Abstract
Mycotoxins in solid foods and feeds jeopardize the public health of humans and animals and cause food security issues. The inefficacy of most preventive measures to control the production of fungi in foods and feeds during the pre-harvest and post-harvest stages incited interest in the mitigation of these mycotoxins that can be conducted by the application of various chemical, physical, and/or biological treatments. These treatments are implemented separately or through a combination of two or more treatments simultaneously or subsequently. The reduction rates of the methods differ greatly, as do their effect on the organoleptic attributes, nutritional quality, and the environment. This critical review aims at summarizing the latest studies related to the mitigation of mycotoxins in solid foods and feeds. It discusses and evaluates the single and combined mycotoxin reduction treatments, compares their efficiency, elaborates on their advantages and disadvantages, and sheds light on the treated foods or feeds, as well as on their environmental impact.
1. Introduction
In a world full of economic, health, and environmental crises, food security concerns have become one of the most important dilemmas of our era. Fungal infection and the resulting production of mycotoxins in crops are major problems caused by climate change as a result of global warming [1,2]. Cereals and grains are considered highly susceptible to such types of infection during the pre-harvest and post-harvest stages of their production; their availability has a vital role in preventing hunger and food insecurity [3]. Mycotoxins, the secondary metabolites of fungi, are considered a food safety challenge, threatening the lives of humans and animals due to their immune toxicity, carcinogenicity, hepatotoxicity, nephrotoxicity, mutagenicity, and teratogenicity [4,5]. The pathogenicity and the toxigenic potentials of many fungal species, such as Aspergillus, Claviceps, Fusarium, Penicillium, and Alternaria, have been reported in various crops [6]. Their occurrence and presence in a specific food product at a specific geographic region depend on extrinsic factors related to environmental conditions fluctuation, such as temperature and relative humidity, which explain the effect of global climate change on the formation of these mycotoxins in agricultural commodities [7,8,9]. The type and the number of mycotoxins in foods and feeds are directly related to many intrinsic factors, such as the moisture content, the pH, the composition of the food, and many other extrinsic factors, such as the relative humidity and the storage temperature [10]. The most commonly known mycotoxins to contaminate foods and feeds are aflatoxins AFs (AFB1, AFB2, AFG1, AFG2, AFM1), ochratoxin (OTA), trichothecenes (deoxynivalenol: DON, nivalenol: NIV, T-2 toxin: T-2, HT-2 toxin: HT-2), zearalenone (ZEN), fumonisins B1 (FB1), enniatins (EN) [11,12,13], moniliformin (MON), beauvericin (BEA), and fusaproliferin (FUS) [14,15].
According to Food and Agriculture Organization (FAO) reports, 25% of the crops in the world are contaminated by mycotoxins [5,16,17]. Eskola et al., found that this percentage is underestimated and that 60 to 80% of crops are contaminated by mycotoxins [18]. In the United States, aflatoxin contamination causes great losses in the corn industry, reaching up to USD 1.68 billion. According to the Rapid Alert System for Food and Feed (RASFF), most rejection notifications at the EU border are due to mycotoxin contamination [19]. Regulations for mycotoxins are not available worldwide, especially in African countries. Mycotoxins in food and feed are extensively regulated in Europe. At the same time, aflatoxins in foods, particularly AFB1, are the most commonly regulated mycotoxins in many countries. Total aflatoxin limits in food were established in 2003 in 48 countries [20,21]. The maximum acceptable levels of the total AFs are 4μ/kg in the European Union and 20 μg/kg in the United States [16]. Globally, the maximum levels for AFs (B1, B2, G1, and G2), AFM1, and OTA in food are regulated by the codex standard CXS 193-1995 and established by the Codex Alimentarius Commission of the Food [18].
“Prevention is better than cure”, and this should be the first strategy for reducing mycotoxins in feeds and foods [22]. This preventive strategy aims at controlling the fungal growth and the production of these metabolites in foods and feeds in the pre-harvest and post-harvest stages by applying good agricultural practices and monitoring storage and processing conditions [23,24]. Practically, complete prevention of the formation of mycotoxins in crops is not feasible, which has triggered the need for alternative strategies aiming at decreasing or eliminating the amount of already produced mycotoxins in food and feed materials [25,26,27].
Chemical, physical, and biological technologies and treatments have been established and studied to mitigate mycotoxins in foods [28,29]. The success and efficiency of the method used to reduce mycotoxins depend on the food or feed characteristics [10]. Many studies have provided insights on a number of techniques designed for the detoxification of mycotoxins in liquid medium [30,31], such as in milk and dairy products using lactic acid bacteria biofilm [32], chitin, and shrimp shells [33] or by chemical treatment, such as ozonation [34] in fruit juices and wine [35,36] and in solid foods and feeds [37,38]. These technologies can be implemented separately, one by one, or combined in order to attain additive or synergistic effects in the reduction of mycotoxins in food or feed.
In this review, we focus on mitigating mycotoxins in solid foods and feeds only. We evaluate the effectiveness of chemical, physical, and biological treatments applied to solid foods and feeds to reduce mycotoxins when implemented separately and/or subsequently or simultaneously combined, as shown in Figure 1. In addition, we evaluate the effect of the different treatment modalities on the quality of the treated food materials providing their advantages and disadvantages.
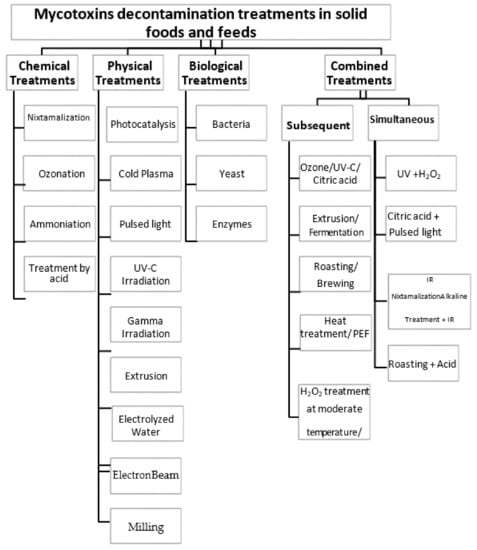
Figure 1.
Flow chart of the different mycotoxin decontamination methods discussed in this review.
2. Single Detoxification Treatments Used in Solid Foods and Feeds
In this section, we summarize the chemical, physical, and biological treatments applied separately, their effect on the reduction rates of different mycotoxins, and the quality of the treated materials.
2.1. Chemical Treatments
Chemical decontamination is used in many industries [26]. It can be used for the destruction of mycotoxins or their neutralization [39]. Many chemical agents are used for the decontamination of solid foods and feed, such as limewater [40], organic acids [41], ozone [42], and ammonia [43]. All these treatments are discussed below in this section (Table 1).

Table 1.
Chemical treatments for the reduction of mycotoxins in solid foods and feeds.
2.1.1. Nixtamalization
Nixtamalization is a traditional process used for maize. It is a chemical treatment based on the alkaline hydrolysis of aflatoxins by the addition of lime and subsequent cooking for a predefined time. This process causes the opening of the lactone ring of aflatoxins leading to their inactivation by the high pH medium and heating process. The efficiency of this process is related to many factors, such as the quantity of the lime used, the temperature of the process, and the contact time between the solution and the grains [60]. The disadvantage of the traditional nixtamalization process (TNP) is the generation of a large quantity of wastewater and a large amount of nejayote (water containing solid fractions of maize tip cap, pericarp, germ, and aflatoxins). Nejayote imposes a safety problem because it is reused in some regions as animal feed, for another nixtamalization process, or to water plants [51].
Inconsistent results have been shown concerning the use of the traditional nixtamalization process to reduce aflatoxins in corn. High reduction rates of aflatoxin B1 (AFB1) and aflatoxicol of 96% and 70%, respectively, were achieved by Anguiano–Ruvalcaba et al., supporting the use of TNP to mitigate aflatoxins in corn [61]. Another study was conducted in the Huasteca Potosina region in the central part of Mexico to measure the efficiency of TNP and showed that this process is not efficient enough to mitigate the aflatoxins in maize grains [51].
To determine the optimal pH for the alkaline treatment of maize dough used for tortilla production, an alkaline treatment at pH 10.2 was appropriate to achieve the total elimination of aflatoxin AFB1 with a resting time of 30–40 min at room temperature. [52].
The cooking ingredients used to perform the nixtamalization may have a critical role in mitigating aflatoxins and decreasing the detrimental effect on food and feed quality, as well as the environment [53]. A study was conducted on maize, and two cooking solutions were used (1% slaked lime or 1% traditional liquid ash). Their efficiencies were similar, and the reduction rates of 90% and 80% of aflatoxins and fumonisins, respectively, were achieved by soaking the maize grains. The flours prepared from the treated grains showed a decreased peak viscosity, as compared with the non-nixtamalized maize flours, associated with a slight reduction in the fat, sugar, protein, and dietary fiber contents. The ash and the niacin content were increased, and the acceptability of the products produced using the treated grains by consumers was high, making this method a cost-effective alternative to fumonisins and aflatoxins detoxification of maize [50].
Another study showed that calcium hydroxide is consistently used for cooking maize grains and for producing nixtamal, but this causes environmental pollution issues due to the high pH of the wastewater and the byproducts ensuing from this process. Alternatively, the authors proposed the use of different cooking solutions, such as sodium and potassium hydroxides, that can be used as an alternative to calcium hydroxide after showing their effective reducing effect on fusarium mycotoxins [53].
2.1.2. Ozonation
Ozone (O3) is a greenhouse gas made of three oxygen atoms. It is present naturally in the atmosphere (friend O3) or generated by human beings (foe O3) [26]. It can be produced by several methods, including UV-irradiation, electrical discharge of oxygen, and electrolysis of water. It is a highly reactive molecule having a high oxidizing effect (redox potential = 2.07 V), and it is used to mitigate many types of contaminants in food [62].
Ozone is used to reduce mycotoxins in food and feed, and it showed sanitation and antimicrobial effectiveness against viruses, bacteria, spores, and fungi [44]. The application of ozone to reduce or eliminate mycotoxins in foods and feeds can be performed by fumigation or in solution; the latter method is faster, as confirmed by a study where ozone solution at a concentration of 10 mg·L−1 was used to treat a 1 μg mL−1 of deoxynivalenol (DON) solution for 30 s achieving a degradation rate of 54.2%. Meanwhile, the degradation rate was higher in scabbed wheat (moisture content = 17%) reaching 57.3%, when treated for 12 h with ozone gas (Concentration = 60 mg L−1). The high degradation rate of DON can be established by increasing the concentration of ozone in solution and gas and by prolonging the application time [47].
Ozone fumigation of sun-dried herbs and spices showed that the main factors for achieving high microbial reduction and high aflatoxin degradation are the concentration of ozone and the exposure times. Ozone fumigation at 3 ppm for 210 min showed a considerable decrease in the total aflatoxins concentration by 93.75% in licorice and by 90% in peppermint. The disadvantage of this method is the reduction of the essential oil of chamomile by 57.14% and peppermint by 26.67% [44].
The degradation of trichothecene mycotoxins by aqueous ozone showed a pH sensitivity with maximum effectiveness at acidic pH of 4–6 and no effectiveness at alkaline pH of 9 [46].
Ozone treatment can be implemented during the storage of crops in a silo. In a previous study aiming at the decontamination of filamentous fungi, it was difficult to achieve homogeneity of ozone concentration in the silo during rice treatment following the application of 0.393 kg O3 m−3 rice. The highest concentration of ozone was in the lower part of the silo, at the proximity of the ozone’s inlet, suggesting a strong reduction of fungi in this area, while the effect of ozone gradually decreased while moving toward the upper parts of the silo [49].
Ozone is naturally unstable, leaving no residues in foods and feeds after its transformation into oxygen in the treated samples. It has a GRAS status (generally recognized as safe). It causes no waste and does not impose pollution problems [44,47]. At the industrial scale, the cost of ozone must be considered in developing countries [63].
Ozone gas applied to the rice silo did not damage the rice quality. No significant changes were perceived, especially in starch modifications, lipid peroxidation, protein profile, and microstructure alteration [49]. Concurrently, the treatment of parboiled rice during the maceration stage showed many advantages in the quality of the treated rice, such as higher head rice yield, higher luminosity and hardness, decreased cooking time, percentage of defective grains, and the abundance of soluble protein [45].
As shown in Table 1, the ozonation appears to be more effective in reducing the microbial load than in reducing mycotoxins already produced in food or feed. Higher aflatoxin reduction rates are achieved in powdered herbs and spices than in intact grains, wheat, and rice. The organoleptic and nutritional characteristics are affected differently in diverse food matrices. They range from no significant modification, or a slight improvement in quality attributes of wheat and rice, to a detrimental effect on the essential oil of herbs and spices [44,47,48,49].
2.1.3. Ammoniation
Ammonia (NH3) is a gas stored in water solution or pressurized bottles. It is used to detoxify mycotoxins in different food matrices. Most studies have focused on aflatoxins [37]. Many studies supported the use of ammonia to detoxify aflatoxins in foods and feeds and proposed it as an effective, economic alternative [55,56]. High reduction rates of aflatoxins have resulted in ammoniation reaching 96 and 99% [54].
Ammonia is more effective against aflatoxins G1 and G2 than aflatoxins B1 and B2. This is confirmed by a study that demonstrated that the degradation rate was 95% for aflatoxin G1, 93% for aflatoxin G2, 85% for aflatoxin B1, and 83% for aflatoxin B2 in artificially contaminated maize crops [56].
The highest efficiency of ammoniation in aflatoxin detoxification is achieved by the use of 0.5 to 2.0% ammonia at moisture levels between 12 and 16% and under pressure (45–55 psi) at high temperatures reaching 80–100 °C for 20 to 60 min where the recovery of the ammonia is conducted by evaporation at the end of the process [54,64].
The degradation of deoxynivalenol (DON) in contaminated wheat kernels was confirmed, and the achieved degradation rates were 75% or higher. The initial concentrations of DON in the kernels were up to 2000 μg/kg and vapor ammonia was implemented at a high temperature reaching 90 °C for 2 h. The toxicity of the ammoniation products is lower than DON [37].
Aflatoxins-contaminated corn is detoxified by the use of aqua-ammonia (liquid) or anhydrous ammonia (gas). The treatment with aqua-ammonia imposes the drying of the crops before storage. Ammoniation could affect the organoleptic characteristics of the treated corn by causing grain darkness as a result of the caramelization of sugar (altrose) caused by the increase in the temperature during treatment [55].
2.1.4. Acid
Food-grade acids can be used for the degradation of many mycotoxins [62]. These acids affect mycotoxins differently. Ochratoxins are reduced through their conversion to phenylalanine and lactone acid. Aflatoxins could be reduced by an acid-catalyzed addition of water to the vinyl ether double bond of AFB1 and AFG1, and they will be converted to their hemiacetal [58].
The high efficiency of food-grade acids in the reduction of aflatoxins is confirmed when used at a concentration of 9% for 15 min at two moisture levels (10 ± 3% and 16 ± 3%), reaching 99% for citric acid, 99.9% for lactic acid, and 96.07% for propionic acid. The most favorable results are obtained following the use of citric acid because of its efficiency in the reduction of the four aflatoxins (AFB1, AFB2, AFG1, AFG2) without the formation of any hazardous residues or metabolites [57].
The conversion rate of AFB1 to AFB2a by citric acid solution (1 M) reached more than 97% when implemented at room temperature for 96 h. This rate increased to 98% and the process was accelerated so that it could be accomplished in only 20 min when boiling was used [59].
Another study evaluated the effect of citric acid and lactic acid solution on the reduction of DON and its derivatives. This study showed that the 5% solutions of both acids are effective in reducing the DON and its derivative 15Ac-DON but have no or small effect on zearalenone, fumonisins, and culmorin [58]. The organic acid may affect the quality of some products causing discoloration and slight changes in odor and taste [59]. The high cost of organic acids is a challenge for their use in the detoxification of feeds [62].
The instability of ozone may support its safety in being used for the degradation of mycotoxins in food and feed without leaving harmful residues in the treated materials. The results shown from different studies in Table 1 put forward the efficiency of the ozonation and ammoniation. Ammonia was able to achieve higher reduction rates of AF and DON than ozone in different food materials. It is worth noting that ammoniation has not been approved by the FDA and may cause many sensorial quality problems [55], and its effect on aflatoxins may be reversible in an acid medium such as the gastrointestinal tract [54]. Many studies proved the high efficiency of citric acid solution in the reduction of mycotoxins and, especially, aflatoxins, reaching 99% with few detrimental effects on food quality. The limitation of its scalability and its use at the industrial level is related to the high cost of these acids [58].
Nixtamalization is a processing step of maize that can be used as a means to eliminate or reduce the number of mycotoxins [65]. Contradictory results about the effectiveness of the traditional nixtamalization process (TNP) are presented in Table 1. Maureen et al., proposed the high efficiency of this process to reduce AF (up to 90%) [50], while Rodríguez–Aguilar et al., declared that TNP is not efficient in reducing the AF in maize and confirmed the contribution of this process to environmental pollution through the high amount of wastewater generated during its execution [51,52]. A potential solution to this harmful effect on the environment could be attributed to the change in the cooking ingredient [53]. The absence of the ideal chemical treatment for the mitigation of mycotoxins imposed the necessity to find other alternatives to be discussed in the next section.
2.2. Physical Treatments
Many traditional methods, such as cleaning and sorting, are used. These methods are capable of physical separation by removing the contaminated portions from the crops and preventing the transfer of the pathogens to the non-contaminated portions. These are not able to neutralize or degrade the mycotoxins already produced in the crops; they only isolate the contaminated portions [28,66,67]. Several industrial processes require the use of conventional cooking at temperatures below 100 °C. Most mycotoxins are heat stable, and it is not possible to mitigate them by these conventional heat treatment processes [68].
The physical detoxification methods explained in this section (Table 2) include thermal treatments or invasive methods such as extrusion [68], and non-thermal treatments or non-invasive methods such as photocatalysis [69], cold plasma [70,71], electrolyzed oxidizing water [72], and irradiation [73,74]. These technologies are beneficial since they are safely used for many food matrices without causing negative effects on the nutritional and organoleptic quality of treated food. The limited scalability at the industrial level may be considered a disadvantage of many physical treatments [75].

Table 2.
Physical treatments for the reduction of mycotoxins in solid foods and feeds.
2.2.1. Photocatalytic Treatment
The use of UV-visible irradiation combined with a semi-conductive photocatalyst showed high efficiency in the reduction of aflatoxins in a liquid medium [62]. DON was degraded in contaminated wheat samples by 72.8% following the use of photocatalyst UCNP@TiO2 (8 mg mL−1) for 90 min with a ratio of wheat to liquid of 1:2 [76].
The photocatalytic efficiency NaYF4:Yb,Tm@TiO2 on the degradation of DON was greater in solution than in wheat. This decrease in efficiency may be caused by the attachment of toxins to starch or proteins in the wheat, or to other wheat components, or even the shielding effect of wheat grains that hinder the light from reaching all contaminated surfaces. A complete degradation was achieved in a solution containing 10 μg mL−1 of DON when treated with simulated sunlight using NaYF4:Yb,Tm@TiO2 (6 mg mL−1) at pH 7 for 60 min. This rate was decreased to 69.8% when artificially contaminated wheat was treated with UCNPs aqueous solution (Ratio 1:1) by illuminating the samples with a Xe lamp for 120 min [69].
Many factors make the use of these techniques more advantageous. They are completely inorganic and do not result in the formation of secondary metabolites, causing pollution. They are cost-effective and easy to apply, requiring mild conditions [95]. Photocatalysis did not cause significant changes in the starch, the protein contents, or the amino acid value in wheat. However, there are also many disadvantages, such as the increased yellowness and decreased whiteness of the wheat flour, the damaged surfaces of starch granules when a prolonged illumination time is applied, and decreased fatty acid value, wet gluten content, and pasting properties of wheat [76].
2.2.2. Cold Plasma
The common states of matter are solid, liquid, and gas; plasma, the fourth, uncommon state, is formed by supplying enough energy to substances to assure the transition from the solid to the ionized state [96].
Cold atmospheric plasma (CAP) is a non-thermal technology that has shown its efficiency in reducing fungal pathogens and their toxins [64]. This technology is a successful alternative to the traditional treatments (heat treatment, wet chemistry, or UV-irradiation) usually implemented. These treatments proved their inefficiency in mitigating AF without affecting the food and feed quality [77].
Complete degradation of aflatoxin B1 is achieved by the effect of CAP-RONS (Reactive Oxygen Plasma and Nitrogen Species), which have a high oxidative potential and high affinity to react with the vinyl bonds in organic molecules. These RONS are yielded by the generation of non-equilibrium atmospheric in ambient air. The same degradation rate is achieved by applying the same CAP system to contaminated corn kernels. CAP treatment is faster than UV-C treatment and is significantly more efficient in AFB1 reduction than UV-C treatment [77].
A different study explored the effect of low-pressure dielectric barrier discharge (DBD) plasma on the degradation of T-2 and HT-2 toxins in oat flour by using different working gases. Only partial degradation of T-2 and HT-2 toxins was achieved by applying this technology, and all the experiments yielded similar results to those obtained by the thermal treatments usually applied during food processing, such as cooking, extrusion, or roasting. None of the used gases in this study could completely detoxify the oat flour samples. The highest degradation rate of T-2 and HT-2 toxins reached 43.25% and 29.23%, respectively, after treating the samples with nitrogen for 30 min. CAP treatments using molecular oxygen and air as working gases did not affect these toxins [78].
Another study confirmed the results of the previous one that DBD plasma is not capable of achieving complete detoxification of AFB1 and FB1 in food matrices. In this study, spiked maize kernels were exposed to a pulsed dielectric barrier discharge (DBD) plasma jet for 10 min. The detoxification rate was 65% for maize grains spiked with AFB1 with an initial concentration of 1.25 ng/g and 64% for maize grains spiked with FB1 with an initial concentration of 259 ng/g [79].
Roasted coffee beans artificially contaminated with ochratoxin A (OTA) were treated with cold plasma for 30 min, and the degradation rate reached 50%. This result was satisfactory as per the EU standards. The brine shrimp lethality assay was used to evaluate toxicity, and the result was “Toxic” for the untreated beans and “Slightly Toxic” for the treated ones [80].
CAP has a low detrimental effect on the organoleptic and nutritional quality of foods and feeds. This treatment could explain this treatment’s low penetration depth, so the degradation may affect only the superficial layers and protect all internal components. The generated RONS may affect the antioxidants and lipids present in the product [79]. SBD plasma has been shown to be more efficient than DBD plasma in the reduction of aflatoxins and other mycotoxins (Table 2), which seems to be more practical and scalable at the agri-food industrial level [77].
2.2.3. Pulsed Light
Pulsed light is a non-thermal treatment used to improve food safety and maintain the quality of food products by preventing the effect of heat treatment adopted in other techniques. It is generated by the flash repetition of non-coherent, broad-spectrum, high-intensity light [97]. It includes infrared, ultraviolet, and visible rays. It has been FDA approved since 1996 to be used for the superficial decontamination of food products (maximum fluence 12 J cm−2) [62,98].
This technology achieved higher reduction rates of AFB1 and AFB2 in rice bran than in rough rice because of its high efficiency on the surface and external parts [83]. Another study showed a positive relationship between the aflatoxins degradation rate and the initial concentrations in solid medium and the intensity of pulsed light treatment [82].
The effect of pulsed light on red pepper powder to mitigate microorganisms and mycotoxins such as total AF, AFB1, and OTA was investigated. The application of 61 pulses at high fluence (9.1 J/cm2) for 20 s effectively reduced the yeast and molds and the total plate count in red pepper powder. The same treatment parameters were applied to cause the reduction of total aflatoxins by 50.9%, aflatoxin B1 by 67.2%, and ochratoxin A by 36.9%. Total phenols increased apparently and significantly, while the total color was slightly changed [81].
2.2.4. UV-C Irradiation
UV light is another non-thermal treatment used as an alternative to thermal and chemical treatments to reduce the negative effects on the quality of treated foods and prevent the formation of residues and byproducts. UV light is classified into different bands according to the wavelength used. UV-C, which ranges from 200 to 280 nm, is commonly used because of its high efficiency against microorganisms, specifically at 250 and 260 nm [99].
The effect of UV-C irradiation on different types of rice was studied using UV irradiation at 254 nm for 1 and 3 h (moisture content of rice = 13%). The one-hour treatment was able to achieve a dose of 2.06 KJ cm−2, causing fungal decontamination and mycotoxin reduction in black and red rice without affecting the cooking and color characteristics. In contrast, the three-hour treatment increased the dose to 6.18 KJ/cm2 and increased the efficiency with a reduction of the total phenolic compounds. In brown rice, only the high dose achieved by the three-hour treatment was effective in reducing the fungal decontamination while causing undesirable browning of grains [73].
Low penetrability and the shadowing effect are two hurdles to the success of UV-C irradiation [100]. To overcome these problems and to increase the efficiency of UV irradiation, a customized rotational cylindrical chamber was established by Shen and Singh. In this study, the authors used a UV indicator applied to peanut kernels and treated with UV-C irradiation at 2.3 mW cm−2 for 2 h with a continuous rotational movement at 11 rpm. The uniformity of the UV-C treatment was significantly improved when the reduction percentage of AFB1 was increased by 23.4% [85].
In another study, innovative vibrational decontamination equipment was designed for the decontamination of maize and peanut to increase the efficiency of this technology. UV-C irradiation was applied at a range of 1080 to 8370 mJ cm−2. After incubation for 10 days, the samples irradiated with 8370 mJ cm−2 showed the lowest count of A. flavus in peanuts and maize. AFB1 reduction rates reached 43% and 51% for maize and peanut, respectively [84].
2.2.5. Gamma Irradiation
Gamma irradiation is a treatment that can be used to disinfect crops by reducing the number of fungi or by mitigating mycotoxins already produced by the fungi in these crops [64]. A gamma source, such as cobalt-60, must be used to generate very high-energy photons. These photons are capable of killing spoilage and pathogenic microorganisms by causing damage to their DNA. The free radicals and ions that occur after the interaction of the energy with water molecules present naturally in food products or crops will attack microbial DNA [101,102].
The important role of water in the successful use of gamma irradiation was supported by a study that investigated the degradation rate of OTA in aqueous solution and different food products (wine, grape juice, and wheat flour). The sensitivity of OTA irradiated at 30.5 kGy reached the maximum in water solutions. It was also demonstrated that OTA is highly resistant to the same irradiation dose in solid matrices or dry foods [87].
Another study confirmed the use of gamma irradiation to inhibit A. flavus and A. ochraceus and reduce AF and OTA in maize. In this study, low doses of 6 kGy lead to the inhibition of mold growth. The reduction of the formed AFB1 by 40.1%, AFB2 by 33.3%, and OTA by 61.1% in maize required higher doses (20 kGy) [86]. A different gamma irradiation study was performed on sorghum and showed that the reduction of natural fungi in sorghum reached 90% at 3 kGy with maximum reduction rates of 59% for AFB1 and 32% for OTA realized at 10 kGy [88].
2.2.6. Extrusion
Mycotoxin reduction could result from food processing operations, such as extrusion, which can simultaneously improve product quality and increase food safety levels by reducing toxins [103]. Conventional cooking treatments (conducted at temperatures below 100 °C) cannot participate in the mitigation of mycotoxins in food products because most of these toxins are heat stable. Alternative cooking treatments, such as extrusion, are performed at higher temperatures and show efficiency in reducing mycotoxin contamination [68].
A study conducted by Massarolo et al. used a single-screw extruder at 50 ng g−1 to reduce aflatoxins on spiked cornmeal samples. The reduction rates of all aflatoxins were higher in the samples after the addition of high amylose corn starch, reaching 89.9% for AFB1 and AFG2, 88.6% for AFB2, and 75% for AFG1. Extrusion may cause possible interactions of the toxins with food components, decreasing their bio-accessibility. Their availability in the small intestine increased significantly after digestion [90]. Another study was conducted by Janić Hajnal et al., and focused on the effect of co-rotating twin-screw extruder on other mycotoxins (DON, 3- and 15-AcDON, HT-2, TEN, and AME) in whole grain triticale flour. The optimal reduction rate of all studied mycotoxins was achieved at a screw speed of 650 rpm with a feed rate of 30 kg/h and moisture content of 20 g/100 g. A higher reduction rate was found in AME, while the lowest rate was detected for DON [89].
2.2.7. Electrolyzed Oxidizing Water
EOW is prepared by introducing tap water and salt into an electrolysis chamber. It is considered a sanitizer or disinfectant because of its bactericidal and fungicidal effects. It is characterized by its specific pH value, its oxidation-reduction potential, ORP, and the available chlorine concentration, ACC [104]. The physicochemical properties of EOW used to treat foods are ACC from 10 to 100 ppm and ORP from −800 mV to higher than 1000 mV. The pH depends on the type of water used in the research, acid, slightly acid, neutral, or alkaline electrolyzed water [104,105]. Research was conducted to study the effect of different pH values of EOW on fungal elimination and DON reduction in wheat grains. This study showed that for acid-electrolyzed water, the optimal pH for reducing fungi was 2.5 and 5.5 to eliminate DON. For alkaline electrolyzed water, the optimal pH value to eliminate DON was 9.5, while pH values between 8.5 and 12.5 were effective for eliminating fungi also. The optimal pH values of the alkaline EOW (pH 9.5) and the acid EOW (pH 5.5) did not affect the wheat characteristics such as color, moisture content, and protein and gluten contents. The starch morphology also did not change significantly. A beneficial effect was caused by the acid-electrolyzed water on wheat flour, causing higher stability, increased farinograph quality numbers, and lowered softening degree [91].
2.2.8. Electron Beam Irradiation
The electron beam is a type of ionizing irradiation [99]. It is generated by the use of a safe dose of an electric accelerator [63]. It is a non-invasive, non-thermal, and eco-friendly detoxification method used for cereal-based products in order to reduce microbial and mycotoxin contamination [106,107]. This irradiation treatment was used to decontaminate naturally contaminated red pepper powder. Low doses of 6 and 10 kGy reduced the yeasts and mold count and the total plate counts by 3, 4.4, and 4.5 log CFU/g, respectively. A higher dose of 30 kGy achieved a 25% reduction in OTA. Electron beam irradiation is more effective in the reduction of microorganisms than mycotoxins. It is worth noting that this treatment had low detrimental effects on the quality of the treated pepper powder, causing a slight change in color and less than 15% reduction of total phenols, carotenoids, and antioxidant activity [92]. Another study conducted by Kim et al. used irradiation as a non-thermal decontamination method for an uncooked Korean cereal product called Saengshik. Electron beam irradiation was conducted at 10 kGy and showed an increase in the total phenolics and a decrease in the total carotenoids and chlorophylls with preservation of antioxidant capacity when the irradiation was conducted at doses lower than 10 kGy [108].
2.2.9. Milling
The milling process is effective in reducing mycotoxins in feeds and foods [68,109]. The weakness of this method lies in the redistribution of mycotoxins in the resulting fractions of milling and their concentration in the products intended for animal feed [67].
Scarpino et al., showed that cleaning maize grains may cause a reduction in the fungal metabolites by 1.2 to 2 times. In this study, the milling process of maize kernel caused an unequal redistribution of the mycotoxins in the different maize fractions and concentrated most mycotoxins in the germ. The highest mycotoxin contents were found in animal feed products, and the healthier products are large flaking grits [93]. A study was conducted using this principle by implementing dry and wet de-germination to maize and showed that the latter was more efficient for decreasing fumonisins in the milled products. Cleaning the kernels reduced FBs by 42%. Furthermore, the tempering degermination process of the uncleaned kernels achieved high reduction rates as compared to the dry degermination, reaching 94% for the largest-sized flaking grits. This process was able to facilitate the separation of the horny endosperm from the fine milling fractions. [94].
By evaluating the results of the different chemical treatments in Table 1 and the results of the different physical treatments in Table 2, we can conclude that chemical treatments are able to achieve the highest degradation rates of different mycotoxins in solid foods and feeds. In contrast, the physical treatments achieve lower degradation rates, but their effects on the quality of treated materials are smaller.
The shadowing or shielding effect is the principal limitation related to the use of photocatalysis and UV-C irradiation in the reduction protocols of mycotoxins in solid food materials. Many studies (Table 2) tried to overcome this limitation by rotating the irradiated peanuts to ensure UV uniformity, but no significant increase in the reduction rates occurred. Photocatalysis achieved higher reduction rates of DON, reaching a total elimination of DON in wheat [76]. CAP is a superficial treatment with low penetration depth. It showed good efficiency in reducing AFT and AFB1 without deterioration in the quality of the treated product. Furthermore, SBD plasma was more effective than DBD plasma since it achieved the complete elimination of AFB1 [77,79]. As working gas, nitrogen achieved the highest reduction rates as compared to oxygen and air when using the DBD system [78]. The pulsed light effectiveness was superficial and showed greater AF reduction rates when applied to rice bran than to rough rice. The AFB reduction rates are defined by the PL intensity and the initial concentration of mycotoxins in the food to be treated [83]. Gamma irradiation showed good effectiveness in reducing mycotoxins in food containing a high amount of water and reducing the fungal load in solid food. Low gamma irradiation doses were able to eliminate fungi and reduce mycotoxin formation in maize, but higher doses were required to reduce the already produced OTA in this material. Mycotoxins were not completely eliminated in any of the mentioned studies (Table 2) [86,87,88]. The electron beam showed its efficiency as a disinfectant by the reduction of different microorganisms such as bacteria, yeasts, and molds, but it was not effective in the reduction of OTA in red pepper powder [92]. The milling process resulted in the reduction of mycotoxins in many edible fractions of maize but caused the concentration of these fungal metabolites in the germ, especially in the fractions used as animal feeds [93].
2.3. Biological Treatments
Biocontrol showed high efficiency in the prevention of AFs formation in the pre-harvest stage when non-aflatoxigenic biological control strains are inoculated in the fields and competed with aflatoxinenic strains of Aspergillus for nutrients and place and causing their exclusion [110,111]. The studies discussed in this section aimed to mitigate the already formed mycotoxins in feeds and foods by biological treatments and not to prevent their formation in crops (Table 3).
Most studies about the mitigation of mycotoxins by biological means focused on the treatment of liquid food or milk [32,33,112], assessing the effect of yeast, bacteria, or their enzymes on the mycotoxins in buffers or solutions [30,113,114]. Biological detoxification could be the result of binding the targets by adsorption mechanisms or by degradation. This detoxification of mycotoxins can be conducted using microorganisms (bacteria, biofilm, or yeast) or their metabolites and enzymes [115]. In this section, we screen various studies using biological control strategies to mitigate the mycotoxins in solid food and feeds (Table 3).
ZEN-detoxifying bacillus strains were used to detoxify highly contaminated maize with an initial concentration of 5 mg kg−1 of ZEN. The degradation of ZEN is related directly to the esterase activity, which has been found in all tested strains, with the maximum activity in B1 and B2 strains. The highest ZEN degradation rate was attained in B2 strains, reaching 56%. B2 strains showed their efficiency in the detoxification of other mycotoxins with different rates—AFB1: 3.8%, DON: 25%, FB1: 39.5%, T2 toxin: 9.5%. The presence of ZEN enhanced the fermentation process of the contaminated maize compared to the non-contaminated grains [116].
CotA laccase is found in the endospore coat of Bacillus. It protects spores from UV light and hydrogen peroxide and has an oxidizing capacity. CotA laccase was immobilized onto chitosan microspheres and used to degrade ZEN in artificially contaminated cornmeal samples. The free CotA laccase form achieved a degradation rate of 70%, while the immobilized form was faster and more effective, achieving a higher degradation rate reaching 90%. The most important advantage is the reuse of the immobilized enzyme. Guo et al., showed that the degradation rate decreased to 54% following multiple uses of the immobilized CotA laccase in the third cycle, reaching only 21% in the fifth one [117]. Lactic acid bacteria (LAB) were used to mitigate mycotoxins in wheat-based products. The Pediococcus acidilactici LUHS29 strain achieved the highest reduction rates of mycotoxins when used alone in sourdough fermentation for 48 h. It removed 15-AcDON, AOH, D3G, toxins H-2 and HT-2, completely removed ENNB1, and reduced the DON by 44–69%. The combined fermentation using this LAB with Lactobacillus Plantarum LUHS135 strain showed great efficiency and increased the reduction rate of DON to 79–100% [118]. In a study conducted by Alberts et al., enzymatic detoxification was examined using Fumonisin Esterase FumD to degrade FB in maize. This enzyme can hydrolyze and remove the tricarballylic acid groups when added to maize during the conditioning step (for 250 min) during the dry milling process. The use of 40 U/kg of FumD in maize resulted in a 99% degradation of FBT in total hominy feed but did not accomplish any degradation of FBT in super maize meal [119].
The fungal growth and/or the mycotoxin production was controlled in bread using specific yeast strains and achieving reduction rates varying between 16.4 and 33.4% for DON, 18.5 and 36.2% for NIV, and 14.3 and 35.4% for ZEA [120]. The heat treatment of peanut samples at 100 °C for 15 min before solid-state fermentation by Zygosaccharomyces rouxii showed great efficiency in the mitigation of AFB1, and the reduction rate reached 97.52% [121].

Table 3.
Microbial and enzymatic treatments for the reduction of mycotoxins in solid foods and feeds.
Table 3.
Microbial and enzymatic treatments for the reduction of mycotoxins in solid foods and feeds.
| Treatment | Feeds/Foods | Contaminants | Experimental Parameters | Reduction Rates | Advantages | References |
|---|---|---|---|---|---|---|
| Bacteria: ZEN-detoxifying Bacillus (ZDB) strains | Maize | ZEN | The highest level of ZEN degradation | B2 strain-reduction rate = 56% |
| [116] |
| B2 strain detoxifies other mycotoxins | Reduction rates: AFB1: 3.8%; DON: 25%; FB1: 39.5%; T2 toxin: 9.5% |
| ||||
| Bacteria: Bacillus licheniformis spore CotA laccaseapplication of immobilized laccase in contaminated corn meal | Corn meal | ZEN | Treatment with immobilized CotA laccase onto chitosan microspheres for 12-h | Degradation rate: 90% |
| [117] |
| Treatment with free CotA laccase for 12-h | Degradation rate: 70% | |||||
| Reuse of immobilized enzymes for 5 cycles | Decreased degradation rate on each after each cycle: Cycle 1: 90%; Cycle 2: 77%; Cycle 3: 54%; Cycle 4: 30%; Cycle 5: 21% | |||||
| Bacteria—Fermentation: Lactic acid bacteria | Wheat-based products | DON 15 -AcDON AOH D3G, toxins H-2 and HT-2: Enniatin ENNB1 | Pediococcus acidilactici LUHS29 strain | The strongest mycotoxins decontamination effect |
| [118] |
| Prolonged fermentation at 35 °C for 48 h with Pediococcus acidilactici LUHS29 strain | DON: 44–69% 15-AcDON, AOH, D3G, toxins H-2 and HT-2: Removal Enniatin: 5–70% ENNB1: complete removal | |||||
| Combined fermentation (Lactic acid bacteria 7 (JCM 1149) and Pediococcus acidilactici LUHS29 (DSM 20284)) | Complete elimination or effective reduction of DON: 79–100% | |||||
| Enzyme | Maize | FB | FB degradation during dry milling of maize |
| [119] | |
| Fumonisin esterase FumD | Enzyme concentration: 40 U/kg | Reduction rates FBT:
| ||||
| Yeast | Wheat grains and bread | Fusarium Mycotoxins: DON, NIV ZEN | Bread prepared by baking with the addition of an inoculum of the test yeast | Reduction rates: DON: 16.4% to 33.4%; NIV:18.5% to 36.2%; ZEA: 14.3% to 35.4% |
| [120] |
| Yeast | Peanut meal | AFB1 | Peanut samples are heated at 40, 60, 80, 100, or 110 °C for 10 min | [121] | ||
| The residual rates after heat treatment at the following temperature for 10 min: (T:% of residual AFB1 | 80 °C: 61.08%; 100 °C: 63.46%; 110 °C: 49.63% | |||||
| The residual rates after fermentation by Z. rouxii: (Temperature: % of residual AFB1) | (40 °C:32.73%)-(60 °C:20.85%)-(80 °C:16.18%)-(100 °C:5.13%)-(110 °C:5.10%) | |||||
| 100 °C | The optimal temperature achieved the highest reduction rate | |||||
| Peanut samples are heated at 100 °C for 5, 10, 15, or 20 min | ||||||
| The residual rates after heating at 100 °C for different times: (time: % of residual AFB1) | (5 min: 21.06%)-(10 min: 5.13%)-(15 min: 2.48%)-(20 min: 2.44%) | |||||
| 15 min | The optimal time | |||||
| Optimal treatment (100 °C -15 min): | Residual % of AFB1: 2.48% | |||||
3. Subsequent Detoxification Treatments Used in Solid Foods and Feeds
The efficiency of the above-mentioned techniques on mycotoxin reduction showed great variability when implemented singly [122]. The additive or synergistic effect of using many combined treatments subsequently are summarized in Table 4 and evaluated below.

Table 4.
Subsequent techniques to mitigate mycotoxins in solid foods and feeds.
3.1. O3, UV-C, and Citric Acid
Ozone and acid treatment, when implemented individually, can reduce AFB1 and AFG1 more than AFB2 and AFG2. In contrast to O3 and acid treatment, UV-C by itself has great efficiency in the degradation of AFB2 and AFG2. This difference made the combination of the three treatments a great opportunity to increase the degradation rates of aflatoxins in contaminated pistachio samples. The subsequent treatments of the contaminated pistachio with 3N citric acid, followed by O3 exposure for half an hour, and UV-C irradiation for 36 h, achieved high reduction rates of more than 90% for AFB1 and AFB2 and more than 99% for AFG1 and AFG2. This combination did not cause significant changes in the organoleptic and nutritional quality of the pistachio compared to non-treated pistachio samples [123].
3.2. Extrusion and Fermentation
Extrusion is a type of high-temperature treatment, and as discussed previously, it can decrease the number of mycotoxins in cereals [68]. Contradictory results were indicated by Zokaityte et al. They found that extrusion may affect the mycotoxin levels differently by increasing, decreasing, or not changing their concentrations in the samples. The combination of extrusion at different temperatures (115 and 130 °C), over different screw speeds (16, 20, and 25 rpm), with fermentation for 24 h at 30 °C by using 2 strains of LAB (Lactobacillus casei and Lactobacillus paracasei) and their effects have also been studied. This combination increased the amount of lactic acid and decreased bacterial contamination as a result of pH reduction. The effect of extrusion on different mycotoxins contradicted the results obtained in other studies. The 15-DON concentration increased in all extruded samples, and the fermentation of the samples decreased them to acceptable levels. The capacity of fermentation to decrease mycotoxin levels in the food or feed samples may be caused by the binding capacity of LAB. Mycotoxin types and their initial concentrations in the food matrix, the physicochemical characteristics of this matrix, and the fermentation variables, such as temperature and duration, play a major role in determining the binding percentages [124].
3.3. Roasting and Brewing
The combination of roasting and brewing of naturally contaminated coffee beans by using the traditional Qatari method was studied to show its effect on the reduction of AF and OTA. The roasting temperature is the main factor affecting the reduction rates of AF and OTA in the coffee beans. The reduction rates were proportional to the roasting temperature. The maximum reduction rates achieved with the high roasting scheme were 61.52% and 57.43% for AFs and OTA, respectively. Brewing alone was effective in reducing OTA more than AF. Brewing showed high efficiency in the reduction of both mycotoxins in roasted coffee beans by a low roast scheme. The best combination was defined at a high roast scheme with traditional brewing, and the cumulative reduction rates were 62.38% for AFs and 64.7% for OTA. It is worth noting that roasting temperatures applied to coffee beans in Arab countries are lower than those applied in other countries to preserve the traditional organoleptic characteristics such as color and flavor [125].
3.4. PEF and Thermal Treatment
The effect of the thermal process, the pulsed electric field, and the combination of both treatments on the reduction of AFT and AFB1 were studied. Following the optimization of both treatment modalities, mycotoxins were affected by the thermal process time at alkaline pH, the thermal process temperature at neutral, and acid pH values when this process was implemented individually. The highest reduction rates were obtained after treatment at 110.36 °C for 15 min at pH 10, reaching 96.696% and 95.473% for AFB1 and AFT, respectively. On the other hand, PEF treatment was also optimized, and the highest reduction rates were achieved at a pulse width of 65 μs and output voltage of 26%. It seems that the combination of both treatments did not achieve a great improvement in the reduction rates of AFT and AFB1. As compared with the optimal thermal treatment implemented alone, this combination increased the reduction rates by 0.185% for AFB1 and 0.248% for AFT [126].
3.5. H2O2 Treatment at Moderate Temperature after Roasting
The effect of H2O2 on aflatoxins reduction in peanuts was investigated and showed higher efficiency following its application at 50 °C instead of room temperature (20 °C), while the reduction rate increased from 30% to 73%. The same H2O2 treatment (30 g/hg H2O2 at 50 °C) was implemented on unroasted peanuts for 8 h, achieving a higher AF reduction rate of 86%. The combination of this treatment with pre-roasting the peanuts at 140 °C for 10 min caused the inactivation of catalase and increased the reduction rate slightly to reach 90%. The constructive points of this combination were the preservation of the oil quality of the treated peanuts, the absence of significant weight loss, and the conservation of the peanut’s form since the temperature did not reach that of starch gelatinization. Moreover, the combination is eco-friendly, leaving no H2O2 residues after air drying the treated peanuts at 35 °C for 12 h [127].
4. Simultaneous Detoxification Treatments Used in Solid Foods and Feeds
In this section, we discuss many combined treatments applied simultaneously to food or feed matrices, and these combinations are represented in Table 5.

Table 5.
Simultaneous techniques to mitigate mycotoxins in solid foods and feeds.
4.1. UV with H2O2
UV-C is used in combination with H2O2 to degrade aflatoxins in peanuts. It represents an eco-friendly technique leaving no toxic or harmful byproducts, with unique residual compounds limited to water and oxygen. The simultaneous application of these two treatments for 1 h (UV-C: 2.76 mW/cm2—H2O2: 1 g/hg) accelerated the degradation rates of AF in both whole peanut kernels and milled kernels to 30% and 60%, respectively. The advanced oxidative processes (UV and H2O2) affected the quality of the oil in milled kernels and caused the darkening of the whole kernels [128].
4.2. Pulsed Light with Citric Acid
The combination of pulsed light with citric acid showed great efficiency on AFT, AFB1, and AFB2 in peanuts, with reduction rates reaching about 98.2%, 98.9%, and 98.1%, respectively. The chemical quality did not show significant changes as a result of this combined effect, but a significant change in the color of peanuts occurred [129]. In previously discussed studies about pulsed light treatments, the reduction rates of AFT, AFB1, and AFB2 were in the range of 39.2 to 90.2% in red pepper powder and rice with different fluence ranges applied [81,83]. Therefore, combining an acid with pulsed light can be considered a beneficial combination, demonstrating higher efficiency in mitigating aflatoxins than each treatment alone.
4.3. Infrared with Alkaline Treatment
There are contradictory results concerning the nixtamalization efficiency in reducing aflatoxins in maize by using the traditional process. Rodríguez–Aguilar et al., proposed the non-efficiency of the traditional nixtamalization process (TNP) in the elimination of aflatoxins from contaminated maize [51]. Meanwhile, Zavala–Franco et al., found that TNP can degrade aflatoxins in maize by 98.35%. The same study proposed using infrared as an alternative to heat treatment in the nixtamalization process. The applied protocol satisfactorily achieved a degradation rate of 93.82%. No formation of AFB1-Lys occurred with the infrared nixtamalization process. This combination of alkaline treatment with infrared seems to be promising for mitigating mycotoxins while generating fewer toxic materials than the traditional process [130].
4.4. Roasting with Acid
Aflatoxin levels can be reduced by using high-temperature treatment, such as roasting. Degradation rates in the range of 50 to 70% in peanuts and the range of 40 to 80% in maize were achieved [68]. Roasting pistachio nuts at 120 °C for 1 h is optimized when used in combination with the addition of citric acid and lemon juice. The high amount of used acids increased the AFB1 degradation rate to reach 93.1%, which negatively affected the physical quality of the pistachio. Decreasing the acid amount by half decreased the degradation rate to 49.2% but maintained the desired appearance of the treated pistachio [131].
5. Comparison between the Different Mycotoxin Decontamination Treatments
In general, chemical treatments achieved higher reduction rates of mycotoxins than physical treatments in solid foods and feeds. This effectiveness is accompanied by many side effects, such as the detrimental impacts on the quality of the treated food materials (ammoniation) and the formation of unavoidable chemical residues causing an environmental problem (nixtamalization). All the chemical treatments presented in this review showed possible scalability, except the acid treatment, due to the high cost of using organic acids. The physical treatments showed lower degradation rates. This can be seen in the shielding effect in the case of irradiation or the presence of the skin on some foods, such as peanuts. It is worth noting that these physical treatments usually have low penetrability where the effect remains superficial, treating a thin layer. The biological treatments of solid foods or feeds were commonly less available than other treatments. They showed good results and achieved high reduction rates with a beneficial effect of LAB on fermentation by increasing lactic acid production in maize. Combined fermentation using two LAB strains achieved higher reduction rates than those using each strain individually. The results obtained using the biological decontamination treatments prove its suitability to be considered an alternative to physical and chemical treatments by providing a safe, eco-friendly, and cost-effective method with a minimal negative effect on the quality of treated materials.
Concerning the combined treatment and by comparing the subsequent treatments in Table 4 and the simultaneous treatments in Table 5, we can spotlight many successful combinations, such as the subsequent application of O3/UV-C/citric acid and high concentration H2O2 treatment at moderate temperature/roasting, which achieved a reduction of AFs in pistachio and peanuts, respectively. The reduction of AFs by PEF/heat treatment attained high reduction rates in agar, but it was decreased when implemented in dry food, hypothesizing that the presence of water contributes to its success in AF elimination. Roasting/brewing was able to reduce mycotoxins without reaching the complete elimination of AFs and OTA from coffee beans. All the simultaneous treatments mentioned in this review showed their success in reducing or eliminating AFs; reduction rates exceeding 93% were accomplished by implementing citric acid with pulsed light to peanuts, IR nixtamalization to maize, and roasting with acid to pistachio.
6. Conclusions
In this review, we screened, evaluated, and discussed different chemical, physical, biological, and combined techniques to mitigate mycotoxins in solid foods and feeds. Many chemical treatments showed their effectiveness by achieving approximately a total elimination of AFs under certain conditions, such as optimized nixtamalization, ammoniation, and acid. Physical treatments such as photocatalysis and cold plasma were able to achieve the complete elimination of DON and AFB1, respectively. Chemical treatments showed higher reduction rates of mycotoxins than physical treatments, but the latter treatments were favorable from a quality perspective. Their effect was superficial, causing minimal changes in the quality of treated materials. Biological treatments are considered safe, eco-friendly, and cost-effective methods for mitigating mycotoxins. Zygosaccharomyces rouxii or a combination of LAB strains attained high reduction rates. Two or more treatments were used subsequently or simultaneously in order to find a synergistic effect of the combination to achieve high reduction rates in solid food materials without inducing any extreme impacts in each one. Nine combinations are presented in the last two tables, showing the higher reduction rates of aflatoxins (>90%) achieved by the following two combinations when implemented subsequently: O3/UV-C/citric acid and high H2O2 concentration treatment at moderate temperature/roasting. Other combinations were applied simultaneously, also showing their efficiency in the reduction of aflatoxins (>93%), such as citric acid with pulsed light and roasting with acid. These combinations affected the physical characteristics of the treated nuts. Future research should focus on the optimization of physical treatments to increase their mycotoxin reduction efficiency and on the elaboration of more combination possibilities to find the best synergistic effect to protect the product quality and the environment. It is highly important to focus more on implementing detoxification techniques on naturally contaminated materials than spiked or artificially contaminated materials. Natural contamination may be caused by several mycotoxins and may occur differently. Furthermore, these studies should examine the effectiveness of these techniques at the industrial scale more than at the laboratory scale.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate Change, Food Security and Mycotoxins: Do We Know Enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Gomez, K.S.; Castañeda Roldán, E.; Ávila Sosa, R.; Munguía-Pérez, R. Mycotoxins and Climate Change. In The Impact of Climate Change on Fungal Diseases; Frías-De-León, M.G., Brunner-Mendoza, C., del Rocío Reyes-Montes, M., Duarte-Escalante, E., Eds.; Fungal Biology Book Series; Springer International Publishing: Cham, Switzerland, 2022; pp. 239–256. ISBN 978-3-030-89664-5. [Google Scholar]
- Matumba, L.; Namaumbo, S.; Ngoma, T.; Meleke, N.; De Boevre, M.; Logrieco, A.F.; De Saeger, S. Five Keys to Prevention and Control of Mycotoxins in Grains: A Proposal. Glob. Food Secur. 2021, 30, 100562. [Google Scholar] [CrossRef]
- Puri, S.; Shingh, S.; Tiwari, P. Mycotoxins: A Threat to Food Security and Health. Int. J. Appl. Sci. Biotechnol. 2019, 7, 298. [Google Scholar] [CrossRef]
- Marc, R.A. Implications of Mycotoxins in Food Safety; IntechOpen: Cluj-Napoca, Romania, 2022; ISBN ISBN 978-1-83962-904-4. [Google Scholar]
- Hassan, H.F.; Koaik, L.; Khoury, A.E.; Atoui, A.; El Obeid, T.; Karam, L. Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; Obidiegwu, J.E.; Chilaka, A.C.; Atanda, O.O.; Mally, A. Mycotoxin Regulatory Status in Africa: A Decade of Weak Institutional Efforts. Toxins 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Mycotoxins in Lebanese Food Basket—Final.Pdf. 2022. Available online: https://www.usj.edu.lb/intranet/actu/pdf/11610_1952.pdf (accessed on 10 August 2022).
- Pleadin, J.; Frece, J.; Markov, K. Chapter Eight—Mycotoxins in Food and Feed. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 89, pp. 297–345. [Google Scholar]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional Impact of Mycotoxins in Food Animal Production and Strategies for Mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2019; ISBN 978-1-78984-874-8. [Google Scholar]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef]
- Fapohunda, S.O.; Anjorin, T.S.; Sulyok, M.; Krska, R. Profile of Major and Emerging Mycotoxins in Sesame and Soybean Grains in the Federal Capital Territory, Abuja, Nigeria. Eur. J. Biol. Res. 2018, 8, 121–130. [Google Scholar]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent Advances on Toxicity and Determination Methods of Mycotoxins in Foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Altomare, C.; Logrieco, A.F.; Gallo, A. Mycotoxins and Mycotoxigenic Fungi: Risk and Management. A Challenge for Future Global Food Safety and Security. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; pp. 64–93. ISBN 978-0-323-85180-0. [Google Scholar]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination Status of Major Mycotoxins in Agricultural Product and Food Stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Stroka, J.; Gonçalves, C. Mycotoxins in Food and Feed: An Overview. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–419. ISBN 978-0-12-814045-1. [Google Scholar]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Novacco, M.; Ottoboni, M.; Tretola, M.; Dell’Orto, V. Mycotoxins in Wheat and Mitigation Measures. In Wheat Improvement, Management and Utilization; Wanyera, R., Owuoche, J., Eds.; InTech: Milan, Italy, 2017; ISBN 978-953-51-3151-9. [Google Scholar]
- Wan, J.; Chen, B.; Rao, J. Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-based Food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef]
- Piotrowska, M. Microbiological Decontamination of Mycotoxins: Opportunities and Limitations. Toxins 2021, 13, 819. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxin Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Uliana, R.D.; Coppa, C.F.S.C.; Lee, S.H.I.; Kamimura, E.S.; Oliveira, C.A.F.; Corassin, C.H. Aflatoxin M1: Biological Decontamination Methods in Milk and Cheese. Food Sci. Technol. 2022, 42, e22920. [Google Scholar] [CrossRef]
- Assaf, J.C.; Atoui, A.; Khoury, A.E.; Chokr, A.; Louka, N. A Comparative Study of Procedures for Binding of Aflatoxin M1 to Lactobacillus Rhamnosus GG. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2018, 49, 120–127. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin Removal by Lactobacillus Spp. and Their Application in Animal Liquid Feed. Toxins 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Khoury, A.E.; Chokr, A.; Louka, N.; Atoui, A. A Novel Method for Elimination of Aflatoxin M1 in Milk Using Lactobacillus Rhamnosus GG Biofilm. Int. J. Dairy Technol. 2019, 72, 248–256. [Google Scholar] [CrossRef]
- Assaf, J.C.; El Khoury, A.; Atoui, A.; Louka, N.; Chokr, A. A Novel Technique for Aflatoxin M1 Detoxification Using Chitin or Treated Shrimp Shells: In Vitro Effect of Physical and Kinetic Parameters on the Binding Stability. Appl. Microbiol. Biotechnol. 2018, 102, 6687–6697. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of High Hydrostatic Pressure (HPP) and Pulsed Electric Field (PEF) Technologies on Reduction of Aflatoxins in Fruit Juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Nan, M.-N.; Bi, Y.; Qiang, Y.; Xue, H.-L.; Yang, L.; Feng, L.-D.; Pu, L.-M.; Long, H.-T.; Prusky, D. Electrostatic Adsorption and Removal Mechanism of Ochratoxin A in Wine via a Positively Charged Nano-MgO Microporous Ceramic Membrane. Food Chem. 2022, 371, 131157. [Google Scholar] [CrossRef]
- Borràs-Vallverdú, B.; Ramos, A.J.; Marín, S.; Sanchis, V.; Rodríguez-Bencomo, J.J. Deoxynivalenol Degradation in Wheat Kernels by Exposition to Ammonia Vapours: A Tentative Strategy for Detoxification. Food Control 2020, 118, 107444. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Miah, K. Kinetics of Deoxynivalenol Flux in Wheat Kernels Steeped in Different Solutions for Improved Food Safety. Food Control 2022, 133, 108606. [Google Scholar] [CrossRef]
- Wu, N.; Ou, W.; Zhang, Z.; Wang, Y.; Xu, Q.; Huang, H. Recent advances in detoxification strategies for zearalenone contamination in food and feed. Chin. J. Chem. Eng. 2021, 29, 168–177. [Google Scholar] [CrossRef]
- Gilbert Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Aflatoxin B1 in Nixtamalized Maize in Mexico; Occurrence and Accompanying Risk Assessment. Toxicol. Rep. 2019, 6, 1135–1142. [Google Scholar] [CrossRef]
- Méndez-Albores, A.; Arámbula-Villa, G.; Loarca-Piña, M.G.F.; Castaño-Tostado, E.; Moreno-Martínez, E. Safety and Efficacy Evaluation of Aqueous Citric Acid to Degrade B-Aflatoxins in Maize. Food Chem. Toxicol. 2005, 43, 233–238. [Google Scholar] [CrossRef]
- Mallakian, S.; Rezanezhad, R.; Jalali, M.; Ghobadi, F. The Effect of Ozone Gas on Destruction and Detoxification of Aflatoxin. Bull. Société R. Sci. Liège 2017, 86, 1–6. [Google Scholar] [CrossRef]
- Park, D.; Price, W. Reduction of Aflatoxin Hazards Using Ammoniation. Rev. Environ. Contam. Toxicol. 2001, 171, 139–175. [Google Scholar] [CrossRef]
- Ouf, S.A.; Ali, E.M. Does the Treatment of Dried Herbs with Ozone as a Fungal Decontaminating Agent Affect the Active Constituents? Pollut. 2021, 277, 116715. [Google Scholar] [CrossRef]
- Da Luz, S.R.; Almeida Villanova, F.; Tuchtenhagen Rockembach, C.; Dietrich Ferreira, C.; José Dallagnol, L.; Luis Fernandes Monks, J.; de Oliveira, M. Reduced of Mycotoxin Levels in Parboiled Rice by Using Ozone and Its Effects on Technological and Chemical Properties. Food Chem. 2022, 372, 131174. [Google Scholar] [CrossRef]
- Young, J.C.; Zhu, H.; Zhou, T. Degradation of Trichothecene Mycotoxins by Aqueous Ozone. Food Chem. Toxicol. 2006, 44, 417–424. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of Ozone Treatment on Deoxynivalenol and Quality Evaluation of Ozonised Wheat. Food Addit. Contam. Part A 2015, 32, 544–553. [Google Scholar] [CrossRef]
- McDonough, M.X.; Campabadal, C.A.; Mason, L.J.; Maier, D.E.; Denvir, A.; Woloshuk, C. Ozone Application in a Modified Screw Conveyor to Treat Grain for Insect Pests, Fungal Contaminants, and Mycotoxins. J. Stored Prod. Res. 2011, 47, 249–254. [Google Scholar] [CrossRef]
- Savi, G.D.; Gomes, T.; Canever, S.B.; Feltrin, A.C.; Piacentini, K.C.; Scussel, R.; Oliveira, D.; Machado-de-Ávila, R.A.; Cargnin, M.; Angioletto, E. Application of Ozone on Rice Storage: A Mathematical Modeling of the Ozone Spread, Effects in the Decontamination of Filamentous Fungi and Quality Attributes. J. Stored Prod. Res. 2020, 87, 101605. [Google Scholar] [CrossRef]
- Maureen, N.; Kaaya, A.N.; Kauffman, J.; Narrod, C.; Atukwase, A. Enhancing Nutritional Benefits and Reducing Mycotoxin Contamination of Maize through Nixtamalization. J. Biol. Sci. 2020, 20, 153–162. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Solís-Mercado, J.; Flores-Ramírez, R.; Díaz-Barriga, F.; Zuki-Orozco, A.; Cilia-López, V.G. Aflatoxins and the Traditional Process of Nixtamalisation in Indigenous Communities from the Huasteca Potosina Region. World Mycotoxin J. 2020, 13, 391–399. [Google Scholar] [CrossRef]
- Moreno-Pedraza, A.; Valdés-Santiago, L.; Hernández-Valadez, L.J.; Rodríguez-Sixtos Higuera, A.; Winkler, R.; Guzmán-de Peña, D.L. Reduction of Aflatoxin B1 during Tortilla Production and Identification of Degradation By-Products by Direct-Injection Electrospray Mass Spectrometry (DIESI-MS). Salud Publica Mex. 2015, 57, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, J.O.; De Saeger, S.; De Boevre, M.; Adegoke, G.O.; Audenaert, K.; Croubels, S.; Antonissen, G.; Vermeulen, K.; Gbashi, S.; Njobeh, P.B. Effect of Selected Cooking Ingredients for Nixtamalization on the Reduction of Fusarium Mycotoxins in Maize and Sorghum. Toxins 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Assessment of an Application on a Detoxification Process of Groundnut Press Cake for Aflatoxins by Ammoniation. EFSA J. 2021, 19, e07035. [Google Scholar] [CrossRef] [PubMed]
- Sumner, P.; Hammond, C. Treating Aflatoxin-Contaminated Corn with Ammonia; University of Georgia: Athens, GA, USA, 2009. [Google Scholar]
- Nyandieka, H.S.; Maina, J.O.; Nyamwange, C. Detoxification of Aflatoxin in Artificially Contaminated Maize Crop by Ammoniation Procedures. Discov. Innov. 2009, 21, 77. [Google Scholar] [CrossRef]
- Jubeen, F.; Sher, F.; Hazafa, A.; Zafar, F.; Ameen, M.; Rasheed, T. Evaluation and Detoxification of Aflatoxins in Ground and Tree Nuts Using Food Grade Organic Acids. Biocatal. Agric. Biotechnol. 2020, 29, 101749. [Google Scholar] [CrossRef]
- Humer, E.; Lucke, A.; Harder, H.; Metzler-Zebeli, B.; Böhm, J.; Zebeli, Q. Effects of Citric and Lactic Acid on the Reduction of Deoxynivalenol and Its Derivatives in Feeds. Toxins 2016, 8, 285. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Effect of Dietary Acids on the Formation of Aflatoxin B 2a as a Means to Detoxify Aflatoxin B 1. Food Addit. Contam. Part A 2016, 33, 1456–1467. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef]
- Anguiano-Ruvalcaba, G.L.; Vargas-Cortina, A.V.y.; Peña, D.G.-D. Inactivation of aflatoxin B1 and aflatoxicol through traditional “nixtamalización” of corn and their regeneration by acidification of corn dough. Salud Pública México 2005, 47, 369–375. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel Strategies for Degradation of Aflatoxins in Food and Feed: A Review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Nunes, V.M.; Moosavi, M.; Mousavi Khaneghah, A.; Oliveira, C.A. Innovative Modifications in Food Processing to Reduce the Levels of Mycotoxins. Curr. Opin. Food Sci. 2021, 38, 155–161. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- Cabrera-Meraz, J.; Maldonado, L.; Bianchini, A.; Espinal, R. Incidence of Aflatoxins and Fumonisins in Grain, Masa and Corn Tortillas in Four Municipalities in the Department of Lempira, Honduras. Heliyon 2021, 7, e08506. [Google Scholar] [CrossRef]
- Murugesan, P.; Brunda, D.K.; Moses, J.A.; Anandharamakrishnan, C. Photolytic and Photocatalytic Detoxification of Mycotoxins in Foods. Food Control 2021, 123, 107748. [Google Scholar] [CrossRef]
- Hoffmans, Y.; Schaarschmidt, S.; Fauhl-Hassek, C.; Van der Fels-Klerx, H. (Ine) Factors during Production of Cereal-Derived Feed That Influence Mycotoxin Contents. Toxins 2022, 14, 301. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Li, Q.; Wang, J.; Zhou, Y.; Duan, N.; Niazi, S.; Wang, Z. Photocatalysis and Degradation Products Identification of Deoxynivalenol in Wheat Using Upconversion Nanoparticles@TiO2 Composite. Food Chem. 2020, 323, 126823. [Google Scholar] [CrossRef]
- Ott, L.C.; Appleton, H.J.; Shi, H.; Keener, K.; Mellata, M. High Voltage Atmospheric Cold Plasma Treatment Inactivates Aspergillus Flavus Spores and Deoxynivalenol Toxin. Food Microbiol. 2021, 95, 103669. [Google Scholar] [CrossRef]
- Marshall, H.; Meneely, J.P.; Quinn, B.; Zhao, Y.; Bourke, P.; Gilmore, B.F.; Zhang, G.; Elliott, C.T. Novel Decontamination Approaches and Their Potential Application for Post-Harvest Aflatoxin Control. Trends Food Sci. Technol. 2020, 106, 489–496. [Google Scholar] [CrossRef]
- Gonçalves Lemos, J.; Stefanello, A.; Olivier Bernardi, A.; Valle Garcia, M.; Nicoloso Magrini, L.; Cichoski, A.J.; Wagner, R.; Venturini Copetti, M. Antifungal Efficacy of Sanitizers and Electrolyzed Waters against Toxigenic Aspergillus. Food Res. Int. 2020, 137, 109451. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.D.; Lang, G.H.; da SIlva Lindemann, I.; da Silva, N.T.; Hoffmann, J.F.; Ziegler, V.; de Oliveira, M. Postharvest UV-C Irradiation for Fungal Control and Reduction of Mycotoxins in Brown, Black, and Red Rice during Long-Term Storage. Food Chem. 2021, 339, 127810. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santaescolástica, C.; Fraeye, I.; Barba, F.J.; Gómez, B.; Tomasevic, I.; Romero, A.; Moreno, A.; Toldrá, F.; Lorenzo, J.M. Application of Non-Invasive Technologies in Dry-Cured Ham: An Overview. Trends Food Sci. Technol. 2019, 86, 360–374. [Google Scholar] [CrossRef]
- Liu, Y.; Joseph Hubert, G.; Gong, Y.Y.; Orfila, C. A Review of Post-Harvest Approaches to Reduce Fungal and Mycotoxin Contamination of Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, F.; Li, Q.; Zhou, Y.; He, C.; Duan, N. Detoxification of DON by Photocatalytic Degradation and Quality Evaluation of Wheat. RSC Adv. 2019, 9, 34351–34358. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the Pathways of Air Plasma Induced Aflatoxin B1 Degradation and Detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef]
- Kiš, M.; Milošević, S.; Vulić, A.; Herceg, Z.; Vukušić, T.; Pleadin, J. Efficacy of Low Pressure DBD Plasma in the Reduction of T-2 and HT-2 Toxin in Oat Flour. Food Chem. 2020, 316, 126372. [Google Scholar] [CrossRef]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A Holistic Study to Understand the Detoxification of Mycotoxins in Maize and Impact on Its Molecular Integrity Using Cold Atmospheric Plasma Treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef]
- Casas-Junco, P.P.; Solís-Pacheco, J.R.; Ragazzo-Sánchez, J.A.; Aguilar-Uscanga, B.R.; Bautista-Rosales, P.U.; Calderón-Santoyo, M. Cold Plasma Treatment as an Alternative for Ochratoxin A Detoxification and Inhibition of Mycotoxigenic Fungi in Roasted Coffee. Toxins 2019, 11, 337. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Harmeling, H.; Emire, S.; Teshome, P.G.; Toepfl, S.; Aganovic, K. Pulsed Light Treatment Reduces Microorganisms and Mycotoxins Naturally Present in Red Pepper (Capsicum annuum L.) Powder. J. Food Process Eng. 2021, 45, e13948. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Khir, R.; Wu, B.; Zhou, C.; Pan, Z.; Ma, H. Degradation Kinetics of Aflatoxin B 1 and B 2 in Solid Medium by Using Pulsed Light Irradiation: Degradation Kinetics of Aflatoxins in Solid Medium Using Pulsed Light Irradiation. J. Sci. Food Agric. 2018, 98, 5220–5224. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of Pulsed Light Treatment for Degradation and Detoxification of Aflatoxin B1 and B2 in Rough Rice and Rice Bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Udovicki, B.; Stankovic, S.; Tomic, N.; Djekic, I.; Smigic, N.; Trifunovic, B.S.; Milicevic, D.; Rajkovic, A. Evaluation of Ultraviolet Irradiation Effects on Aspergillus Flavus and Aflatoxin B1 in Maize and Peanut Using Innovative Vibrating Decontamination Equipment. Food Control 2022, 134, 108691. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Effect of Rotating Peanuts on Aflatoxin Detoxification by Ultraviolet C Light and Irradiation Uniformity Evaluated by AgCl-Based Dosimeter. Food Control 2021, 120, 107533. [Google Scholar] [CrossRef]
- Khalil, O.A.A.; Hammad, A.A.; Sebaei, A.S. Aspergillus Flavus and Aspergillus Ochraceus Inhibition and Reduction of Aflatoxins and Ochratoxin A in Maize by Irradiation. Toxicon 2021, 198, 111–120. [Google Scholar] [CrossRef]
- Calado, T.; Fernández-Cruz, M.L.; Cabo Verde, S.; Venâncio, A.; Abrunhosa, L. Gamma Irradiation Effects on Ochratoxin A: Degradation, Cytotoxicity and Application in Food. Food Chem. 2018, 240, 463–471. [Google Scholar] [CrossRef]
- Ben Amara, A.; Mehrez, A.; Ragoubi, C.; Romero-González, R.; Garrido Frenich, A.; Landoulsi, A.; Maatouk, I. Fungal Mycotoxins Reduction by Gamma Irradiation in Naturally Contaminated Sorghum. J. Food Process. Preserv. 2022, 46, e16345. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Babic, J.; Pezo, L.; Banjac, V.; Colovic, R.; Kos, J.; Krulj, J.; Vrtač, K.; Jakovac-Strajn, B. Effects of Extrusion Process on Fusarium and Alternaria Mycotoxins in Whole Grain Triticale Flour. LWT 2021, 155, 112926. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Mendoza, J.R.; Verma, T.; Kupski, L.; Badiale-Furlong, E.; Bianchini, A. Fate of Aflatoxins in Cornmeal during Single-Screw Extrusion: A Bioaccessibility Approach. LWT 2021, 138, 110734. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, F.; Zhou, X.; Zhang, J.; Ding, Y. Using Acid and Alkaline Electrolyzed Water to Reduce Deoxynivalenol and Mycological Contaminations in Wheat Grains. Food Control 2018, 88, 98–104. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Kießling, M.; Emire, S.A.; Teshome, P.G.; Töpfl, S.; Aganovic, K. Influence of Electron Beam Treatment on Naturally Contaminated Red Pepper (Capsicum annuum L.) Powder: Kinetics of Microbial Inactivation and Physicochemical Quality Changes. Innov. Food Sci. Emerg. Technol. 2021, 67, 102588. [Google Scholar] [CrossRef]
- Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Blandino, M. Fate of Regulated, Masked, Emerging Mycotoxins and Secondary Fungal Metabolites during Different Large-Scale Maize Dry-Milling Processes. Food Res. Int. 2021, 140, 109861. [Google Scholar] [CrossRef]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Photocatalytic Degradation of Aflatoxin B1 by Activated Carbon Supported TiO2 Catalyst. Food Control 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.-H.; Sun, D.-W. Blocking and Degradation of Aflatoxins by Cold Plasma Treatments: Applications and Mechanisms. Trends Food Sci. Technol. 2021, 109, 647–661. [Google Scholar] [CrossRef]
- Gómez-López, V.; Noguera-Artiaga, L.; Figueroa, F.; Girón, F.; Carbonell-Barrachina, A.; Gabaldon, J.; Perez-Lopez, A. Effect of Pulsed Light on Quality of Shelled Walnuts. Foods 2022, 11, 1186. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Pulsed Light Processing of Foods for Microbial Safety. Food Qual. Saf. 2017, 1, 187–202. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Tao, Y.; Mujumdar, A.S.; Pan, Z.; Chen, C.; Yang, X.-H.; Liu, Z.-L.; Wang, H.; Xiao, H.-W. Recent Advances in Non-Thermal Decontamination Technologies for Microorganisms and Mycotoxins in Low-Moisture Foods. Trends Food Sci. Technol. 2020, 106, 104–112. [Google Scholar] [CrossRef]
- Shen, M.; Singh, R. Detoxification of Aflatoxins in Foods by Ultraviolet Irradiation, Hydrogen Peroxide, and Their Combination—A Review. LWT 2021, 142, 110986. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative Technologies to Manage Aflatoxins in Foods and Feeds and the Profitability of Application—A Review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, N.; Yusop, S.M.; Rahman, I.A.; Dauqan, E.; Abdullah, A. Efficacy of Gamma Irradiation in Improving the Microbial and Physical Quality Properties of Dried Chillies (Capsicum Annuum L.): A Review. Foods 2022, 11, 97. [Google Scholar] [CrossRef]
- Awuchi, C.; Nyakundi Ondari, E.; Ofoedu, C.; Chacha, J.; Rasaq, W.; Morya, S.; Okpala, C. Grain Processing Methods’ Effectiveness to Eliminate Mycotoxins: An Overview. Asian J. Chem. 2021, 33, 2267–2275. [Google Scholar] [CrossRef]
- Villarreal-Barajas, T.; Vázquez-Durán, A.; Méndez-Albores, A. Effectiveness of Electrolyzed Oxidizing Water on Fungi and Mycotoxins in Food. Food Control 2022, 131, 108454. [Google Scholar] [CrossRef]
- Rebezov, M.; Saeed, K.; Khaliq, A.; Rahman, S.J.U.; Sameed, N.; Semenova, A.; Khayrullin, M.; Dydykin, A.; Abramov, Y.; Thiruvengadam, M.; et al. Application of Electrolyzed Water in the Food Industry: A Review. Appl. Sci. 2022, 12, 6639. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi Moosavi, M.; Oliveira, C.A.F.; Vanin, F.; Sant’Ana, A.S. Electron Beam Irradiation to Reduce the Mycotoxin and Microbial Contaminations of Cereal-Based Products: An Overview. Food Chem. Toxicol. 2020, 143, 111557. [Google Scholar] [CrossRef]
- Mohammadi, X.; Matinfar, G.; Khaneghah, A.M.; Singh, A.; Pratap-Singh, A. Emergence of Cold Plasma and Electron Beam Irradiation as Novel Technologies to Counter Mycotoxins in Food Products. World Mycotoxin J. 2021, 14, 75–83. [Google Scholar] [CrossRef]
- Kim, G.-R.; Ramakrishnan, S.R.; Ameer, K.; Chung, N.; Kim, Y.-R.; Kwon, J.-H. Irradiation Effects on Chemical and Functional Qualities of Ready-to-Eat Saengshik, a Cereal Health Food. Radiat. Phys. Chem. 2020, 171, 108692. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of Processing on Mycotoxin Stability in Cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef]
- Reis, T.A.; Oliveira, T.D.; Zorzete, P.; Faria, P.; Corrêa, B. A Non-Toxigenic Aspergillus Flavus Strain Prevents the Spreading of Fusarium Verticillioides and Fumonisins in Maize. Toxicon 2020, 181, 6–8. [Google Scholar] [CrossRef]
- Molo, M.S.; Heiniger, R.W.; Boerema, L.; Carbone, I. Trial Summary on the Comparison of Various Non-Aflatoxigenic Strains of Aspergillus Flavus on Mycotoxin Levels and Yield in Maize. Agron. J. 2019, 111, 942–946. [Google Scholar] [CrossRef]
- Du, G.; Liu, L.; Guo, Q.; Cui, Y.; Chen, H.; Yuan, Y.; Wang, Z.; Gao, Z.; Sheng, Q.; Yue, T. Microbial Community Diversity Associated with Tibetan Kefir Grains and Its Detoxification of Ochratoxin A during Fermentation. Food Microbiol. 2021, 99, 103803. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of Yeasts and Yeast Derivatives for the Biological Control of Toxigenic Fungi and Their Toxic Metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Li, X.; Tang, H.; Yang, C.; Meng, X.; Liu, B. Detoxification of Mycotoxin Patulin by the Yeast Rhodotorula Mucilaginosa. Food Control 2019, 96, 47–52. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Savvaidis, I.; Chokr, A.; Louka, N.; Atoui, A. Detoxification Approaches of Mycotoxins: By Microorganisms, Biofilms and Enzymes. Int. J. Food Contam. 2022, 9, 3. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of Zearalenone (ZEN)-Detoxifying Bacillus in Animal Feed Decontamination through Fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Y.; Ma, Q.; Ji, C.; Zhao, L. Detoxification of the Mycoestrogen Zearalenone by Bacillus Licheniformis Spore CotA Laccase and Application of Immobilized Laccase in Contaminated Corn Meal. LWT 2022, 163, 113548. [Google Scholar] [CrossRef]
- Zadeike, D.; Vaitkeviciene, R.; Bartkevics, V.; Bogdanova, E.; Bartkiene, E.; Lele, V.; Juodeikiene, G.; Cernauskas, D.; Valatkeviciene, Z. The Expedient Application of Microbial Fermentation after Whole-Wheat Milling and Fractionation to Mitigate Mycotoxins in Wheat-Based Products. LWT 2021, 137, 110440. [Google Scholar] [CrossRef]
- Alberts, J.F.; Davids, I.; Moll, W.-D.; Schatzmayr, G.; Burger, H.-M.; Shephard, G.S.; Gelderblom, W.C.A. Enzymatic Detoxification of the Fumonisin Mycotoxins during Dry Milling of Maize. Food Control 2021, 123, 107726. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Reduction of the Fusarium Mycotoxins: Deoxynivalenol, Nivalenol and Zearalenone by Selected Non-Conventional Yeast Strains in Wheat Grains and Bread. Molecules 2022, 27, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, Y.; Kong, Q.; Ma, Y.; Liu, Y. Detoxification of Aflatoxin B1 by Zygosaccharomyces Rouxii with Solid State Fermentation in Peanut Meal. Toxins 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Mathad, G.N.; Oliveira, C.A.F.; Mousavi Khaneghah, A. Combinations of Emerging Technologies with Fermentation: Interaction Effects for Detoxification of Mycotoxins? Food Res. Int. 2021, 141, 110104. [Google Scholar] [CrossRef] [PubMed]
- Babaee, R.; Karami-Osboo, R.; Mirabolfathy, M. Evaluation of the Use of Ozone, UV-C and Citric Acid in Reducing Aflatoxins in Pistachio Nut. J. Food Compos. Anal. 2022, 106, 104276. [Google Scholar] [CrossRef]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Bartkevics, V.; Pugajeva, I.; Bērziņa, Z.; Gruzauskas, R.; Sidlauskiene, S.; et al. The Influence of Combined Extrusion and Fermentation Processes on the Chemical and Biosafety Parameters of Wheat Bran. LWT 2021, 146, 111498. [Google Scholar] [CrossRef]
- Al Attiya, W.; Hassan, Z.U.; Al-Thani, R.; Jaoua, S. Prevalence of Toxigenic Fungi and Mycotoxins in Arabic Coffee (Coffea Arabica): Protective Role of Traditional Coffee Roasting, Brewing and Bacterial Volatiles. PLoS ONE 2021, 16, e0259302. [Google Scholar] [CrossRef]
- Subramanian, V.; Shanmugam, N.; Ranganathan, K.; Kumar, S.; Reddy, R. Effect of Combination Processing on Aflatoxin Reduction: Process Optimization by Response Surface Methodology. J. Food Process. Preserv. 2017, 41, e13230. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Detoxifying Aflatoxin Contaminated Peanuts by High Concentration of H2O2 at Moderate Temperature and Catalase Inactivation. Food Control 2022, 142, 109218. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Decomposing Aflatoxins in Peanuts Using Advanced Oxidation Processes by UV and H2O2. Food Bioprocess Technol. 2022, 15, 1647–1657. [Google Scholar] [CrossRef]
- Abuagela, M.; Marshall, M.; Yagiz, Y.; Mostafa, H.; Iqdiam, B. Combined Effects of Citric Acid and Pulsed Light Treatments to Degrade B-Aflatoxins in Peanut. Food Bioprod. Process. 2019, 117, 396–403. [Google Scholar] [CrossRef]
- Zavala-Franco, A.; Arámbula-Villa, G.; Ramírez-Noguera, P.; Salazar, A.M.; Sordo, M.; Marroquín-Cardona, A.; de Dios Figueroa-Cárdenas, J.; Méndez-Albores, A. Aflatoxin Detoxification in Tortillas Using an Infrared Radiation Thermo-Alkaline Process: Cytotoxic and Genotoxic Evaluation. Food Control 2020, 112, 107084. [Google Scholar] [CrossRef]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Khaneghah, A.M.; Campagnollo, F.B.; Anderson, S.S. Removal of Aflatoxin B1 by Roasting with Lemon Juice and/or Citric Acid in Contaminated Pistachio Nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).