Molecular mechanisms conferring acetic acid resistance in acetic acid bacteria. Int. J. Food Microbiol. 2008, 125, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.B.; Zhu, H.J.; Yang, F.; Xiao, K.; Zhang, L.; Zhang, M.L.; Peng, Y.S.; Wang, C.; Li, D.S.; et al. Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation. Food Sci. Hum. Wellness 2022, 11, 1409–1418. [Google Scholar] [CrossRef]
- Barja, F.; Andrés-Barrao, C.; Ortega Pérez, R.; Cabello, E.M.; Chappuis, M.-L. Physiology of Komagataeibacter spp. During Acetic Acid Fermentation. In Acetic Acid Bacteria: Ecology and Physiology; Matsushita, K., Toyama, H., Tonouchi, N., Okamoto-Kainuma, A., Eds.; Springer: Tokyo, Japan, 2016; pp. 201–221. [Google Scholar]
- Andres-Barrao, C.; Saad, M.M.; Cabello Ferrete, E.; Bravo, D.; Chappuis, M.L.; Ortega Perez, R.; Junier, P.; Perret, X.; Barja, F. Metaproteomics and ultrastructure characterization of Komagataeibacter spp. involved in high-acid spirit vinegar production. Food Microbiol. 2016, 55, 112–122. [Google Scholar] [CrossRef]
- Yasir, M.; Al-Zahrani, I.A.; Bibi, F.; Abd El Ghany, M.; Azhar, E.I. New insights of bacterial communities in fermented vegetables from shotgun metagenomics and identification of antibiotic resistance genes and probiotic bacteria. Food Res. Int. 2022, 157, 111190. [Google Scholar] [CrossRef]
- Tamang, J.P.; Kharnaior, P.; Pariyar, P.; Thapa, N.; Lar, N.; Win, K.S.; Mar, A.; Nyo, N. Shotgun sequence-based metataxonomic and predictive functional profiles of Pe poke, a naturally fermented soybean food of Myanmar. PLoS ONE 2021, 16, e0260777. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Yang, L.; Fan, W.L.; Xu, Y. Metaproteomics insights into traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2506–2529. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ita, R.E.; Egong, E.J.; Udofia, L.E.; Mgbechidinma, C.L.; Akan, O.D. Metaproteomics insights into fermented fish and vegetable products and associated microbes. Food Chem.-Mol. Sci. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Lee, S.; Park, H.; Jo, S.; Kim, S.; Rahim, M.A.; Ul-Haq, A.; Barman, I.; Lee, Y.; Seo, A.; et al. Characteristics and Microbiome Profiling of Korean Gochang Bokbunja Vinegar by the Fermentation Process. Foods 2022, 11, 3308. https://doi.org/10.3390/foods11203308
Seo H, Lee S, Park H, Jo S, Kim S, Rahim MA, Ul-Haq A, Barman I, Lee Y, Seo A, et al. Characteristics and Microbiome Profiling of Korean Gochang Bokbunja Vinegar by the Fermentation Process. Foods. 2022; 11(20):3308. https://doi.org/10.3390/foods11203308
Chicago/Turabian StyleSeo, Hoonhee, Saebim Lee, Hyuna Park, Sujin Jo, Sukyung Kim, Md Abdur Rahim, Asad Ul-Haq, Indrajeet Barman, Youngkyoung Lee, Ayoung Seo, and et al. 2022. "Characteristics and Microbiome Profiling of Korean Gochang Bokbunja Vinegar by the Fermentation Process" Foods 11, no. 20: 3308. https://doi.org/10.3390/foods11203308
APA StyleSeo, H., Lee, S., Park, H., Jo, S., Kim, S., Rahim, M. A., Ul-Haq, A., Barman, I., Lee, Y., Seo, A., Kim, M., Jung, I.-y., & Song, H.-Y. (2022). Characteristics and Microbiome Profiling of Korean Gochang Bokbunja Vinegar by the Fermentation Process. Foods, 11(20), 3308. https://doi.org/10.3390/foods11203308








