A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
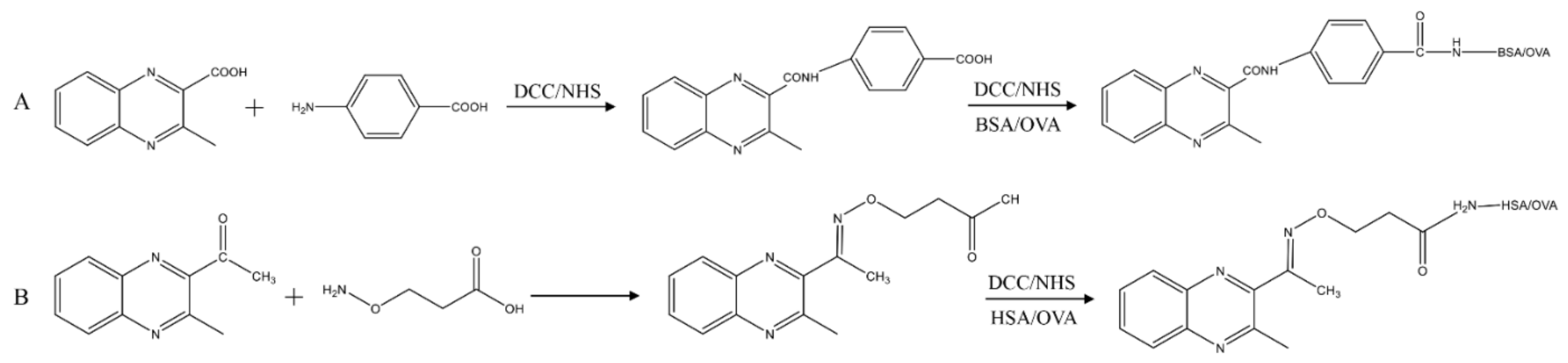
2.2. Antigen Design and Preparation
2.2.1. Synthesis of Antigens MQCA–PABA–BSA/OVA
2.2.2. Synthesis of Antigens DMEQ–AOAA–HSA/OVA
2.3. Preparation of mAb
2.4. Development of ic-ELISA Analysis
2.5. Validation of ic-ELISA Analysis
3. Results and Discussion
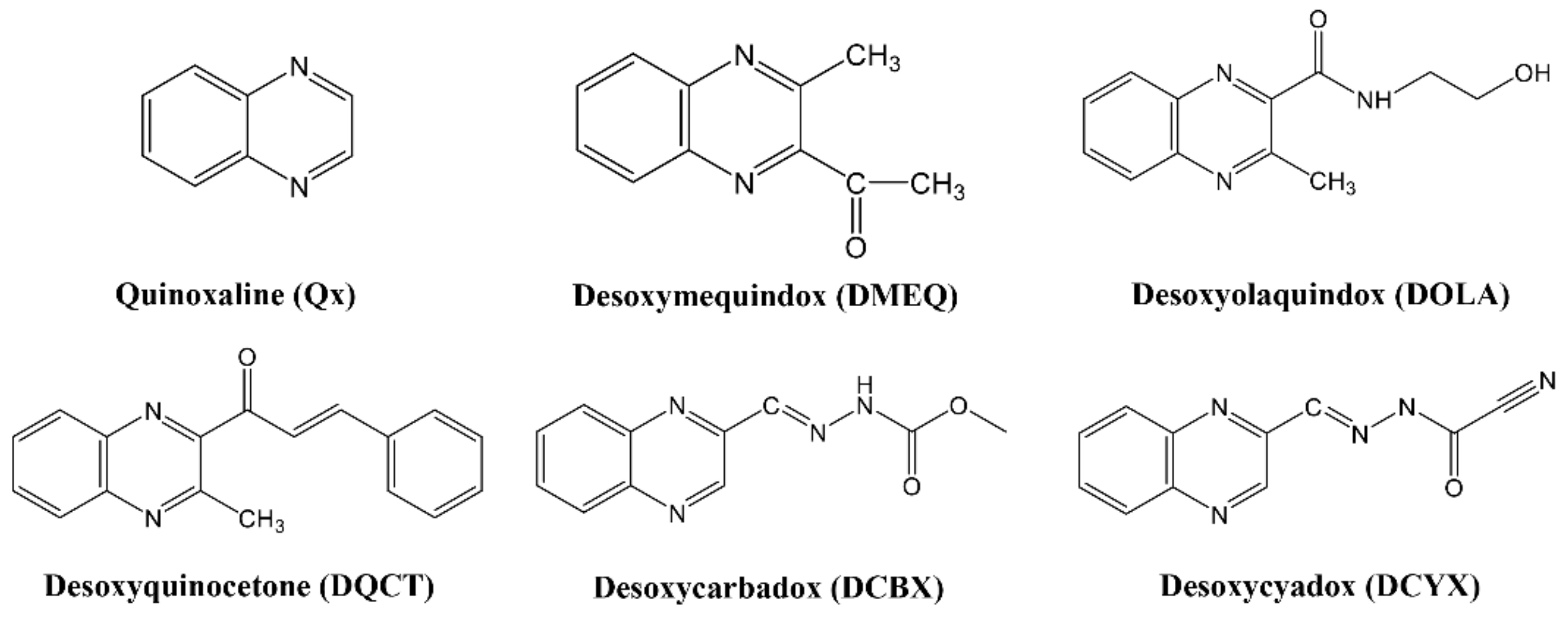
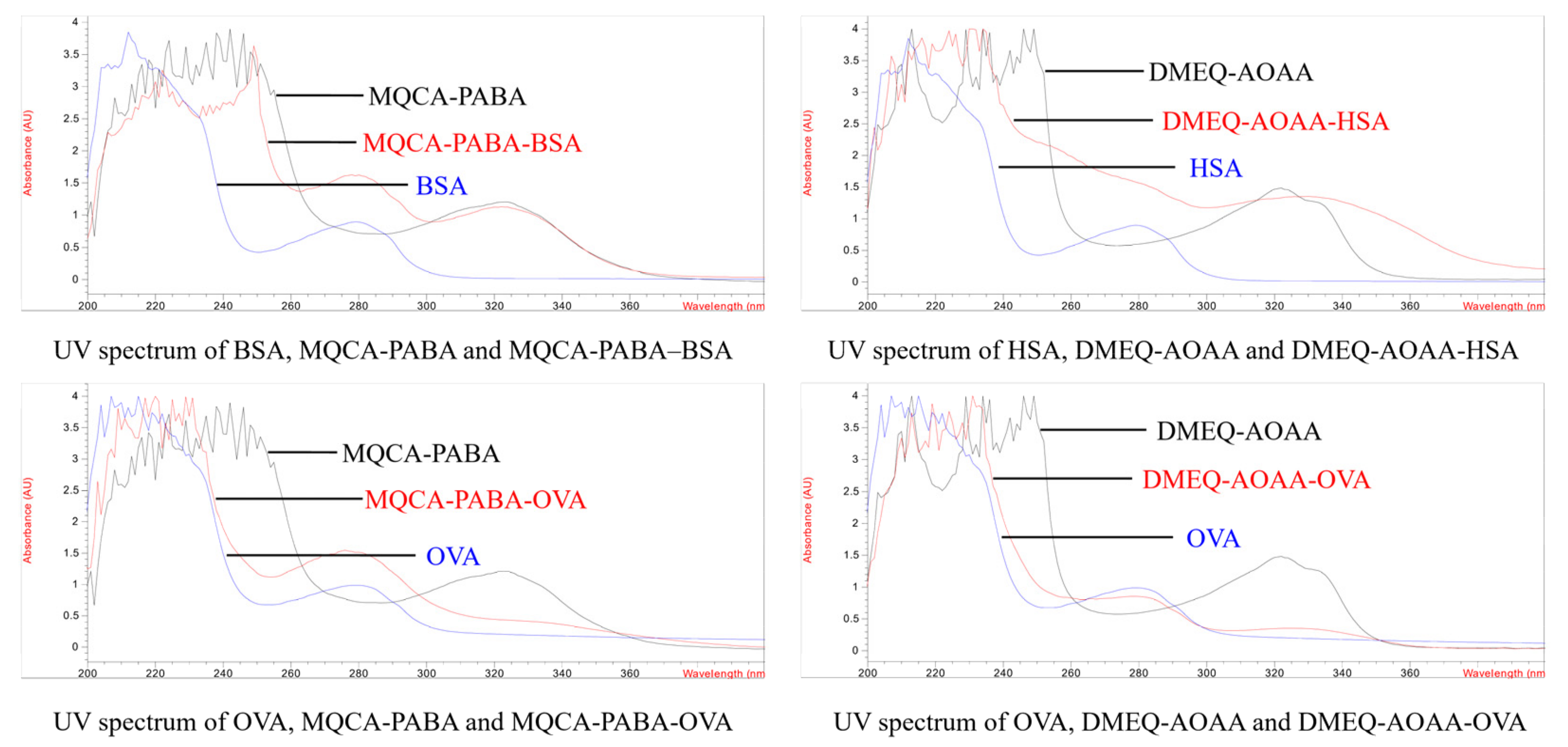
3.1. Antigen Design and Characterization
3.2. Characterization of mAb
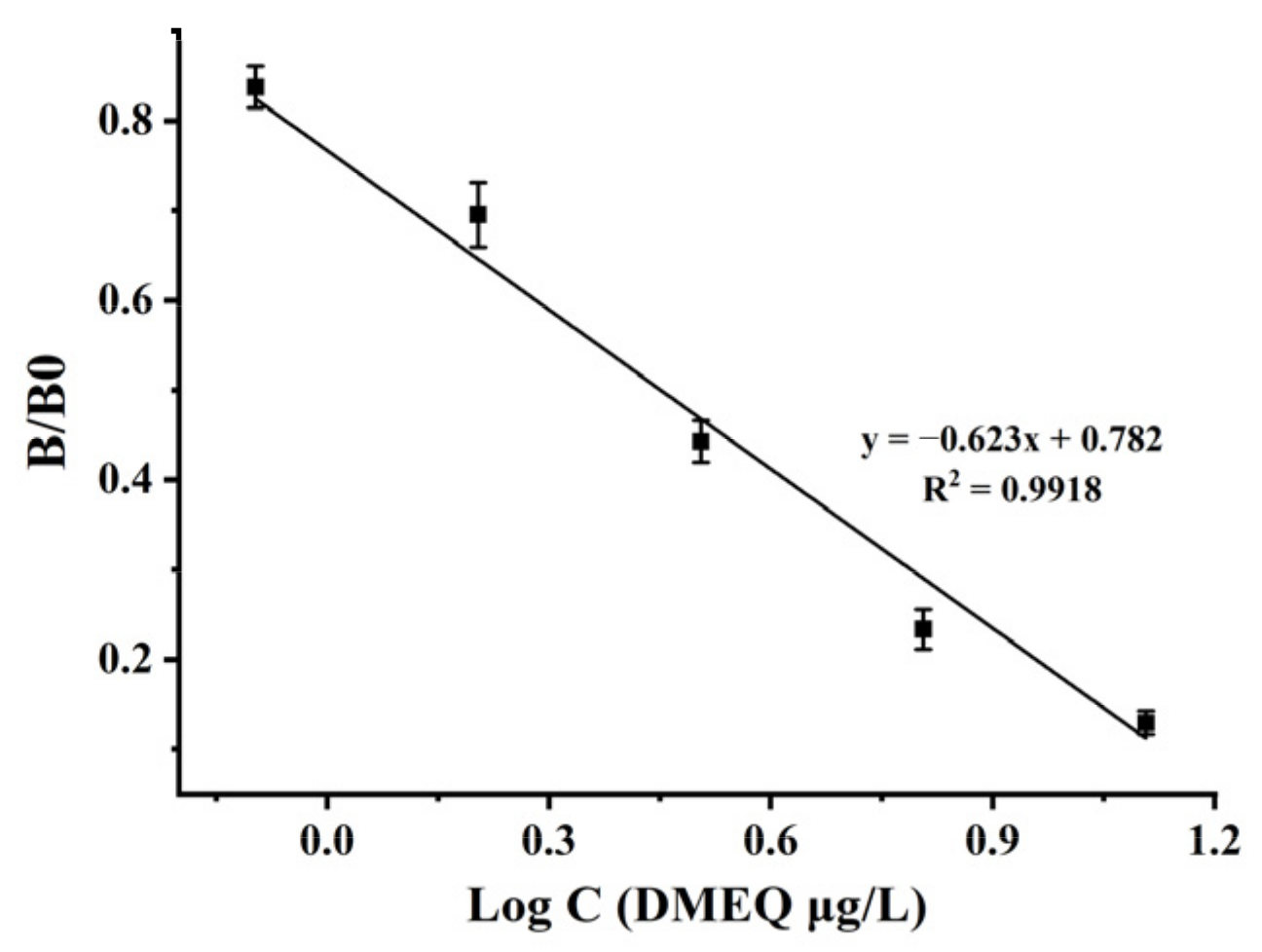
3.3. Performance of the ic-ELISA Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.Y.; Huang, L.L.; Chen, D.M.; Yuan, Z.H. Metabolism of mequindox in liver microsomes of rats, chicken and pigs. Rapid Commun. Mass Spectrom. 2010, 24, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Lei, Z.X.; Gu, C.Q.; Guo, J.C.; Yu, H.R.; Fatima, Z.N.; Zhou, K.X.; Shabbir, M.A.B.; Maan, M.K.; Wu, Q.H.; et al. Mequindox induces apoptosis, DNA damage, and carcinogenicity in Wistar rats. Food Chem. Toxicol. 2019, 127, 270–279. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Lei, Z.X.; Wu, Q.; Awais, I.; Shabbir, M.A.B.; Ahmed, S.; Fatima, Z.; Wang, X.; Pan, Y.H.; Xie, S.Y.; et al. The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats. Front. Pharmacol. 2018, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, A.; Wang, X.; Liu, Z.Y.; Wang, Y.L.; Huang, X.J.; Liu, Y.; Yu, H.; Zhang, H.F.; Li, T.T.; Yang, C.H.; et al. Long-term mequindox treatment induced endocrine and reproductive toxicity via oxidative stress in male Wistar rats. Toxicol. Appl. Pharm. 2011, 252, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, A.; Wang, X.; Tu, H.G.; Zhang, W.; Dai, M.H.; Peng, D.P.; Wang, Y.L.; Huang, L.L.; Chen, D.M.; Mannan, S.; et al. Genotoxicity evaluation of Mequindox in different short-term tests. Food Chem. Toxicol. 2013, 51, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Lei, Z.X.; Wu, Q.; Huang, D.Y.; Xie, S.Y.; Wang, X.; Pan, Y.H.; Yuan, Z.H. Mequindox Induced Genotoxicity and Carcinogenicity in Mice. Front. Pharmacol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Yin, F.J.; Pan, Y.H.; Chen, D.M.; Li, J.; Wan, D.; Liu, Z.L.; Yuan, Z.H. Metabolism, Distribution, and Elimination of Mequindox in Pigs, Chickens, and Rats. J. Agric. Food Chem. 2015, 63, 9839–9849. [Google Scholar] [CrossRef]
- Tan, H.L.; Pan, Y.H.; Chen, D.M.; Tao, Y.F.; Zhou, K.X.; Liu, Z.L.; Yuan, Z.H.; Huang, L.L. Discovery of the Marker Residue of Olaquindox in Pigs, Broilers, and Carp. J. Agric. Food Chem. 2019, 67, 6603–6613. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wang, Y.L.; Huang, L.; Tao, Y.F.; Yuan, Z.H.; Chen, D.M. Simultaneous determination of five quinoxaline-1,4-dioxides in animal feeds using ultrasonic solvent extraction and high-performance liquid chromatography. Anal. Chim. Acta 2006, 569, 97–102. [Google Scholar] [CrossRef]
- He, Q.Q.; Fang, B.H.; Su, Y.J.; Zeng, Z.L.; Yang, J.W.; He, L.M.; Zeng, D.P. Simultaneous determination of quinoxaline-1,4-dioxides in feeds using molecularly imprinted solid-phase extraction coupled with HPLC. J. Sep. Sci. 2013, 36, 301–310. [Google Scholar] [CrossRef]
- You, Y.L.; Song, L.T.; Li, Y.S.; Wu, Y.T.; Xin, M. Simple and Fast Extraction-Coupled UPLC-MS/MS Method for the Determination of Mequindox and Its Major Metabolites in Food Animal Tissues. J. Agric. Food Chem. 2016, 64, 2394–2404. [Google Scholar] [CrossRef]
- Li, Y.S.; Liu, K.L.; Beier, R.C.; Cao, X.Y.; Shen, J.Z.; Zhang, S.X. Simultaneous determination of mequindox, quinocetone, and their major metabolites in chicken and pork by UPLC-MS/MS. Food Chem. 2014, 160, 171–179. [Google Scholar] [CrossRef]
- Le, T.; Xu, J.; He, H.Q.; Niu, X.D.; Chen, Y.; Jia, Y.Y. Development and validation of an enzyme-linked immunosorbent assay for rapid detection of multi-residues of five quinoxaline-1,4-dioxides in animal feeds. Food Agric. Immunol. 2013, 24, 457–466. [Google Scholar] [CrossRef]
- Le, T.; Zhu, L.Q.; Shu, L.H.; Zhang, L. Simultaneous determination of five quinoxaline-1,4-dioxides in animal feeds using an immunochromatographic strip. Food Addit. Contam. A 2016, 33, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Shen, J.Z.; Wang, Z.H.; Jiang, W.X.; Zhang, S.X. A sensitive and specific ELISA for determining a residue marker of three quinoxaline antibiotics in swine liver. Anal. Bioanal. Chem. 2013, 405, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Shen, J.Z.; Wang, Z.H.; Zhang, Q.D.; Dong, X.Y.; Wu, C.; Zhang, S.X. Rapid Screening of Quinoxaline Antimicrobial Growth Promoters and Their Metabolites in Swine Liver by Indirect Competitive Enzyme-Linked Immunosorbent Assay. Food Anal. Method 2013, 6, 1583–1591. [Google Scholar] [CrossRef]
- Peng, D.P.; Wang, Y.L.; Feng, L.; Cao, G.C.; Tao, Y.F.; Liu, Z.L.; Yuan, Z.H. Preparation of Broadly Specific Monoclonal Antibodies for Simultaneous Determination of Fluoroquinolone Residues in Eggs. Food Anal. Method 2016, 9, 3520–3531. [Google Scholar] [CrossRef]
- Chen, X.J.; Li, Z.Z.; Guo, J.Y.; Li, D.M.; Gao, H.L.; Wang, Y.; Xu, C.L. Simultaneous screening for marbofloxacin and ofloxacin residues in animal-derived foods using an indirect competitive immunoassay. Food Agric. Immunol. 2017, 28, 489–499. [Google Scholar] [CrossRef]
- Peng, D.P.; Ye, S.Q.; Wang, Y.L.; Chen, D.M.; Tao, Y.F.; Huang, L.L.; Liu, Z.L.; Dai, M.H.; Wang, X.Q.; Yuan, Z.H. Development and Validation of an Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Screening of Tylosin and Tilmicosin in Muscle, Liver, Milk, Honey and Eggs. J. Agric. Food Chem. 2012, 60, 44–51. [Google Scholar] [CrossRef]
- Yang, H.C.; He, L.M.; Liu, Y.H.; Bian, K.; Hu, F.Y.; Fang, B.H. Determination of Quinoxalines and Their Two Main Metabolites in Environmental Water Samples by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Lett. 2014, 47, 1421–1433. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, X.Y.; Sheng, F.; Kong, D.X.; Wang, Y.L.; Pan, Y.H.; Chen, M.; Tao, Y.F.; Liu, Z.L.; Ahmed, S.; Yuan, Z.H.; et al. Broad-spectrum monoclonal antibody and a sensitive multi-residue indirect competitive enzyme-linked immunosorbent assay for the antibacterial synergists in samples of animal origin. Food Chem. 2019, 280, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Zhang, B.; Zhao, Q.X.; Wang, S.; Zhang, Y. Preparation of a Broad-Spectrum Heterocyclic Aromatic Amines (HAAs) Antibody and Its Application in Detection of Eight HAAs in Heat Processed Meat. J. Agric. Food Chem. 2020, 68, 15501–15508. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.X.; Shen, J.Z.; Zhang, S.X.; Feng, P.S.; Wu, H.X.; Wu, C.M. Comparative Metabolism of Mequindox in Liver Microsomes, Hepatocytes, and Intestinal Microflora of Chicken. Anal. Lett. 2012, 45, 1749–1763. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Lei, Z.X.; Dai, M.H.; Wang, X.; Yuan, Z.H. Toxic metabolites, Sertoli cells and Y chromosome related genes are potentially linked to the reproductive toxicity induced by mequindox. Oncotarget 2017, 8, 87512–87528. [Google Scholar] [CrossRef] [PubMed]
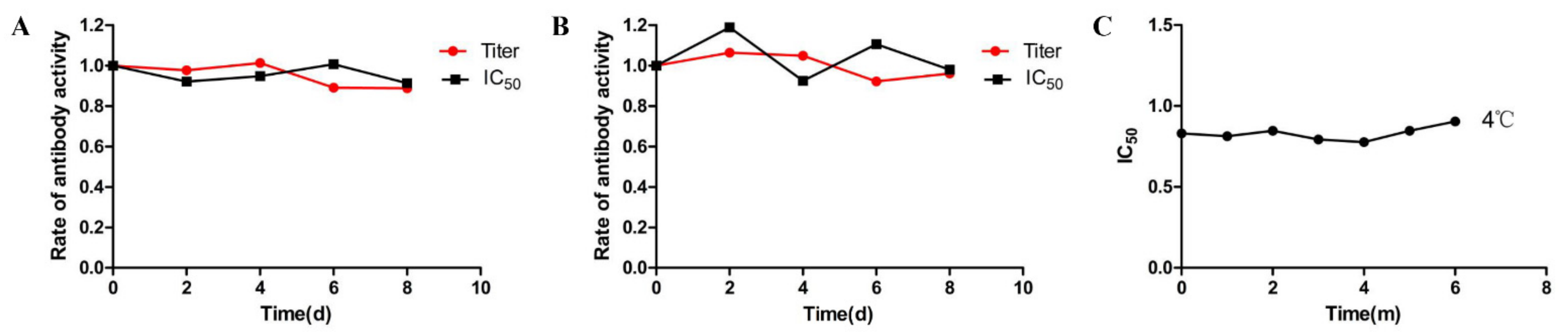
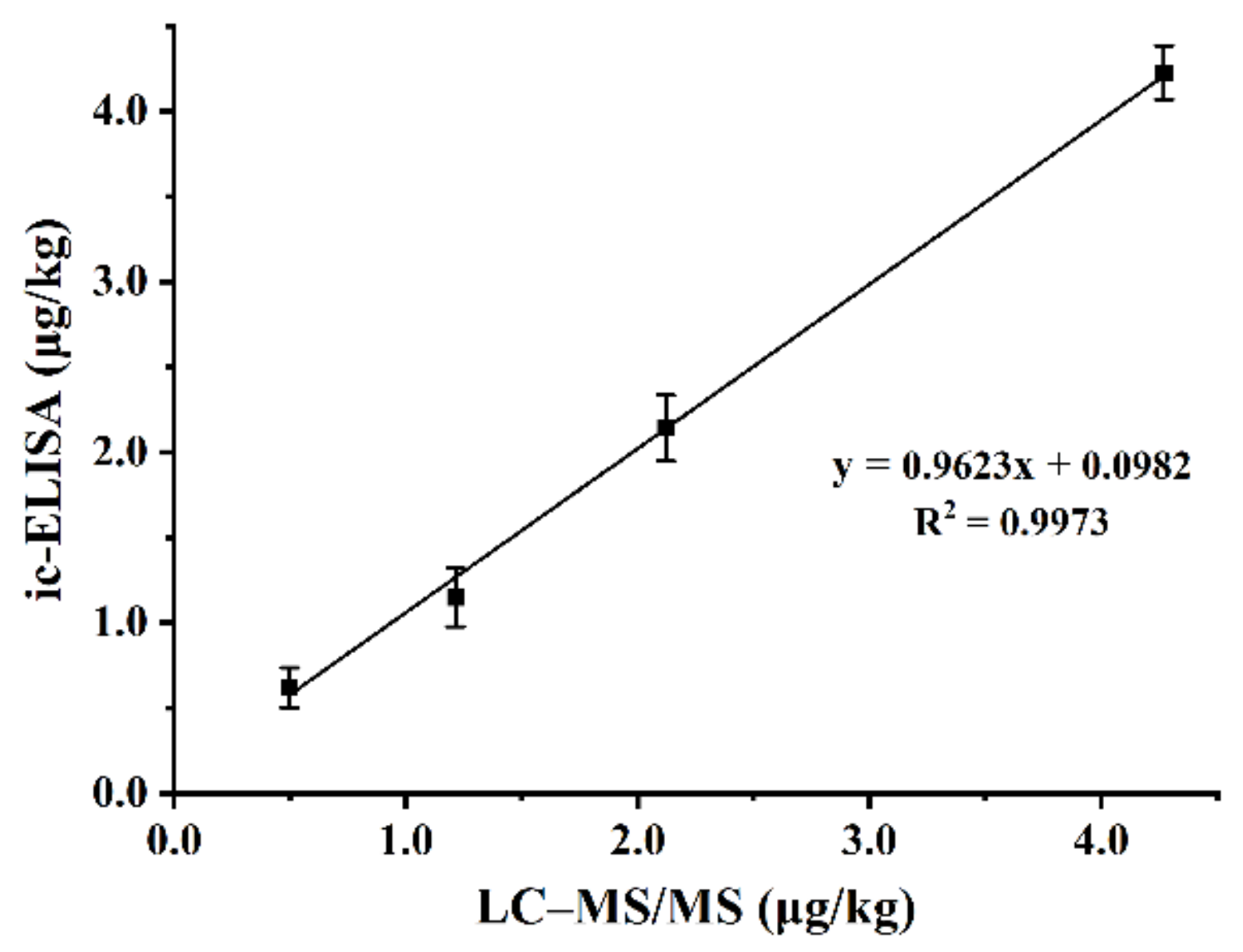
| Immunogen | Coating Antigen | Titre (1:X × 103) | B/B0 1 Values (DMEQ, 100 μg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse 1 | Mouse 2 | Mouse 3 | Mouse 4 | Mouse 1 | Mouse 2 | Mouse 3 | Mouse 4 | ||
| MQCA–PABA–BSA | MQCA–PABA–OVA | 2 | 1.5 | 12 | 3.5 | 0.846 | 0.965 | 0.815 | 0.902 |
| DMEQ–AOAA–OVA | 1 | 0.8 | 2 | 1.5 | 0.516 | 0.469 | 0.568 | 0.766 | |
| DMEQ–AOAA–HSA | MQCA–PABA–OVA | 2 | 3 | 1.8 | 1.5 | 0.851 | 0.921 | 0.956 | 0.827 |
| DMEQ–AOAA–OVA | 3.5 | 5 | 4 | 3 | 0.790 | 0.873 | 0.884 | 0.919 | |
| Competitor | IC50 (µg/L) | CR (%) |
|---|---|---|
| DMEQ | 2.84 | 100 |
| MQCA–PABA | 6.45 | 44 |
| DOLA | 10.52 | 27 |
| MQCA | 142 | 2 |
| DQCT | 218 | 1.3 |
| QCA | 284 | 1 |
| DCBX | >1000 | <0.1 |
| DCYX | >1000 | <0.1 |
| N1-DCYX | >1000 | <0.1 |
| N4-DCYX | >1000 | <0.1 |
| MEQ | >1000 | <0.1 |
| Sample | LOD (µg/kg) | LOQ (µg/kg) | Spiked Level (μg/kg) | Recovery (%) | CVintra-assay (%, n 1 = 3) | Mean Recovery ± SD (%) | CVinter-assay (%, n 1 = 9) |
|---|---|---|---|---|---|---|---|
| Pork | 0.47 | 0.61 | 0.6 | 93.3–95.7 | <10.8 | 94.7 ± 1.2 | 1.3 |
| 1.2 | 87.5–101.8 | <9.7 | 94.3 ± 7.2 | 7.6 | |||
| 2.4 | 86.3–89.8 | <9.3 | 88.6 ± 2.0 | 2.3 | |||
| Swine liver | 0.58 | 0.90 | 0.9 | 89.6–104.0 | <7.6 | 99.0 ± 8.2 | 8.2 |
| 1.8 | 89.0–97.9 | <10.1 | 94.4 ± 4.7 | 5.0 | |||
| 3.6 | 95.9–107.8 | <4.5 | 100.6 ± 6.3 | 6.2 | |||
| Swine kidney | 0.55 | 0.77 | 0.75 | 80.8–86.1 | <8.9 | 82.9 ± 5.7 | 6.9 |
| 1.5 | 79.3–94.8 | <8.8 | 85.6 ± 8.8 | 10.3 | |||
| 3.0 | 73.7–80.8 | <6.0 | 78.2 ± 4.5 | 5.8 | |||
| Chicken | 0.52 | 0.74 | 0.75 | 82.4–99.5 | <8.3 | 89.2 ± 9.1 | 10.2 |
| 1.5 | 93.7–99.2 | <6.6 | 96.0 ± 2.9 | 3.0 | |||
| 3.0 | 81.3–89.5 | <7.8 | 85.1 ± 4.1 | 4.8 | |||
| Chicken liver | 0.54 | 0.77 | 0.75 | 93.9–99.7 | <5.1 | 97.2 ± 4.6 | 4.7 |
| 1.5 | 76.4–82.9 | <2.7 | 79.3 ± 3.2 | 4.0 | |||
| 3.0 | 91.2–101.1 | <8.2 | 97.0 ± 7.5 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Luo, M.; Li, H.; Xiao, J.; He, X.; Liang, J.; Peng, D. A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues. Foods 2022, 11, 3305. https://doi.org/10.3390/foods11203305
Song W, Luo M, Li H, Xiao J, He X, Liang J, Peng D. A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues. Foods. 2022; 11(20):3305. https://doi.org/10.3390/foods11203305
Chicago/Turabian StyleSong, Wanyao, Mengyu Luo, Huaming Li, Jiaxu Xiao, Xiuping He, Jixiang Liang, and Dapeng Peng. 2022. "A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues" Foods 11, no. 20: 3305. https://doi.org/10.3390/foods11203305
APA StyleSong, W., Luo, M., Li, H., Xiao, J., He, X., Liang, J., & Peng, D. (2022). A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues. Foods, 11(20), 3305. https://doi.org/10.3390/foods11203305






