Characterization of Dextran Produced by the Food-Related Strain Weissella cibaria C43-11 and of the Relevant Dextransucrase Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. EPS Production in mMRS
2.3. Purification and Quantification of EPS
2.4. EPS Monosaccharide Composition
2.5. NMR Spectroscopy
2.5.1. Chemicals
2.5.2. Sample Preparation
2.5.3. NMR Experiment
2.6. Analysis of the Dsr Gene
2.7. Transcription Analysis
2.7.1. RNA Extraction and cDNA Synthesis
2.7.2. Real-Time (RT) qPCR
2.8. Statistical Analysis
3. Results
3.1. EPS Quantification and Characterization
3.1.1. EPS Production
3.1.2. Monosaccharide Composition
3.1.3. EPS Characterization (NMR)
3.2. Analysis of the Dsr Gene
3.3. Transcription Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Tech. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Kuipers, O.P.; Smid, E.J. Lactic acid bacteria—A continuing journey in science and application. FEMS Microbiol. Rev. 2017, 41 (Suppl. 1), S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; and Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Di Biase, M.; Bavaro, A.R.; Lonigro, S.L.; Pontonio, E.; Conte, A.; Padalino, L.; Minisci, A.; Lavermicocca, P.; Valerio, F. Lactobacillus plantarum ITM21B fermentation product and chickpea flour enhance the nutritional profile of salt reduced bakery products. Int. J. Food Sci. Nutr. 2019, 70, 701–713. [Google Scholar] [CrossRef]
- De Bellis, P.; Rizzello, C.G.; Sisto, A.; Valerio, F.; Lonigro, S.L.; Conte, A.; Lorusso, V.; Lavermicocca, P. Use of a selected Leuconostoc citreum strain as a starter for making a “yeast-free” bread. Foods 2019, 8, 70. [Google Scholar] [CrossRef]
- De Bellis, P.; Montemurro, M.; D′Imperio, M.; Rizzello, C.G.; Sisto, A.; Lavermicocca, P. Production of a yeast-free focaccia with reduced salt content using a selected Leuconostoc citreum strain and seawater. LWT 2020, 134, 109918. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Di Biase, M.; Conte, A.; Lonigro, S.L.; Caputo, L.; Cedola, A.; Del Nobile, M.A.; Logrieco, A.F.; Lavermicocca, P.; Valerio, F. Weissella cibaria short-fermented liquid sourdoughs based on quinoa or amaranth flours as fat replacer in focaccia bread formulation. Int. J. Food Sci. Technol. 2021, 56, 3197–3208. [Google Scholar] [CrossRef]
- Valerio, F.; Di Biase, M.; Lattanzio, V.M.; Lavermicocca, P. Improvement of the antifungal activity of lactic acid bacteria by addition to the growth medium of phenylpyruvic acid, a precursor of phenyllactic acid. Int. J. Food Microbiol. 2016, 222, 1–7. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In vitro prebiotic activities of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Food 2020, 67, 103853. [Google Scholar] [CrossRef]
- Nácher-Vázquez, M.; Ballesteros, N.; Canales, Á.; Saint-Jean, S.R.; Pérez-Prieto, S.I.; Prieto, A.; Aznar, R.; López, P. Dextrans produced by lactic acid bacteria exhibit antiviral and immunomodulatory activity against salmonid viruses. Carbohyd. Polym. 2015, 124, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Ruas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; Lopez, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohyd. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Besrour-Aouam, N.; Fhoula, I.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Najjari, A.; Prieto, A.; Ruas Madiedo, P.; López, P.; Ouzari, H.I. The role of dextran production in the metabolic context of Leuconostoc and Weissella Tunisian strains. Carbohyd. Polym. 2021, 253, 117254. [Google Scholar] [CrossRef]
- Bounaix, M.S.; Robert, H.; Gabriel, V.; Morel, S.; Remaud-Simeon, M.; Gabriel, B.; Fontagne-Faucher, C. Characterization of dextran-producing Weissella strains isolated from sourdoughs and evidence of constitutive dextransucrase expression. FEMS Microbiol. Lett. 2010, 311, 18–26. [Google Scholar] [CrossRef]
- Byrne, D. Commission Decision of 30 January 2001 on Authorising the Placing on the Market of a Dextran Preparation Produce by Leuconostoc Mesenteroides as a Novel Food Ingredients in Bakery Products under Regulation (EC) No. 258/97 of the European Parliament and of the Council Official Journal European Commission L44; European Commission: Brussels, Belgium, 2001. [Google Scholar]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In situ synthesis of exopolysaccharides by Leuconostoc spp. and Weissella spp. and their rheological impacts in fava bean flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef]
- Wang, Y.; Compaoré-Sérémé, D.; Sawadogo-Lingani, H.; Coda, R.; Katina, K.; Maina, N.H. Influence of dextran synthesized in situ on the rheological, technological and nutritional properties of whole grain pearl millet bread. Food Chem. 2019, 285, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Buksa, K.; Kowalczyk, M.; Boreczek, J. Extraction, purification and characterisation of exopolysaccharides produced by newly isolated lactic acid bacteria strains and the examination of their influence on resistant starch formation. Food Chem. 2021, 362, 130221. [Google Scholar] [CrossRef] [PubMed]
- Tieking, M.; Gänzle, M.G. Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of in-situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Cri. Rev. Food Sci. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ping, W.; Ge, J. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overview of exopolysaccharides produced by Weissella genus—A review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef]
- Besrour-Aouam, N.; Mohedano, M.L.; Fhoula, I.; Zarour, K.; Najjari, A.; Aznar, R.; Prieto, A.; Ouzari, H.-I.; López, P. Different modes of regulation of the expression of dextransucrase in Leuconostoc lactis AV1n and Lactobacillus sakei MN1. Front. Microbiol. 2019, 10, 959. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41 (Suppl. 1), S168–S200. [Google Scholar] [CrossRef]
- Coda, R.; Xu, Y.; Moreno, D.S.; Mojzita, D.; Nionelli, L.; Rizzello, C.G.; Katina, K. Performance of Leuconostoc citreum FDR241 during wheat flour sourdough type I propagation and transcriptional analysis of exopolysaccharides biosynthesis genes. Food Microbiol. 2018, 76, 164–172. [Google Scholar] [CrossRef]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ spent grain as substrate for dextran biosynthesis by Leuconostoc pseudomesenteroides DSM20193 and Weissella confusa A16. Microb. Cell Fact. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Garofalo, C.; Belleggia, L.; Maoloni, A.; Cardinali, F.; Mozzon, M.; Foligni, R.; Aquilanti, L.; Clementi, F. Selection of cereal-sourced lactic acid bacteria as candidate starters for the baking industry. PLoS ONE 2020, 15, e0236190. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, R.; Singh, D.; Bhatt, S.; Gupta, M. Physiological and genomic characterization of an exopolysaccharide-producing Weissella cibaria CH2 from cheese of the western Himalayas. Food Biosci. 2020, 35, 100570. [Google Scholar] [CrossRef]
- Lynch, K.M.; Lucid, A.; Arendt, E.K.; Sleator, R.D.; Lucey, B.; Coffey, A. Genomics of Weissella cibaria with an examination of its metabolic traits. Microbiology 2015, 161, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Werning, M.L.; Notararigo, S.; Nácher, M.; Fernández de Palencia, P.; Aznar, R.; López, P. Biosynthesis, purification and biotechnological use of exopolysaccharides produced by lactic acid bacteria. In Food Additives; El-Samragy, Y., Ed.; Intech: Rijeka, Croatia, 2012; pp. 83–114. [Google Scholar]
- Yu, Y.J.; Chen, Z.; Chen, P.T.; Ng, I.S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gänzle, M.G. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10 M. Int. J. Food Microbiol. 2018, 280, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Favilla, M.; De Bellis, P.; Sisto, A.; de Candia, S.; Lavermicocca, P. Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contami-nating bakery products. Syst. Appl. Microbiol. 2009, 32, 438–448. [Google Scholar] [CrossRef]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of amaranth and quinoa flours on exopolysaccharide production and protein profile of liquid sourdough fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef]
- Minervini, F.; De Angelis, M.; Surico, R.F.; Di Cagno, R.; Gänzle, M.; Gobbetti, M. Highly efficient synthesis of exopolysaccharides by Lactobacillus curvatus DPPMA10 during growth in hydrolyzed wheat flour agar. Int. J. Food Microbiol. 2010, 141, 130–135. [Google Scholar] [CrossRef]
- Cerning, J.C.M.C.; Renard, C.M.G.C.; Thibault, J.F.; Bouillanne, C.; Landon, M.; Desmazeaud, M.; Topisirovic, L. Carbon source requirements for exopolysaccharide production by Lactobacillus casei CG11 and partial structure analysis of the polymer. Appl. Environ. Microb. 1994, 60, 3914–3919. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Jungkunz, S.; Wagner, M.; Vogel, R.F. Optimization of homoexopolysaccharide formation by lactobacilli in gluten-free sourdoughs. Food Microbiol. 2012, 32, 286–294. [Google Scholar] [CrossRef]
- Garbetta, A.; D’Antuono, I.; Melilli, M.G.; Sillitti, C.; Linsalata, V.; Scandurra, S.; Cardinali, A. Inulin enriched durum wheat spaghetti: Effect of polymerization degree on technological and nutritional characteristics. J. Funct. Foods 2020, 71, 104004. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, V.; Salamov, A. Automatic Annotation of Microbial Genomes and Metagenomic Sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 61–78. [Google Scholar]
- Wang, L.; Stegemann, J.P. Extraction of high quality RNA from polysaccharide matrices using cetlytrimethylammonium bromide. Biomaterials 2010, 31, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ogden, T.L. Handling results below the level of detection. Ann. Occup. Hyg. 2010, 54, 255–256. [Google Scholar]
- Keeler, J. Understanding NMR Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-74608-0. [Google Scholar]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella Confusa. Carbohyd. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Verni, M.; Wang, C.; Montemurro, M.; De Angelis, M.; Katina, K.; Rizzello, C.G.; Coda, R. Exploring the microbiota of faba bean: Functional characterization of lactic acid bacteria. Front. Microbiol. 2017, 8, 2461. [Google Scholar] [CrossRef]
- Sáez, G.D.; Sabater, C.; Fara, A.; Zárate, G. Fermentation of chickpea flour with selected lactic acid bacteria for improving its nutritional and functional properties. J. Appl. Microbiol. 2021, 133, 181–199. [Google Scholar] [CrossRef]
- Baruah, R.; Maina, N.H.; Katina, K.; Juvonen, R.; Goyal, A. Functional food applications of dextran from Weissella cibaria RBA12 from pummelo (Citrus maxima). Int. J. Food Microbiol. 2017, 242, 124–131. [Google Scholar] [CrossRef]
- Herman, R.H. Mannose metabolism. II. Am. J. Clin. Nutr. 1971, 24, 556–561. [Google Scholar] [CrossRef]
- Bounaix, M.S.; Gabriel, V.; Morel, S.; Robert, H.; Rabier, P.; Remaud-Simeon, M.; Gabriel, B.; Fontagne-Faucher, C. Biodiversity of exopolysaccharides produced from sucrose by sourdough lactic acid bacteria. J. Agr. Food Chem. 2009, 57, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.N.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A. Structural analysis and characterization of dextran produced by wild and mutant strains of Leuconostoc mesenteroides. Carbohyd. Polym. 2014, 99, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohyd. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Dobruchowska, J.M.; Gerwig, G.J.; Kralj, S.; Grijpstra, P.; Leemhuis, H.; Dijkhuizen, L.; Kamerling, J.P. Structural characterization of linear isomalto-/malto-oligomer products synthesized by the novel GTFB 4,6-α-glucanotransferase enzyme from Lactobacillus reuteri 121. Glycobiology 2012, 22, 517–528. [Google Scholar] [CrossRef]
- Quirasco, M.; Lopez-Munguia, A.; Remaud-Simeon, M.; Monsan, P.; Farres, A. Induction and transcription studies of the dextransucrase gene in Leuconostoc mesenteroides NRRL B-512F. Appl. Environ. Microbiol. 1999, 65, 5504–5509. [Google Scholar] [CrossRef]
- Arskold, E.; Svensson, M.; Grage, H.; Roos, S.; Radstrom, P.; van Niel, E.W. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int. J. Food Microbiol. 2007, 116, 159–167. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.M.; Remaud-Siméon, M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming Weissella cibaria MG1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef]
- Patrone, V.; Al-Surrayai, T.; Romaniello, F.; Fontana, A.; Milani, G.; Sagheddu, V.; Puglisi, E.; Callegari, M.L.; Al-Mansour, H.; Mohamed Waheed Kishk, M.W.; et al. Integrated Phenotypic-Genotypic Analysis of Candidate Probiotic Weissella Cibaria Strains Isolated from Dairy Cows in Kuwait. Probiotics Antimicro. 2021, 13, 809–823. [Google Scholar] [CrossRef]
- Fernandez De Henestrosa, A.R.; Ogi, T.; Aoyagi, S.; Chafin, D.; Hayes, J.J.; Ohmori, H.; Woodgate, R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 2000, 35, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- D′Ari, R. The SOS system. Biochimie 1985, 67, 343–347. [Google Scholar] [CrossRef]
- Brent, R.; Ptashne, M. The lexA gene product represses its own promoter. Proc. Natl. Acad. Sci. USA 1980, 77, 1932–1936. [Google Scholar] [CrossRef]
- Little, J.W.; Mount, D.W.; Yanisch-Perron, C.R. Purified lexA protein is a repressor of the recA and lexA genes. Proc. Natl. Acad. Sci. USA 1981, 78, 4199–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]

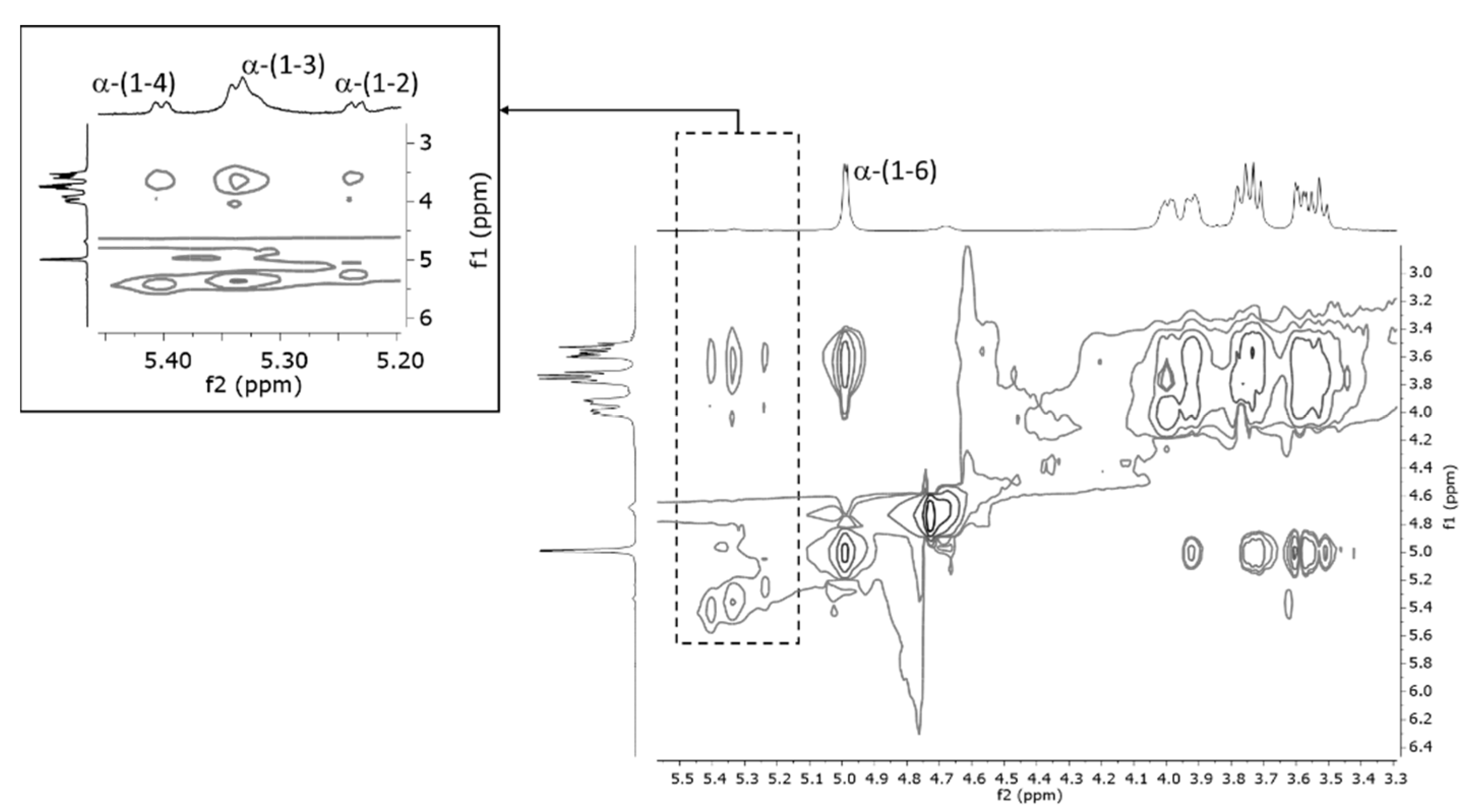
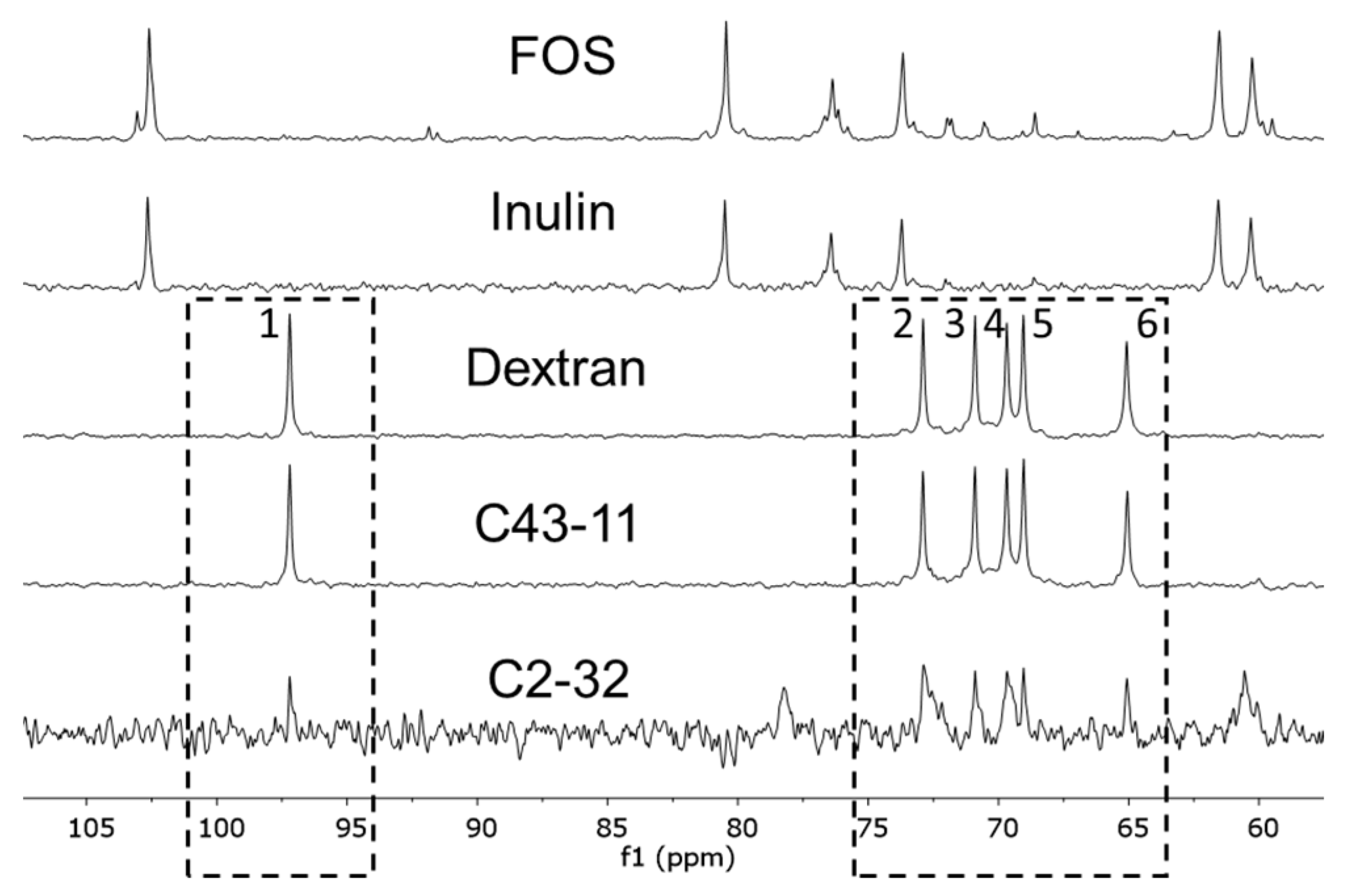

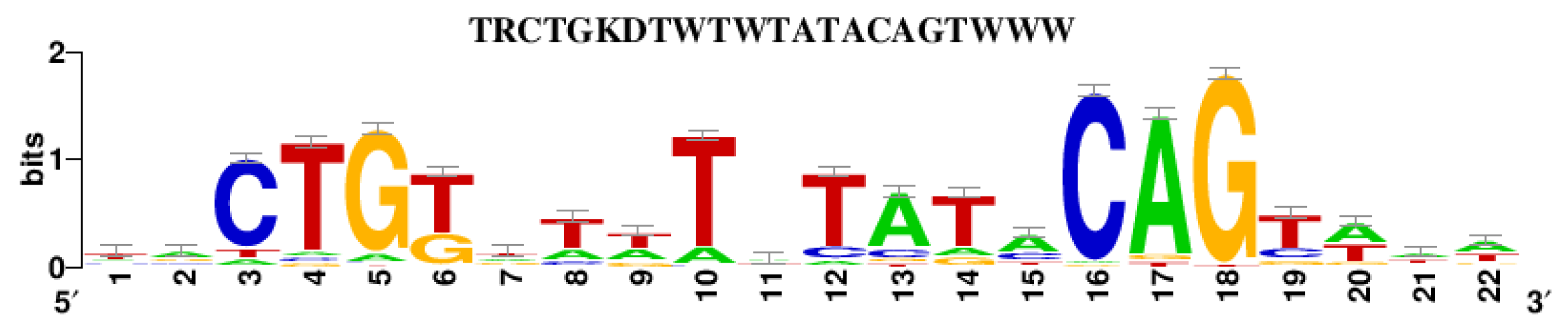
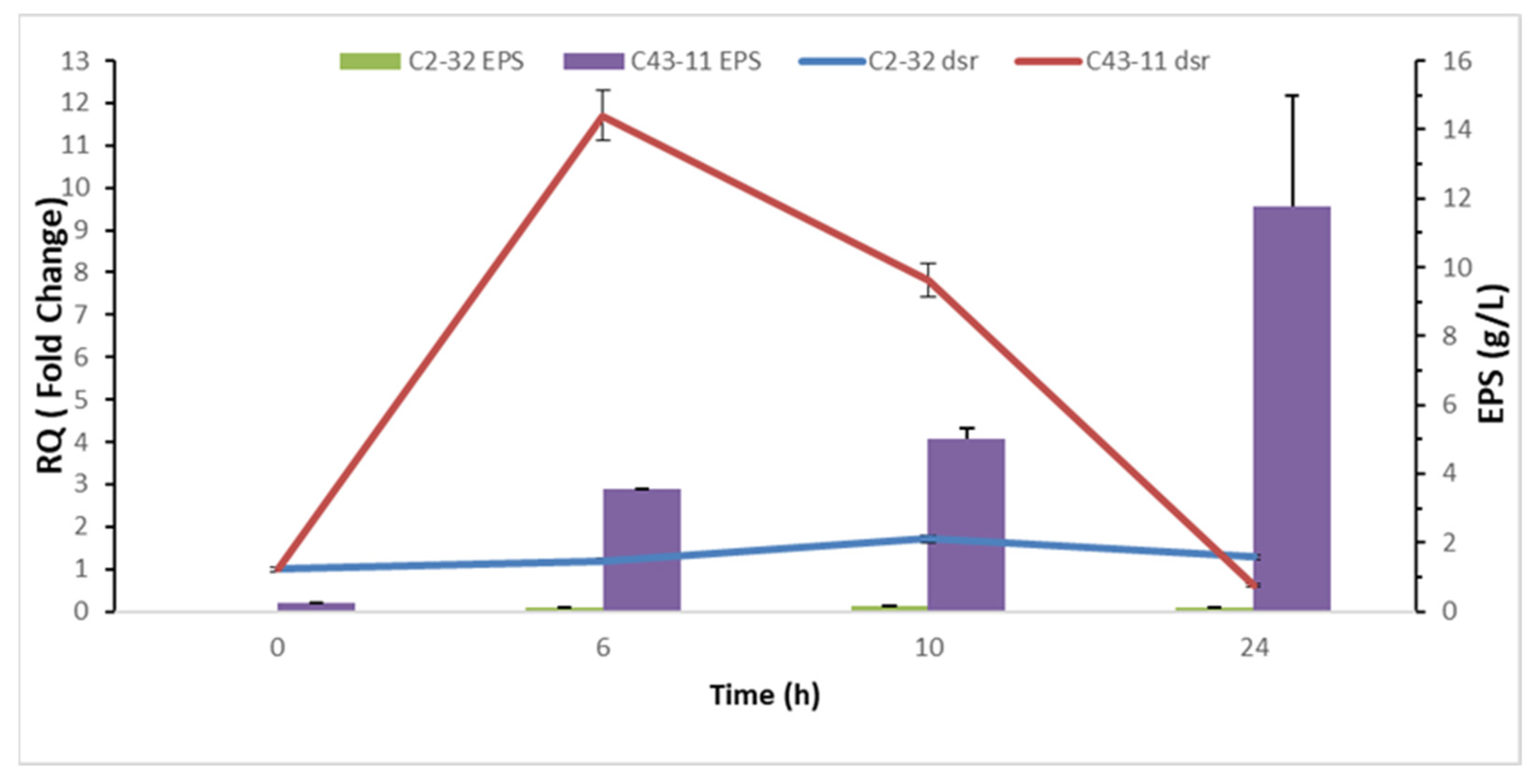
| Culture | T0 | T6 | T10 | T24 |
|---|---|---|---|---|
| Cell Count (log cfu/mL) | ||||
| C2-32 | 7.08 ±0.01 b | 7.20 ± 0.08 b | 7.57 ± 0.03 b | 8.52 ± 0.62 a |
| C2-32 + S | 7.07 ± 0.10 b | 7.40 ± 0.02 b | 7.50 ± 0.09 b | 8.59 ± 0.78 a |
| C43-11 | 7.32 ± 0.03 a | 7.39 ± 0.01 b | 7.68 ± 0.03 b | 8.77 ± 0.78 a |
| C43-11 + S | 7.320 ± 0.06 a | 7.64 ± 0.06 a | 7.89 ± 0.02 a | 9.27 ± 0.29 a |
| EPS (g/L) | ||||
| C2-32 | <LOD b,* | <LOD c,* | <LOD b,* | <LOD b,* |
| C2-32 + S | <LOD b,* | 0.12 ± 0.004 b | 0.14 ± 0.00 b | 0.13 ± 0.00 b |
| C43-11 | <LOD b,* | <LOD c,* | 0.12 ± 0.00 b | <LOD b,* |
| C43-11 + S | 0.23 ± 0.00 a | 3.55 ± 0.02 a | 5.02 ± 0.31 a | 11.74 ± 3.25 a |
| Culture | Glucose | Mannose | Fructose |
|---|---|---|---|
| mg/L | |||
| C43-11 + S | 16299.9 ± 2114.1 a | 203.4 ± 55.4 ab | 779.9 ± 2.7 a |
| C2-32 + S | 259.7 ± 75.9 b | 207.0 ± 19.0 ab | 13.8 ± 4.7 b |
| C43-11 | 138.5 ± 23.3 b | 280.5 ± 23.3 a | 21.0 ± 8.5 b |
| C2-32 | 125.8 ± 22.7 b | 124.9± 10.6 b | 20.0 ± 4.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bellis, P.; Ferrara, M.; Bavaro, A.R.; Linsalata, V.; Di Biase, M.; Musio, B.; Gallo, V.; Mulè, G.; Valerio, F. Characterization of Dextran Produced by the Food-Related Strain Weissella cibaria C43-11 and of the Relevant Dextransucrase Gene. Foods 2022, 11, 2819. https://doi.org/10.3390/foods11182819
De Bellis P, Ferrara M, Bavaro AR, Linsalata V, Di Biase M, Musio B, Gallo V, Mulè G, Valerio F. Characterization of Dextran Produced by the Food-Related Strain Weissella cibaria C43-11 and of the Relevant Dextransucrase Gene. Foods. 2022; 11(18):2819. https://doi.org/10.3390/foods11182819
Chicago/Turabian StyleDe Bellis, Palmira, Massimo Ferrara, Anna Rita Bavaro, Vito Linsalata, Mariaelena Di Biase, Biagia Musio, Vito Gallo, Giuseppina Mulè, and Francesca Valerio. 2022. "Characterization of Dextran Produced by the Food-Related Strain Weissella cibaria C43-11 and of the Relevant Dextransucrase Gene" Foods 11, no. 18: 2819. https://doi.org/10.3390/foods11182819
APA StyleDe Bellis, P., Ferrara, M., Bavaro, A. R., Linsalata, V., Di Biase, M., Musio, B., Gallo, V., Mulè, G., & Valerio, F. (2022). Characterization of Dextran Produced by the Food-Related Strain Weissella cibaria C43-11 and of the Relevant Dextransucrase Gene. Foods, 11(18), 2819. https://doi.org/10.3390/foods11182819








