Meat Quality and Muscle Tissue Proteome of Crossbred Bulls Finished under Feedlot Using Wet Distiller Grains By-Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Slaughtering, Sampling, and Carcass Quality Trait Evaluation
2.2. Aging, Chemical Composition, and Marbling Score Determination of the Meat Samples
2.3. Shear Force, Cooking Loss, and Myofibrillar Proteolysis
2.4. Fatty Acid Profile
2.5. Proteome
2.5.1. Protein Extraction and Precipitation
2.5.2. Protein Separation by Two-Dimensional Polyacrylamide Gel Electrophoresis
2.5.3. Image Processing
2.5.4. Tryptic Digestion of Protein Spots and Identification of Proteins by ESI-MS/MS
2.5.5. Bioinformatics Procedures
2.5.6. Data Analysis
3. Results
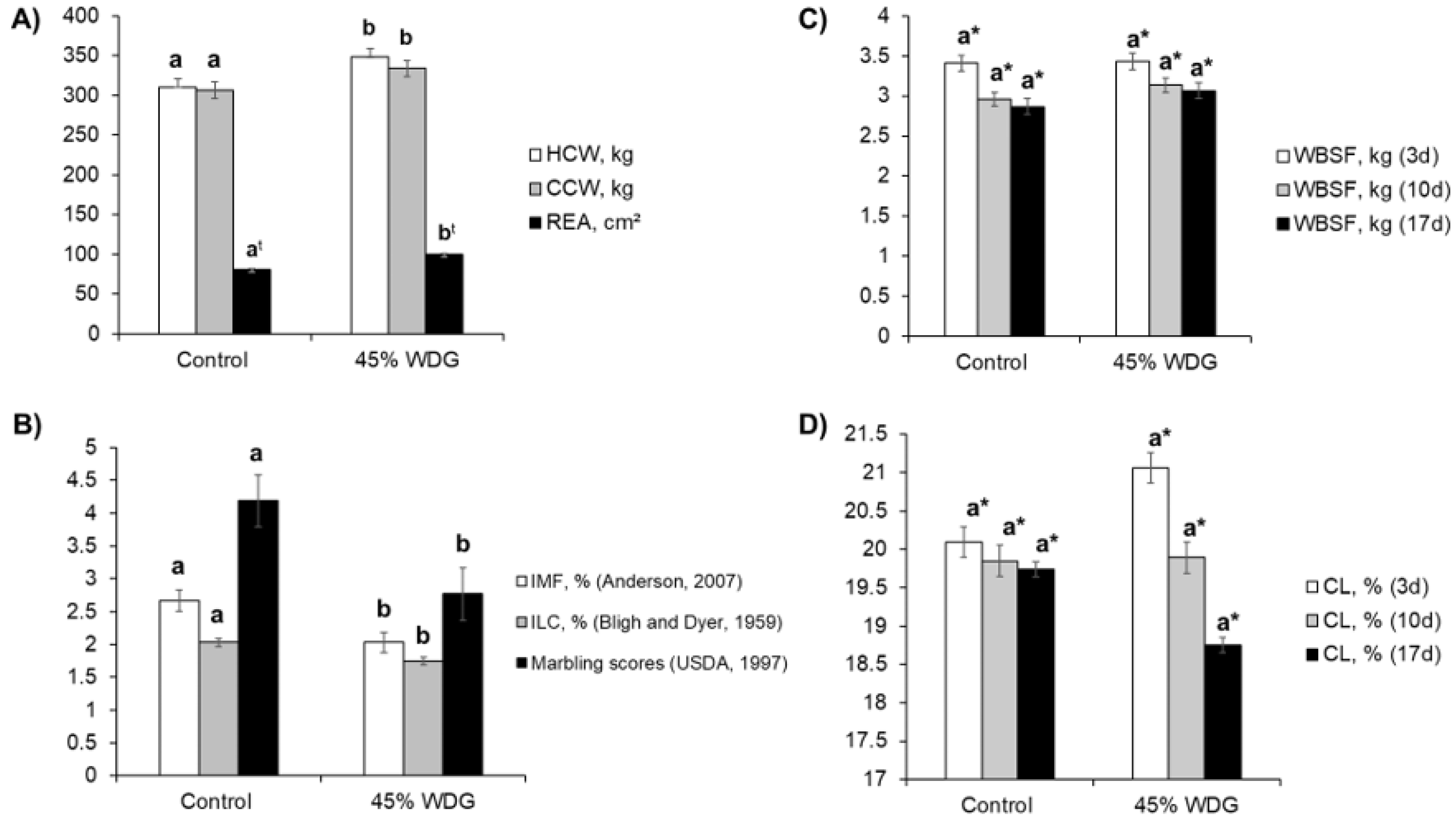
3.1. Carcass and Meat Quality Traits
3.2. Fatty Acid Profile
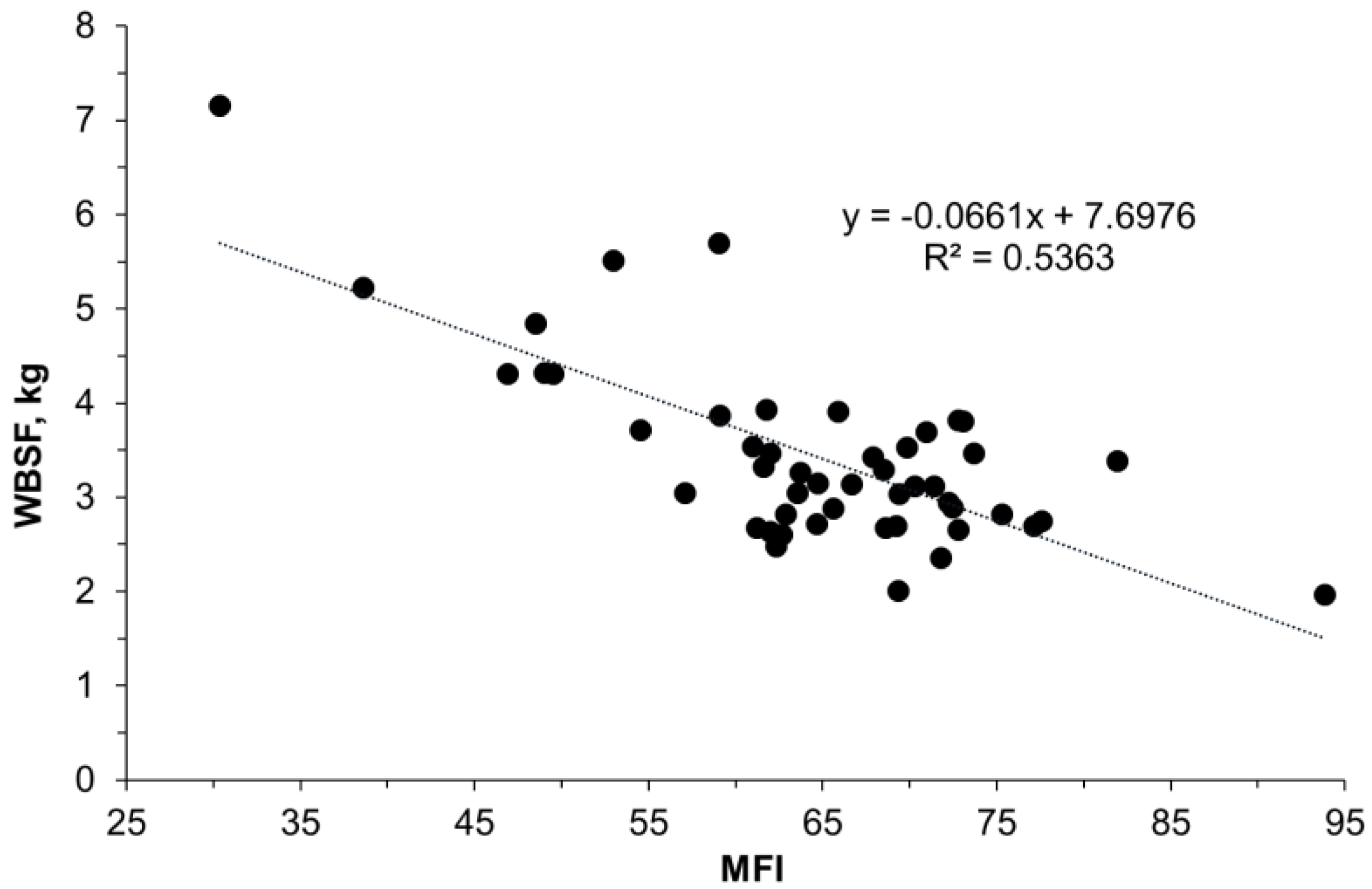
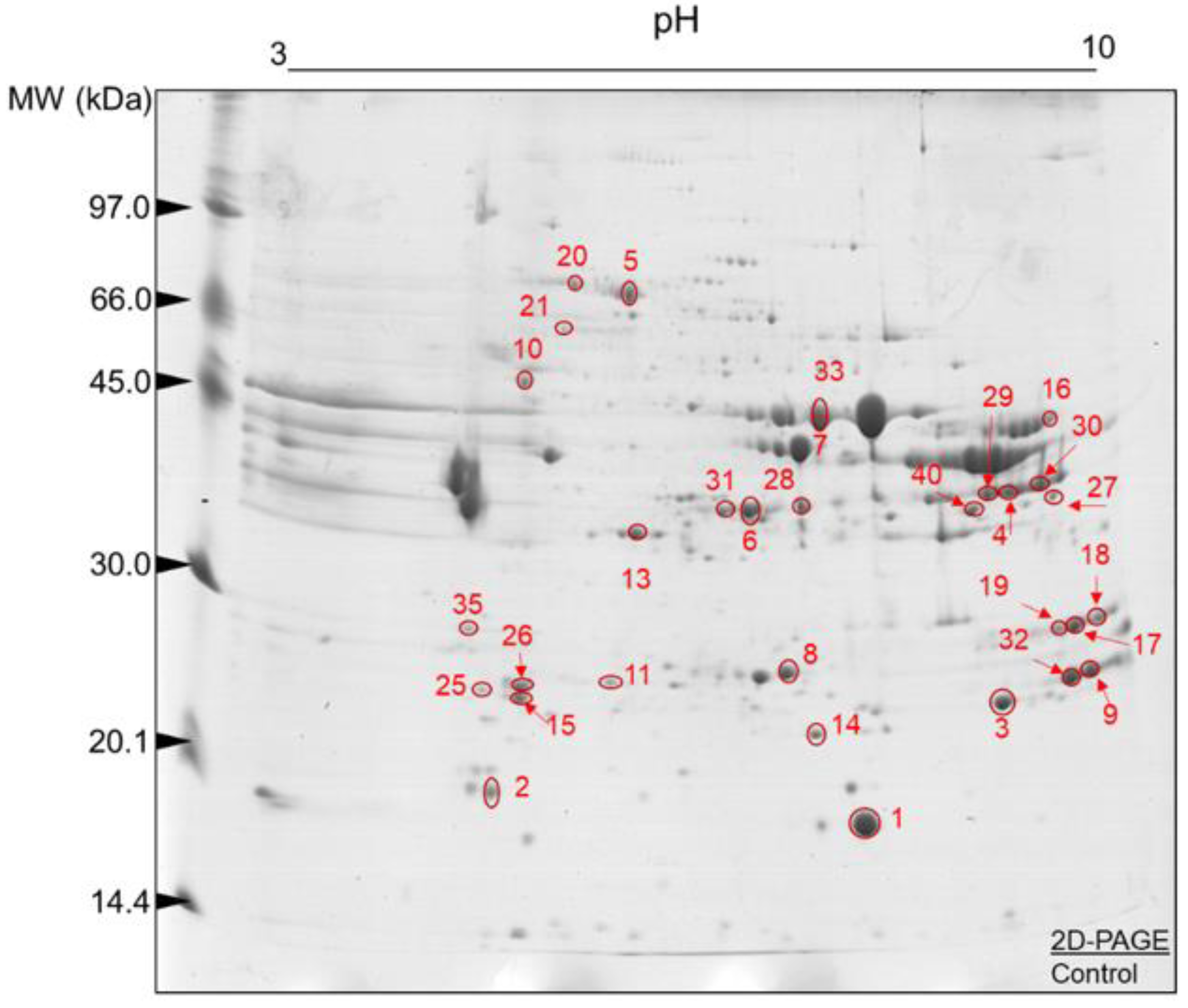
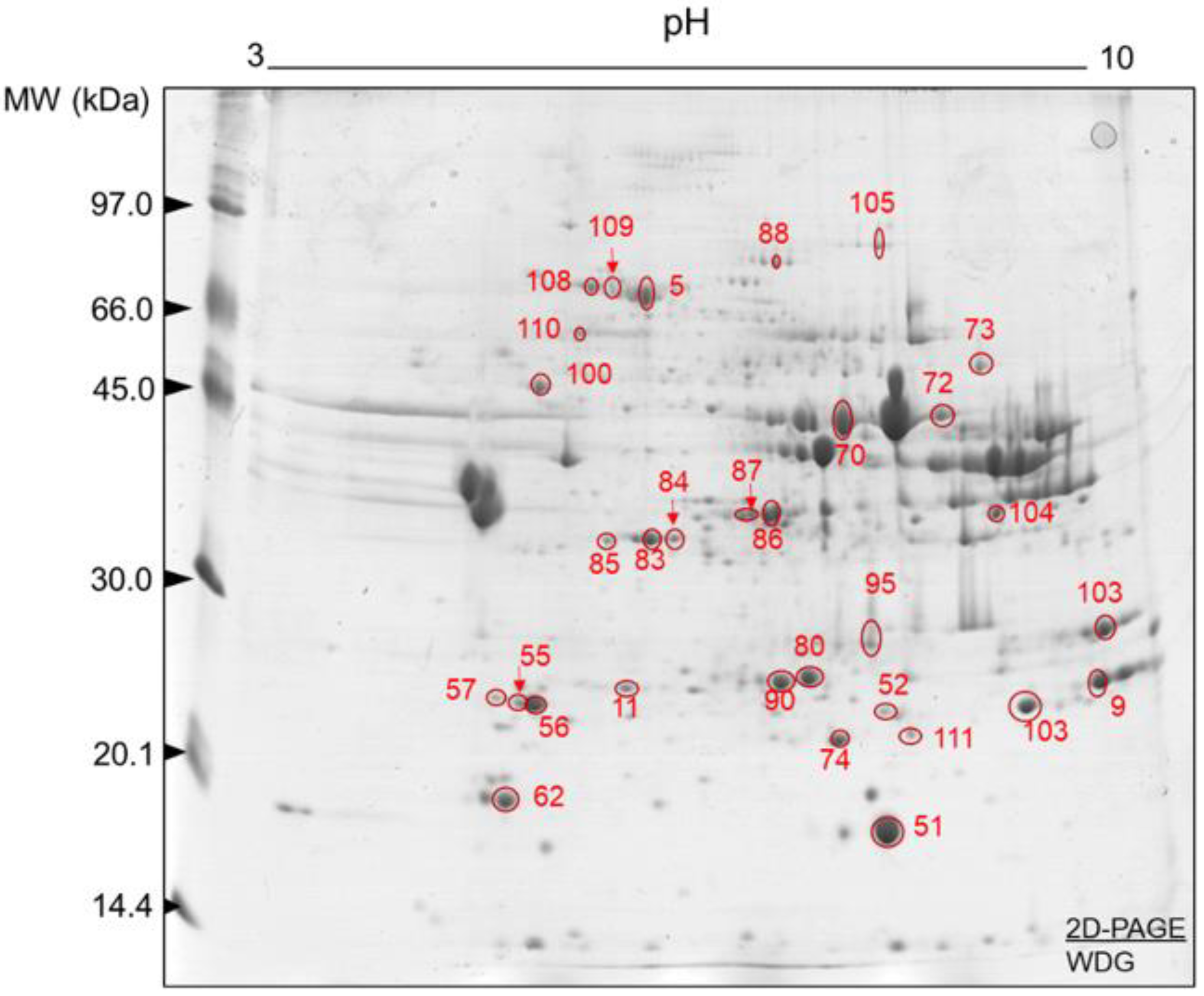
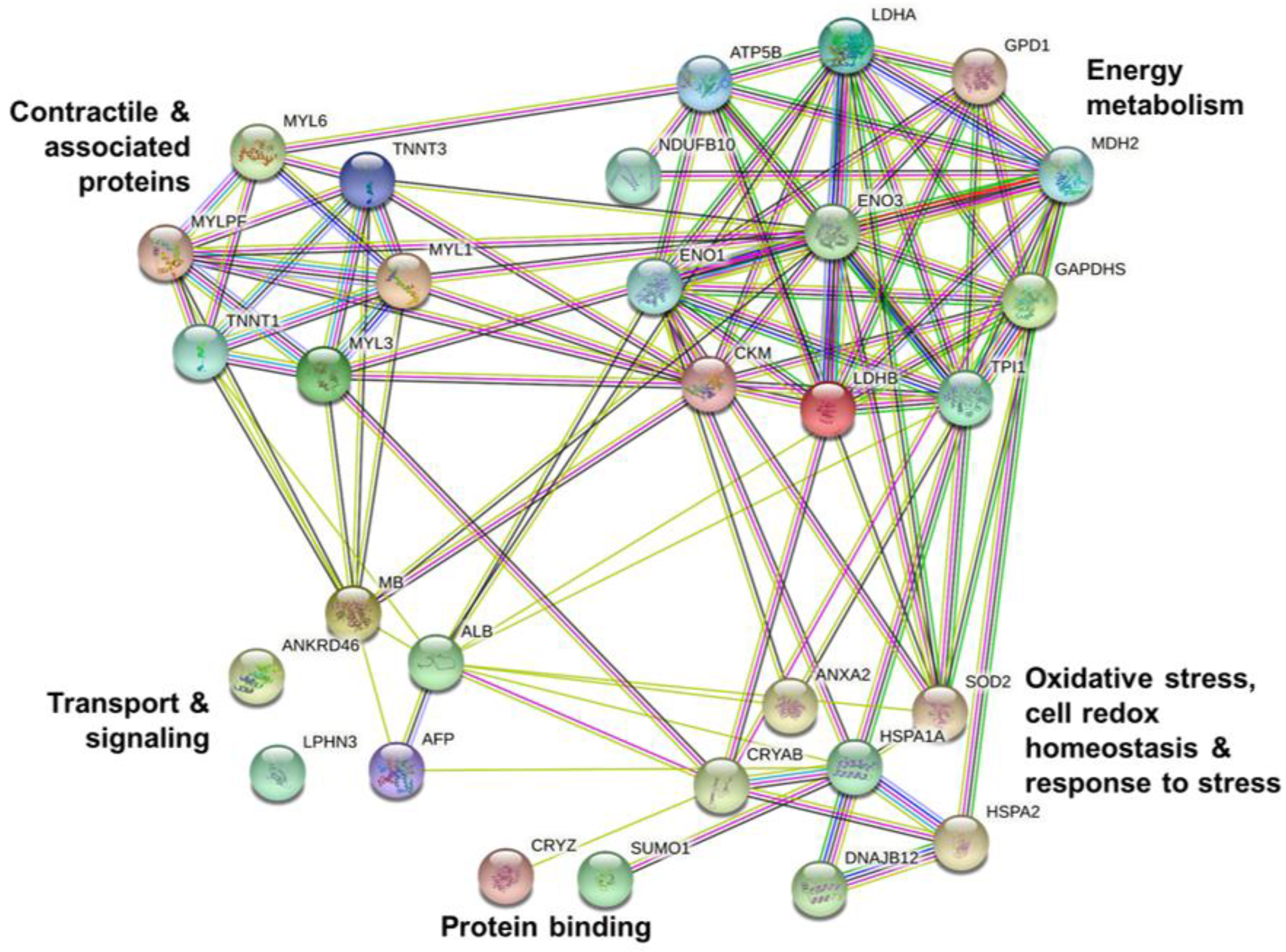
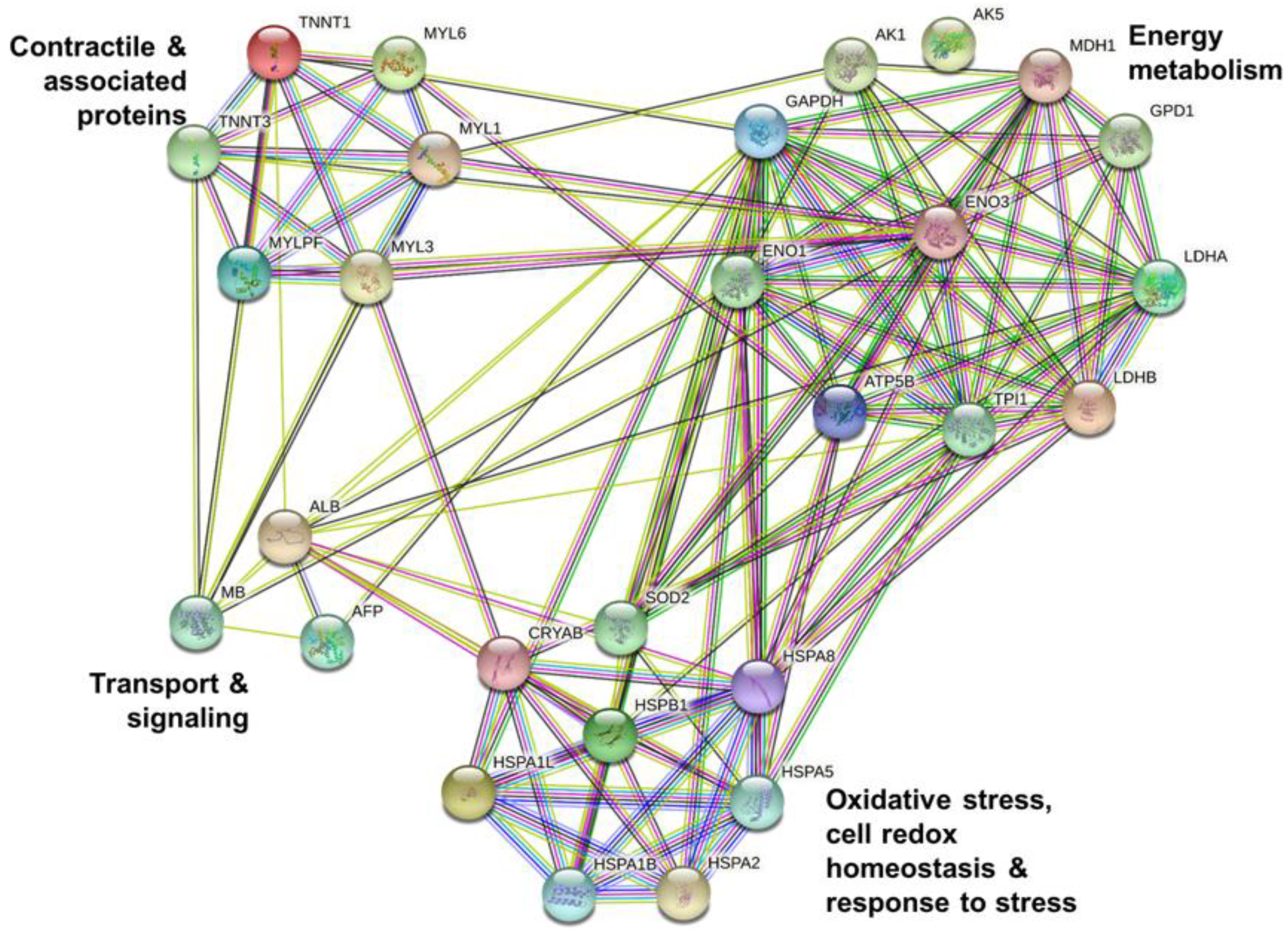
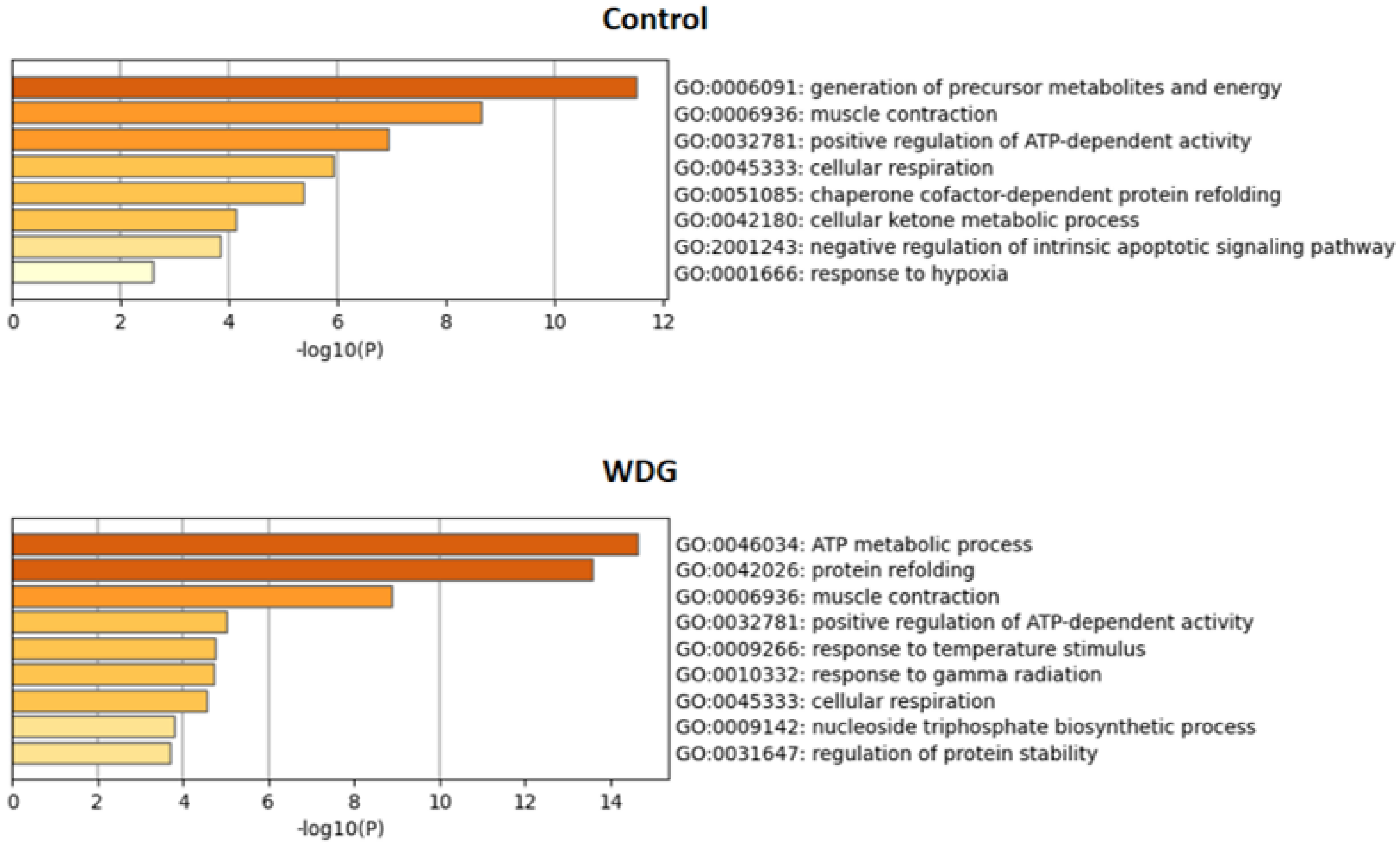
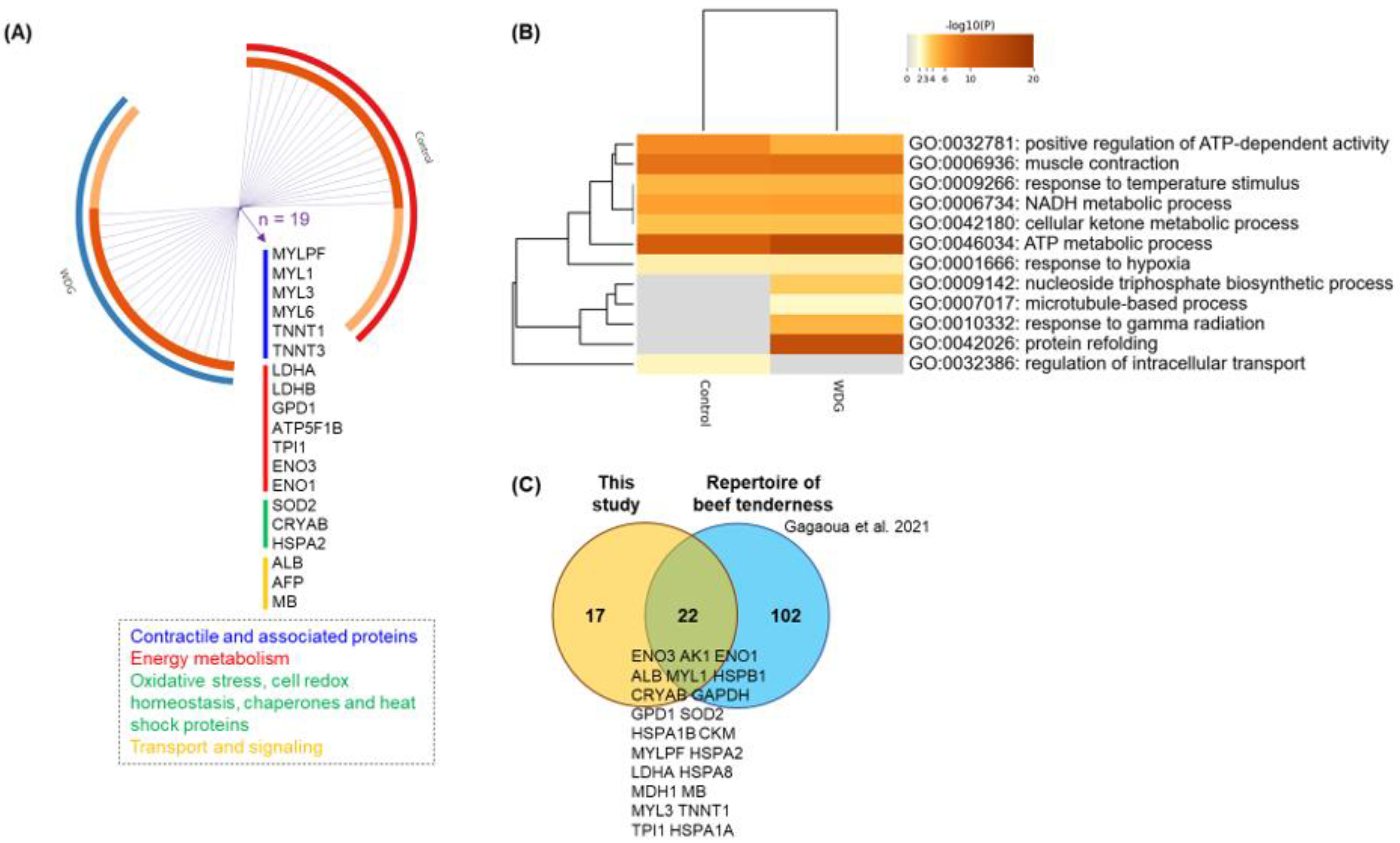
3.3. Muscle Tissue Proteome
4. Discussion
4.1. Carcass and Meat Quality Traits
4.2. Fatty Acid Profile
4.3. Muscle Proteome Data and Changing Molecular Signatures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ott, S.L.; Rask, N. Importance of by-products on the economics of alcohol production from corn. Energy Agric. 1983, 2, 257–266. [Google Scholar] [CrossRef]
- Ponce, C.H.; Cole, N.A.; Sawyer, J.; Da Silva, J.C.B.; Smith, D.R.; Maxwell, C.; Brown, M.S. Effects of wet corn distiller’s grains with solubles and nonprotein nitrogen on feeding efficiency, growth performance, carcass characteristics, and nutrient losses of yearling steers12. J. Anim. Sci. 2019, 97, 2609–2630. [Google Scholar] [CrossRef] [PubMed]
- Keomanivong, F.E.; Ruch, M.C.; Liu, J.-H.; Kirsch, J.D.; Bauer, M.L.; Dahlen, C.R.; Kapphahn, M.; Borhan, S.; Rahman, S.; Swanson, K.C. Influence of dry-rolled corn processing and distiller’s grain inclusion rate on ruminal pH, ammonia and volatile fatty acid concentration, in vitro methane production and enzyme activity. Anim. Feed Sci. Technol. 2017, 228, 132–139. [Google Scholar] [CrossRef]
- Böttger, C.; Südekum, K.-H. Review: Protein value of distillers dried grains with solubles (DDGS) in animal nutrition as affected by the ethanol production process. Anim. Feed Sci. Technol. 2018, 244, 11–17. [Google Scholar] [CrossRef]
- Blom, E.J.; Anderson, D.; Brake, D.W. Increases in duodenal glutamic acid supply linearly increase small intestinal starch digestion but not nitrogen balance in cattle1. J. Anim. Sci. 2016, 94, 5332–5340. [Google Scholar] [CrossRef]
- Klopfenstein, T.J.; Erickson, G.E.; Bremer, V.R. BOARD-INVITED REVIEW: Use of distillers by-products in the beef cattle feeding industry1. J. Anim. Sci. 2008, 86, 1223–1231. [Google Scholar] [CrossRef]
- Schoonmaker, J.P.; Claeys, M.C.; LeMenager, R.P. Effect of increasing distillers grains inclusion on performance and carcass characteristics of early-weaned steers1. J. Anim. Sci. 2013, 91, 1784–1790. [Google Scholar] [CrossRef]
- Nade, T.; Uchida, K.; Omori, K.; Matsubayashi, K.; Kimura, N. Effects of feeding dried distillers grains with solubles (DDGS) on meat quality at the late stage of the fattening period of Holstein steers. Anim. Sci. J. 2012, 83, 310–317. [Google Scholar] [CrossRef]
- Shen, Y.N.; Kim, S.H.; Yoon, D.H.; Lee, H.G.; Kang, H.S.; Seo, K.S. Proteome Analysis of Bovine Longissimus dorsi Muscle Associated with the Marbling Score. Asian-Australasian J. Anim. Sci. 2012, 25, 1083–1088. [Google Scholar] [CrossRef]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al Jammas, M.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J. Proteom. 2019, 200, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Zhu, Y. Proteomics advances in beef production. In Food Proteomics; Academic Press: Cambridge, MA, USA, 2022; pp. 151–182. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Basu, U.; Dodson, M.V.; Basarb, J.A.; Guan, L.L. Proteome differences associated with fat accumulation in bovine subcutaneous adipose tissues. Proteome Sci. 2010, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, D.S.; Gómez, J.F.; Silva, S.L.; Beline, M.; Zhang, X.; Wang, Y.; Pavan, B.; Koulicoff, L.A.; Rosa, A.F.; Goulart, R.S.; et al. Proteome basis for the biological variations in color and tenderness of longissimus thoracis muscle from beef cattle differing in growth rate and feeding regime. Food Res. Int. 2022, 153, 110947. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Aldana, S.; Beggs, M.; Birkey, J.; Conquest, A.; Conway, R.; Hemminger, T.; Herrick, J.; Hurley, C.; Ionita, C.; et al. Determination of Fat, Moisture, and Protein in Meat and Meat Products by Using the FOSS FoodScan Near-Infrared Spectrophotometer with FOSS Artificial Neural Network Calibration Model and Associated Database: Collaborative Study. J. AOAC Int. 2007, 90, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- USDA. Official United States Standards for Grades of Carcass Beef. In United States Standards for Grades of Carcass Beef; Agricultural Marketing Service: Des Moines, ID, USA, 1997; pp. 1–20. Available online: https://www.ams.usda.gov/sites/default/files/media/CarcassBeefStandard.pdf (accessed on 18 December 2017).
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association Educational Foundation: Des Moines, ID, USA, 2015. [Google Scholar]
- Baldassini, W.A.; Neto, O.R.M.; Fernandes, T.T.; Ament, H.d.P.; Luz, M.G.; Santiago, B.M.; Curi, R.A.; Chardulo, L.A.L. Testing different devices to assess the meat tenderness: Preliminary results. J. Food Sci. Technol. 2021, 58, 2441–2446. [Google Scholar] [CrossRef]
- Culler, R.D.; Parrish, F.C., Jr.; Smith, G.C.; Cross, H.R. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 1978, 43, 1177–1180. [Google Scholar] [CrossRef]
- Borges, B.O.; Curi, R.; Baldi, F.; Feitosa, F.L.B.; De Andrade, W.B.F.; Albuquerque, L.; Oliveira, H.; Chardulo, L.A. Polymorphisms in candidate genes and their association with carcass traits and meat quality in Nellore cattle. Pesqui. Agropecuária Bras. 2014, 49, 364–371. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Baldassini, W.A.; Braga, C.P.; Chardulo, L.A.L.; Silva, J.A.I.V.; Malheiros, J.M.; de Albuquerque, L.G.; Fernandes, T.T.; Padilha, P.D.M. Bioanalytical methods for the metalloproteomics study of bovine longissimus thoracis muscle tissue with different grades of meat tenderness in the Nellore breed (Bos indicus). Food Chem. 2015, 169, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Paredi, G.; Mori, F.; de Marino, M.G.; Raboni, S.; Marchi, L.; Galati, S.; Buschini, A.; Fiego, D.P.L.; Mozzarelli, A. Is the protein profile of pig Longissimus dorsi affected by gender and diet? J. Proteom. 2019, 206, 103437. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef]
- Delgado, E.F.; Aguiar, A.P.; Ortega, E.M.M.; Spoto, M.H.F.; Castillo, C.J.C. Brazilian consumers’ perception of tenderness of beef steaks classified by shear force and taste. Sci. Agricola 2006, 63, 232–239. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B. Proteomics to explain and predict meat quality. In New Aspects of Meat Quality; Woodhead Publishing: Cambridge, UK, 2022; pp. 393–431. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Allen, P.; Troy, D.J.; McDonnell, C.K. Objective carcass measurement technologies: Latest developments and future trends. Trends Food Sci. Technol. 2021, 111, 771–782. [Google Scholar] [CrossRef]
- Beretta, V.; Simeone, A.; Franco, J.; Bentancur, O.; Novac, M.; Panizza, V.; Rodríguez, M.V. Using sorghum dry distillers’ grains plus solubles in sorghum-based finishing diets: Feed utilization, cattle performance and carcass traits. Anim. Feed Sci. Technol. 2021, 271, 114731. [Google Scholar] [CrossRef]
- Gagaoua, M.; Duffy, G.; Alvarez, C.; Burgess, C.; Hamill, R.; Crofton, E.; Botinestean, C.; Ferragina, A.; Cafferky, J.; Mullen, A.; et al. Current research and emerging tools to improve fresh red meat quality. Ir. J. Agric. Food Res. 2022, 141, 1–23. [Google Scholar] [CrossRef]
- Hart, K.B.; Ribeiro, F.A.; Henriott, M.L.; Herrera, N.J.; Calkins, C.R. Quality effects on beef strip steaks from cattle fed high-protein corn distillers grains and other ethanol by-products. J. Anim. Sci. 2019, 97, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.; Jenschke, B.; Senaratne, L.; Carr, T.; Erickson, G.; Calkins, C. Effects of finishing diets containing wet distillers grains plus solubles on beef quality attributes and fatty acid profile. Meat Sci. 2018, 136, 16–22. [Google Scholar] [CrossRef]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Teixeira, P.D.; Oliveira, D.M.; Chizzotti, M.L.; Chalfun-Junior, A.; Coelho, T.C.; Gionbelli, M.; Paiva, L.V.; Carvalho, J.R.R.; Ladeira, M.M. Subspecies and diet affect the expression of genes involved in lipid metabolism and chemical composition of muscle in beef cattle. Meat Sci. 2017, 133, 110–118. [Google Scholar] [CrossRef]
- Haddad, G.D.B.S.; Gomes, H.B.; Buchili, A.F.M.; Rodrigues, L.M.; Fontes, P.R.; Ramos, A.D.L.S.; Ramos, E.M. Accelerating the dry aging of bone-in beef from Nellore cattle by the freeze/thaw process. J. Food Process. Preserv. 2022, 46, e16573. [Google Scholar] [CrossRef]
- Hossner, K.L. (Ed.) Development of Muscle, Skeletal System and Adipose Tissue; CABI Publishing: Wallingford, UK, 2005; pp. 55–93. [Google Scholar] [CrossRef]
- Smith, S.B.; Gill, C.A.; Lunt, D.K.; Brooks, M.A. Regulation of Fat and Fatty Acid Composition in Beef Cattle. Asian-Australas. J. Anim. Sci. 2009, 22, 1225–1233. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Tomaz, L.D.A.; Niehues, M.B.; Ladeira, M.; Curi, R.; Chardulo, L.A.; Baldassini, W.; Martins, C.L.; Arrigoni, M.B.; Neto, O.M. The inclusion of de-oiled wet distillers grains in feedlot diets reduces the expression of lipogenic genes and fat content in Longissimus muscle from F1 Angus-Nellore cattle. PeerJ 2019, 7, e7699. [Google Scholar] [CrossRef]
- Graugnard, D.E.; Piantoni, P.; Bionaz, M.; Berger, L.L.; Faulkner, D.B.; Loor, J.J. Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in Angus and Angus × Simmental cattle fed high-starch or low-starch diets. BMC Genom. 2009, 10, 142. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Zolla, L. Meat science: From proteomics to integrated omics towards system biology. J. Proteom. 2013, 78, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L.; Lebret, B.; Ecolan, P.; Louveau, I.; Damon, M.; Prunier, A.; Billon, Y.; Sellier, P.; Gilbert, H. Muscle characteristics and meat quality traits are affected by divergent selection on residual feed intake in pigs1. J. Anim. Sci. 2011, 89, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Paim, T.D.P.; Viana, P.; Van Tilburg, M.F.; Moura, A.D.A.; De Souza, J.R.; McManus, C.; Abdalla, A.L.; Louvandini, H. Feeding effects of cottonseed and its co-products on the meat proteome from ram lambs. Sci. Agricola 2019, 76, 463–472. [Google Scholar] [CrossRef]
- Rodrigues, R.T.D.S.; Chizzotti, M.L.; Vital, C.; Baracat-Pereira, M.C.; Barros, E.; Busato, K.; Gomes, R.A.; Ladeira, M.; Martins, T. Differences in Beef Quality between Angus (Bos taurus taurus) and Nellore (Bos taurus indicus) Cattle through a Proteomic and Phosphoproteomic Approach. PLoS ONE 2017, 12, e0170294. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vignato, B.; Coutinho, L.L.; Cesar, A.S.M.; Poleti, M.D.; Regitano, L.C.A.; Balieiro, J.C.C. Comparative muscle transcriptome associated with carcass traits of Nellore cattle. BMC Genom. 2017, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Bonnet, M.; Picard, B. Protein Array-Based Approach to Evaluate Biomarkers of Beef Tenderness and Marbling in Cows: Understanding of the Underlying Mechanisms and Prediction. Foods 2020, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M. Proteomic Investigations of Beef Tenderness. In Proteomics in Food Science: From Farm to Fork; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, C.; Richardson, I.; Hocquette, J.-F.; Picard, B. The associations between proteomic biomarkers and beef tenderness depend on the end-point cooking temperature, the country origin of the panelists and breed. Meat Sci. 2019, 157, 107871. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P.; Gagaoua, M.; Warner, R.D. Insights on meat quality from combining traditional studies and proteomics. Meat Sci. 2021, 174, 108423. [Google Scholar] [CrossRef]
- Gagaoua, M.; Monteils, V.; Picard, B. Data from the Farmgate-to-Meat Continuum Including Omics-Based Biomarkers to Better Understand the Variability of Beef Tenderness: An Integromics Approach. J. Agric. Food Chem. 2018, 66, 13552–13563. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Monteils, V. Assessment of cattle inter-individual cluster variability: The potential of continuum data from the farm-to-fork for ultimate beef tenderness management. J. Sci. Food Agric. 2019, 99, 4129–4141. [Google Scholar] [CrossRef]
| Soybean Meal | Ground Corn | Corn Wet Distiller Grain | |
|---|---|---|---|
| DM (% as fed) | 89 | 88 | 32 |
| EE (% DM) | 2.0 | 4.3 | 4.0 |
| Fatty acids (% of total fatty acids) a | |||
| Myristic C14:0 | 0.13 | 0.06 | 1.48 |
| Palmitic C16:0 | 20.43 | 16.53 | 14.84 |
| Stearic C18:0 | 4.29 | 1.99 | 9.73 |
| Oleic C18:1 c9 | 14.85 | 28.30 | 23.1 |
| Linoleic C18:2 c9-c12 | 50.77 | 49.27 | 2.48 |
| Linolenic C18:3 n3 | 6.17 | 1.22 | 0.78 |
| ΣSFA | 26.07 | 19.55 | 27.35 |
| ΣUFA | 73.79 | 80.46 | 33.26 |
| ΣMUFA | 16.85 | 29.97 | 30.32 |
| ΣPUFA | 56.95 | 50.49 | 2.94 |
| Control | 45% WDG | SEM | p-Value | |
|---|---|---|---|---|
| Intramuscular fat, % | 2.90 | 1.85 | 0.095 | <0.0001 |
| Fatty acids (mg/100 g of meat) a | ||||
| C6:0 | 1.849 | 1.075 | 0.383 | 0.077 |
| C10:0 | 1.829 | 1.149 | 0.219 | 0.037 |
| C11:0 | 0.099 | 0.033 | 0.012 | 0.003 |
| Lauric C12:0 | 2.343 | 1.127 | 0.240 | 0.009 |
| C13:0 iso | 0.341 | 0.103 | 0.055 | 0.001 |
| C14:0 iso | 0.717 | 0.503 | 0.185 | 0.861 |
| Myristic C14:0 | 87.829 | 51.924 | 7.358 | 0.009 |
| C15:0 iso | 1.897 | 1.550 | 0.283 | 0.410 |
| C15:0 anteiso | 4.375 | 3.454 | 0.694 | 0.487 |
| Myristoleic C14:1 c9 | 18.741 | 10.337 | 2.568 | 0.066 |
| Pentadecanoic C15:0 | 16.069 | 6.996 | 1.177 | <0.0001 |
| C16:0 iso | 1.795 | 1.278 | 0.367 | 0.492 |
| Palmitic C16:0 | 733.76 | 468.36 | 24.679 | 0.347 |
| C17:0 iso | 5.196 | 4.753 | 0.526 | 0.567 |
| Palmitoleic C16:1 c9 | 105.11 | 52.987 | 7.378 | 0.001 |
| Margaric C17:0 | 34.703 | 16.794 | 1.807 | 0.001 |
| Heptadecenoic C17:1 | 29.482 | 11.380 | 2.728 | <0.0001 |
| Stearic C18:0 | 378.11 | 262.61 | 17.397 | 0.002 |
| C18:1 trans | 75.933 | 75.957 | 11.334 | 0.998 |
| Oleic C18:1 c9 | 1062.82 | 645.70 | 61.888 | 0.001 |
| C18:1 c11 | 78.606 | 40.235 | 5.234 | 0.001 |
| C18:1 c12 | 8.163 | 5.821 | 0.391 | 0.003 |
| C18:1 c13 | 11.839 | 5.478 | 2.013 | 0.104 |
| C18:1 t16 | 1.855 | 2.101 | 0.427 | 0.694 |
| C18:1 c15 | 1.734 | 1.134 | 0.166 | 0.034 |
| Linoleic C18:2 c9c12 | 131.04 | 99.794 | 18.983 | 0.278 |
| C20:0 | 2.082 | 1.586 | 0.203 | 0.077 |
| C18:3 n6 | 0.851 | 0.532 | 0.205 | 0.303 |
| α-Linolenic C18:3 n3 | 10.389 | 6.348 | 0.882 | 0.012 |
| C20:1 | 3.017 | 1.002 | 0.413 | 0.009 |
| CLA C18:2 c9t11 | 3.877 | 4.772 | 0.826 | 0.465 |
| C18:2 t10c12 | 0.094 | 0.201 | 0.075 | 0.607 |
| C20:2 | 1.306 | 0.921 | 0.219 | 0.248 |
| C20:3 n6 | 5.702 | 4.974 | 1.185 | 0.676 |
| C22:0 | 0.0686 | 0.125 | 0.029 | 0.676 |
| Arachidonic C20:4 n6 | 32.616 | 24.869 | 6.922 | 0.452 |
| EPA C20:5 n3 | 7.576 | 5.204 | 1.434 | 0.276 |
| C24:0 | 0.076 | 0.0552 | 0.021 | 0.505 |
| C24:1 | 2.855 | 1.982 | 0.616 | 0.346 |
| C22:5 | 14.983 | 10.644 | 2.806 | 0.306 |
| C22:6 n3 | 1.500 | 1.273 | 0.325 | 0.634 |
| ΣSFA | 1271.29 | 822.41 | 34.943 | <0.0001 |
| ΣUFA | 1610.15 | 1013.69 | 83.816 | 0.001 |
| ΣMUFA | 1400.21 | 854.16 | 76.630 | 0.001 |
| ΣPUFA | 209.94 | 159.53 | 31.540 | 0.291 |
| ΣMUFA/ΣSFA | 32.418 | 19.179 | 2.519 | 0.001 |
| ΣPUFA/ΣSFA | 4.887 | 3.615 | 0.776 | 0.280 |
| ΣUFA/ΣSFA | 37.305 | 22.794 | 2.854 | 0.004 |
| Σn3 | 19.465 | 12.826 | 2.579 | 0.106 |
| Σn6 | 39.169 | 30.376 | 8.284 | 0.474 |
| ω-6/ω-3 | 54.802 | 42.263 | 6.873 | 0.233 |
| Spot ID | Uniprot ID | Gene Symbol | Full Protein Names | Mascot Score | Protein Coverage (%) | pI/MW Experimental | pI/MW Theoretical |
|---|---|---|---|---|---|---|---|
| Contractile and associated proteins | |||||||
| 02 | Q0P571 | MYLPF | Myosin regulatory light chain 2, skeletal muscle isoform | 35,055.73 | 53.53 | 3.69/19,127.56 | 4.735/19,012.55 |
| 15 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 6287.59 | 63.54 | 3.78/21,045.95 | 4.82/20,931.84 |
| 25 | P85100 | MYL3 | Myosin light chain 3 | 1868.16 | 32.16 | 3.6/22,110.17 | 4.87/21,939.03 |
| 26 | P60661 | MYL6 | Myosin light polypeptide 6 | 354.34 | 10.6 | 4.44/17,101.18 | 4.41/16,930.05 |
| 06 | Q8MKH6 | TNNT1 | Troponin T, slow skeletal muscle | 208.97 | 4.56 | 3.79/31,284.30 | 5.67/31,284.25 |
| 04 | Q8MKI3 | TNNT3 | Troponin T, fast skeletal muscle | 22,402.47 | 19.93 | 9.59/321,259.78 | 6.07/32,125.94 |
| Energy metabolism | |||||||
| 40 | P19858 | LDHA | L-lactate dehydrogenase A chain | 69.01 | 5.12 | 9.97/36,939.87 | 7.85/36,597.64 |
| 28 | Q5E9B1 | LDHB | L-lactate dehydrogenase B chain | 51.97 | 6.59 | 6.56/37,009.55 | 6.13/36,723.64 |
| 29 | Q32LG3 | MDH2 | Malate dehydrogenase, mitochondrial | 257.13 | 18.05 | 9.73/36,124.77 | 8.45/35,668.5 |
| 30 | Q2KJE5 | GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 969.47 | 6.08 | 8.29/43,687.24 | 7.98/43,287.96 |
| 31 | Q5EA88 | GPD1 | Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic | 51,064.30 | 65.90 | 4.26/38,218.10 | 6.54/37,647.67 |
| 32 | Q02373 | NDUFB10 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10 | 2416.24 | 27.27 | 6.95/21,250.06 | 8.41/20,964.84 |
| 10 | P00829 | ATP5F1B | ATP synthase subunit beta, mitochondrial | 125,429.60 | 56.44 | 5.32/56,284.23 | 5.04/56,283.53 |
| 08 | Q5E956 | TPI1 | Triosephosphate isomerase | 408,896.4 | 93.98 | 5.31/26,917.47 | 6.59/26,689.51 |
| 07 | Q3ZC09 | ENO3 | Beta-enolase | 250,468.30 | 69.59 | 4.26/47,438.48 | 7.55/47,096.01 |
| 33 | Q9XSJ4 | ENO1 | Alpha-enolase | 7368.94 | 34.56 | 4.26/47,668.70 | 6.48/47,326.13 |
| 16 | Q9XSC6 | CKM | Creatine kinase M-type | 81.84 | 7.87 | 9.73/4322.14 | 6.80/42,988.95 |
| Oxidative stress, cell redox homeostasis, chaperones and heat shock proteins | |||||||
| 14 | P41976 | SOD2 | Superoxide dismutase [Mn], mitochondrial | 4030.24 | 28.38 | 4.44/24,890.60 | 8.53/24,637.95 |
| 20 | Q27975 | HSPA1A | Heat shock 70 kDa protein 1A | 72,555.56 | 54.45 | 4.07/70,544.33 | 5.64/70,258.51 |
| 21 | P34933 | HSPA2 | Heat shock-related 70 kDa protein 2 | 504.82 | 5.19 | 4.07/70,024.93 | 5.32/69,739.7 |
| 09 | Q58DR2 | DNAJB12 | DnaJ homolog subfamily B member 12 | 58.07 | 8.65 | 4.26/41,568.48 | 8.69/41,340.28 |
| 14 | P02510 | CRYAB | Alpha-crystallin B chain | 16,025.71 | 73.14 | 5.32/20,036.81 | 7.05/20,036.79 |
| 06 | P04272 | ANXA2 | Annexin A2 | 136.22 | 12.68 | 5.23/38,897.28 | 7.03/38,612.07 |
| Protein binding | |||||||
| 17 | Q5E9D1 | SUMO1 | Small ubiquitin-related modifier 1 | 141.4561 | 30.69 | 3.93/11,614.06 | 5.27/17,949.28 |
| 27 | O97764 | CRYZ | Zeta-crystallin | 1171.379 | 37.88 | 9.26/35,553.94 | 8.15/35,382.8 |
| Transport and signaling | |||||||
| 18 | O97827 | ADGRL3 | Adhesion G protein-coupled receptor L3 | 58.17 | 3.04 | 6.56/67,847.39 | 7.82/67,329.78 |
| 35 | Q3SX00 | ANKRD46 | Ankyrin repeat domain-containing protein 46 | 73.30 | 13.16 | 4.17/25,557.93 | 5.44/25,329.79 |
| 05 | P02769 | ALB | Albumin | 14,091.32 | 78.25 | 4.26/71,289.43 | 5.87/69,293.41 |
| 19 | Q3SZ57 | AFP | Alpha-fetoprotein | 137.99 | 1.15 | 6.64/70,412.61 | 5.98/68,587.64 |
| 01 | P02192 | MB | Myoglobin | 373,870.70 | 87.66 | 9.98/170,776.21 | 7.19/17,077.59 |
| Spot ID | Uniprot ID | Gene Symbol | Full Protein Names | Mascot Score | Protein Coverage (%) | pI/MW Experimental | pI/MW Theoretical |
|---|---|---|---|---|---|---|---|
| Contractile and associated proteins | |||||||
| 52 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 68,715.63 | 63.54 | 6.87/20,856.32 | 4.83/20,931.84 |
| 62 | Q0P571 | MYLPF | Myosin regulatory light chain 2, skeletal muscle isoform | 74,702.68 | 63.53 | 3.82/19,126.56 | 4.73/19,012.55 |
| 55 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 68,715.63 | 63.54 | 7.00/21,459.50 | 4.83/20,931.84 |
| 56 | P85100 | MYL3 | Myosin light chain 3 | 1554.92 | 12.56 | 7.6/22,110.17 | 4.87/21,939.03 |
| 57 | P60661 | MYL6 | Myosin light polypeptide 6 | 316.95 | 10.6 | 4.44/17,101.18 | 7.86/52,285.46 |
| 72 | Q8MKH6 | TNNT1 | Troponin T, slow skeletal muscle | 1464.83 | 14.45 | 9.77/31,284.03 | 5.67/31,284.25 |
| 73 | Q8MKI3 | TNNT3 | Troponin T, fast skeletal muscle | 13,253.61 | 19.93 | 9.59/32,126.78 | 6.07/32,125.94 |
| Energy metabolism | |||||||
| 83 | P19858 | LDHA | L-lactate dehydrogenase A chain | 4527.98 | 29.22 | 6.10/36,939.71 | 7.85/36,597.64 |
| 14 | Q5E9B1 | LDHB | L-lactate dehydrogenase B chain | 272.92 | 17.66 | 6.1/37,008.86 | 6.13/36,723.64 |
| 85 | Q3T145 | MDH1 | Malate dehydrogenase, cytoplasmic | 346.09 | 20.36 | 4.07/36,723.40 | 6.25/36,438.19 |
| 86 | P10096 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 59.52 | 8.71 | 6.1/36,096.27 | 8.29/35,868.09 |
| 87 | Q5EA88 | GPD1 | Glycerol-3-phosphate dehydrogenase [NAD(+)] | 239.72 | 23.78 | 4.26/38,218.01 | 6.54/37,647.67 |
| 100 | P00829 | ATP5F1B | ATP synthase subunit beta, mitochondrial | 42,545.85 | 39.77 | 6.78/56,283.25 | 5.04/56,283.53 |
| 70 | Q3ZC09 | ENO3 | Beta-enolase | 6853.10 | 48.62 | 6.29/47,438.25 | 7.55/47,096.01 |
| 70 | Q9XSJ4 | ENO1 | Alpha-enolase | 3591.51 | 17.74 | 6.29/47,668.37 | 6.48/47,326.13 |
| 80 | Q5E956 | TPI1 | Triosephosphate isomerase | 367,356.90 | 93.57 | 6.89/26,917.47 | 6.59/26,689.51 |
| 90 | Q5E956 | TPI1 | Triosephosphate isomerase | 1955.57 | 32.13 | 5.23/26,917.64 | 6.59/26,689.51 |
| 104 | A4IFD0 | Ak5 | Adenylate kinase isoenzyme 5 | 6634.23 | 1.60 | 6.78/63,843.41 | 4.91/63,272.94 |
| 103 | P00570 | AK1 | Adenylate kinase isoenzyme 1 | 8082.40 | 48.45 | 7.53/21,778.04 | 8.35/21,663.94 |
| Oxidative stress, cell redox homeostasis, chaperones and heat shock proteins | |||||||
| 95 | P41976 | SOD2 | Superoxide dismutase [Mn], mitochondrial | 4030.24 | 28.38 | 7.00/24,809.60 | 8.53/24,637.95 |
| 08 | Q27965 | HSPA1B | Heat shock 70 kDa protein 1B | 642.10 | 27.15 | 4.07/70,513.42 | 5.64/70,228.42 |
| 108 | P19120 | HSPA8 | Heat shock cognate 71 kDa protein | 107.21 | 3.69 | 4.07/71,468.73 | 5.25/71,240.51 |
| 109 | P0CB32 | HSPA1L | Heat shock 70 kDa protein 1-like | 188.49 | 9.83 | 3.96/70,788.37 | 5.91/70,389.07 |
| 110 | P34933 | HSPA2 | Heat shock-related 70 kDa protein 2 | 107.21 | 3.77 | 4.07/70,024.93 | 5.32/69,739.7 |
| 111 | Q3T149 | HSPB1 | Heat shock protein beta-1 | 158,366.90 | 86.57 | 6.78/22,450.99 | 6.11/22,393.06 |
| 74 | P02510 | CRYAB | Alpha-crystallin B chain | 90,018.13 | 70.86 | 5.32/20,036.06 | 7.05/20,036.79 |
| 88 | Q0VCX2 | HSPA5 | Endoplasmic reticulum chaperone BiP | 79.91 | 1.68 | 4.07/72,514.18 | 4.93/72,400.03 |
| Transport and signaling | |||||||
| 05 | P02769 | ALB | Albumin | 30,645.09 | 50.74 | 4.26/71,289.30 | 5.87/69,293.41 |
| 105 | Q3SZ57 | AFP | Alpha-fetoprotein | 42.61 | 1.15 | 6.64/70,410.61 | 5.98/68,587.64 |
| 51 | P02192 | MB | Myoglobin | 1483.74 | 16.88 | 4.42/17,077.21 | 7.20/17,077.59 |
| QTL Linked to QTLdb a | Gene Name | Protein Name | UniProtID (Bovine) | Chr. |
|---|---|---|---|---|
| Carcass weight (n = 10) | MDH1 | Malate dehydrogenase, cytoplasmic | Q3T145 | Chr.11 |
| CRYAB | Alpha-crystallin B chain | P02510 V6F832 | Chr.15 | |
| ENO1 | Alpha-enolase | Q9XSJ4 | Chr.16 | |
| MYLPF | Myosin regulatory light chain 2 | Q0P571 | Chr.25 | |
| MDH2 | Malate dehydrogenase, mitochondrial | Q58DR9 Q32LG3 | Chr.25 | |
| HSPB1 | Heat shock protein beta-1 | Q3T149 E9RHW1 | Chr.25 | |
| LDHA | L-lactate dehydrogenase A chain | P19858 | Chr.29 | |
| AK5 | Adenylate kinase isoenzyme 5 | A4IFD0 | Chr.3 | |
| CRYZ | Zeta-crystallin | O97764 | Chr.3 | |
| MB | Myoglobin | A0A1K0FUF3 P02192 | Chr.5 | |
| Ribeye area (n = 3) | CRYAB | Alpha-crystallin B chain | P02510 V6F832 | Chr.15 |
| MB | Myoglobin | A0A1K0FUF3 P02192 | Chr.5 | |
| LDHB | L-lactate dehydrogenase B chain | Q5E9B1 | Chr.5 | |
| Marbling score (n = 6) | HSPA8 | Heat shock cognate 71 kDa protein | P19120 | Chr.15 |
| SUMO1 | Small ubiquitin-related modifier 1 | Q5E9D1 | Chr.2 | |
| HSPA1B HSPA1A | Heat shock 70 kDa protein 1B; Heat shock 70 kDa protein 1A | Q27965 Q27975 | Chr.23 | |
| HSPA1L | Heat shock 70 kDa protein 1-like | P0CB32 | Chr.23 | |
| Ak5 | Adenylate kinase isoenzyme 5 | A4IFD0 | Chr.3 | |
| CRYZ | Zeta-crystallin | O97764 | Chr.3 | |
| Intramuscular fat (n = 1) | GPD1 | Glycerol-3-phosphate dehydrogenase | Q5EA88 | Chr.5 |
| Oleic acid content (n = 1) | ENO3 | Beta-enolase | Q3ZC09 | Chr.19 |
| Omega-3 unsaturated fatty acid content (n = 1) | ANXA2 | Annexin A2 | P04272 | Chr.10 |
| Palmitic acid content (n = 2) | GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | Q2KJE5 | Chr.18 |
| CKM | Creatine kinase M-type | Q9XSC6 | Chr.18 | |
| Palmitoleic acid content (n = 2) | HSPA2 | Heat shock-related 70 kDa protein 2 | P34933 | Chr.10 |
| ENO3 | Beta-enolase | Q3ZC09 | Chr.19 | |
| Palmitoleic acid to palmitic acid ratio (n = 1) | DNAJB12 | DnaJ homolog subfamily B member 12 | Q58DR2 | Chr.28 |
| Stearic acid content (n = 4) | HSPA2 | Heat shock-related 70 kDa protein 2 | HSPA2 | Chr.10 |
| MDH1 | Malate dehydrogenase, cytoplasmic | MDH1 | Chr.11 | |
| HSPA5 | Endoplasmic reticulum chaperone BiP | HSPA5 | Chr.11 | |
| AK1 | Adenylate kinase isoenzyme 1 | AK1 | Chr.11 | |
| Trans-6/9-C18:1 fatty acid content (n = 1) | ATP5F1B | ATP synthase subunit beta, mitochondrial | ATP5F1B | Chr.2 |
| Shear force (n = 8) | HSPA8 | Heat shock cognate 71 kDa protein | P19120 | Chr.15 |
| NDUFB10 | NADH dehydrogenase | M5FHL5 Q02373 | Chr.25 | |
| MYLPF | Myosin regulatory light chain 2 | Q0P571 | Chr.25 | |
| ADGRL3 | Adhesion G protein-coupled receptor L3 | O97827 | Chr.6 | |
| ALB | Albumin | P02769 A0A140T897 | Chr.6 | |
| ADGRL3 | Adhesion G protein-coupled receptor L3 | O97827 | Chr.6 | |
| ALB | Albumin | P02769 A0A140T897 | Chr.6 | |
| AFP | Alpha-fetoprotein | Q3SZ57 | Chr.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassini, W.; Gagaoua, M.; Santiago, B.; Rocha, L.; Torrecilhas, J.; Torres, R.; Curi, R.; Neto, O.M.; Padilha, P.; Santos, F.; et al. Meat Quality and Muscle Tissue Proteome of Crossbred Bulls Finished under Feedlot Using Wet Distiller Grains By-Product. Foods 2022, 11, 3233. https://doi.org/10.3390/foods11203233
Baldassini W, Gagaoua M, Santiago B, Rocha L, Torrecilhas J, Torres R, Curi R, Neto OM, Padilha P, Santos F, et al. Meat Quality and Muscle Tissue Proteome of Crossbred Bulls Finished under Feedlot Using Wet Distiller Grains By-Product. Foods. 2022; 11(20):3233. https://doi.org/10.3390/foods11203233
Chicago/Turabian StyleBaldassini, Welder, Mohammed Gagaoua, Bismarck Santiago, Leone Rocha, Juliana Torrecilhas, Rodrigo Torres, Rogério Curi, Otávio Machado Neto, Pedro Padilha, Felipe Santos, and et al. 2022. "Meat Quality and Muscle Tissue Proteome of Crossbred Bulls Finished under Feedlot Using Wet Distiller Grains By-Product" Foods 11, no. 20: 3233. https://doi.org/10.3390/foods11203233
APA StyleBaldassini, W., Gagaoua, M., Santiago, B., Rocha, L., Torrecilhas, J., Torres, R., Curi, R., Neto, O. M., Padilha, P., Santos, F., Lanna, D. P., & Chardulo, L. A. (2022). Meat Quality and Muscle Tissue Proteome of Crossbred Bulls Finished under Feedlot Using Wet Distiller Grains By-Product. Foods, 11(20), 3233. https://doi.org/10.3390/foods11203233








