Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects
Abstract
1. Introduction
2. Methodology
3. Thymus Genera: An Overview
4. Thymus algeriensis Boiss. and Reut.
4.1. Distribution
4.2. Systematic Classification and Botanical Aspects
4.3. Uses in Folk Medecine
4.4. Phytochemistry
4.4.1. Essential oil Chemical Composition
| R/P * | PP | P/H | S | EX | NC | MC | Ref. |
|---|---|---|---|---|---|---|---|
| Algeria | |||||||
| Biskra province | AP | n.m, Apr | Eo | HDGC-MS | 34 | Camphor (37.29%); 1,8-Cineole (11.12%); Camphene (7.81%); Myrcene (7.13%); Borneol (5.54%); Limonene (3.44%); Germacrene-D (2.31%); β-Caryophyllene (2.30%) | [51] |
| El Hadjeub and El Ghicha/Laghouat | n,m | n.m, Jun | Eo | HDGC-FID, GC-MS | n.m | Carvacrol acetate (14.16%); Limonene (11.49%); α-Pinene (9.26%) | [130] |
| El-Guetfa/M’sila | AP (S, L, F) L | BFlo Flo Aflo Flo, Mar | (1) BFEo (2) Feo (3) AFEo (4) LMAD (5) LHD (6) LSD | HD, GC-MS, GC-FID HD, GC-MS, GC-FID HD, GC-MS, GC-FID HD, SD, MAD GC-FID, GC-MS | 85 | BFEo: Camphor (17.45%); Borneol (13.90%); Camphene (10.73%); Acorenone (8.03%); 1,8-Cineole (5.16%); α-Pinene (4.56%); Geranyl acetate (4.26%); α-Cadinol (4.14%); Bornyl acetate (3.86%); trans-Sabinene hydrate (2.40%) Feo: Camphor (22.60%); Camphene (12.75%); Borneol (11.16%); 1,8-Cineole (5.94%); Acorenone (5.85%); α-Pinene (5.00%); Bornyl acetate (3.86%); Geranyl acetate (2.65%); 7-epi-α-Eudesmol (2.63%) AFEo: Camphor (34.31%); Borneol (14.48%); Camphene (12.86%); 1,8-Cineole (11.21%); Bornyl acetate (4.278%); α-Pinene (2.80%) LMAD: Camphor (20.74%); Borneol (16.74%); Camphene (8.73%); 1,8-Cineole (7.01%); 5-neo-cedranol (6.03%); Bornyl acetate (5.70%); 7-epi-α-Eudesmol (4.13%); Geranyl acetate (3.78%); α-Pinene (3.03%) LHD: Camphor (32.56%); Borneol (17.13%); Camphene (14.88%); 1,8-Cineole (7.88%); Bornyl acetate (5.21%); α-Pinene (3.74%) LSD: Camphor (24.25%); Borneol (22.20%); Perilla aldehyde (13.21%); Bornyl acetate (7.92%); 1,8-Cineole (7.72%); Camphene (7.53%) | [61] |
| El Kantara area/Biskra | AP | n.m, Apr | Eo | HD GC/MS | 35 | Camphor (52.16%); Borneol (12.72%); L-α-terpineol (5.46%); Terpinen-4-ol (4.04%); Germacrene D (3.94%); Linalool (3.71%); Bornyl acetate (2.57%); Caryophyllene (2.55%) | [54] |
| Aures/Batna | AP | n.m/Jun | Eo | SD GC-MS, GC-FID | 35 | Germacrene D (29.60%); β-Caryophyllene (11.00%); -β-Farnesene (7.80%); β-Eudesmol (5.30%); δ-Cadinene (4.00%); Bicyclogermacrene (4.40%); α-Humulene (3.50%); α-Guaiene (2.30%); α-Bulnesene (2.40%); E-Nerolidol (2.40%); Phytol (2.30%) | [140] |
| Djemorah/Biskra | S, L, F, Fr | Flo, Apr/Ma | Eo | HS-SPME GC-MS | 39 | β-Myrcene (13.78%); Camphor (12.29%); Linalyl acetate (9.11%); 1,8-Cineole (6.31%); β-Farnesene (5.23%); α-Terpineol (5.07%); Camphene (4.61%); α-Pinene (4.65%); Bornyl acetate (4.79%) | [131] |
| Laghouat province | L | Flo, Apr/Ma | Eo | HD GC-MS | 29 | α-Terpinyl acetate (47.40%); Neryl acetate (9.60%); α-Pinene (6.80%); α-Terpineol (4.90%); 1,8-Cineole (4.10%); Nerolidol (3.5%); Bornyl acetate (3.1%); Limonene (2.70%) | [132] |
| National Park of Bellazma/Batna | S, L, F | Flo, Mar/Apr | Eo | SD GC-MS, GC-FID | 30 | Elemol (18.38%); Camphor (14.22%); β-Eudesmol (11.50%); α-Caryophyllene (9.68%); Borneol (6.44%); Germacrene isomer (4.55%); Caryophyllene oxide (3.51%); Bornyl acetate (2.41%) | [55] |
| Ain Beida/Oum El Bouaghi | S, F, L, Fr | Flo, n.m | Eo | HD GC-MS | 36 | Camphor (13.62%); 1,8-Cineole (6.00%); Borneol (5.74%); Viridiflorol (4.00%); Linalool (3.93%); α-Terpineol (3.80%); Caryophyllene oxide (3.50%) | [139] |
| Mekhatri/Ain-Defla | AP | n.m, Jun | Eo | HD GC-MS, GC-FID | 34 | γ-Terpinene (14.90 ± 2.80%); Cymene (14.70 ± 2.60%); Carvacrol (8.40 ± 4.20%); Thymol (5.60 ± 1.80%); β-Myrcene (2.70 ± 0.90%) | [134] |
| Selaoua Anouna/Guelma | L, F | Pflo, Mar/Apr Flo, Ma/Jun | SAPFlo SAFlo | HD GC-MS | 19 | SAPFlo: Camphor (33.30%); O-Cymene (6.36%); Isolimonene (5.50%); Eucalyptol (5.31%); Limonene (5.13%); Linalool (4.68%) SAFlo: Verbenone (13.18%); p-Cimene-7-ol (26.98%); Methyl ter buthy ether (19.63%); β-Cymene (7.74%); γ-Terpinene (5.64%); Camphor (3.64%); α-Pinene (3.08%); Pinocarveol (2.67%); Mertenyl acetate (2.59%) | [154] |
| Sidi Aissa/M’sila | AP (L, F, S) | Flo, Apr | Eo | HD GC-MS, GC-FID | 71 | Camphor (22.61%); Camphene (12.78%); Borneol (11.16%); 1,8-Cineole (5.94%); Acorenone (5.84%); α- Pinene (5.01%); Bornyl acetate (3.86%); Geranyl acetate (2.65%); 7-epi-α -Eudesmol (2.63%); | [138] |
| Chrea National Park/Blida 800 m altitude (CHR1) Chrea National Park/Blida 1500 m altitude (CH2) El-Asnam/Blida (ALAS) | AP | Flo, July | (1) CHR1Eo (2) CHR2Eo (3) ALASEo | HD GC-MS, GC-FID | 53 49 46 | CHR1Eo: Thymol (29.50%); p-Cymene (13.00%); γ-Terpinene (6.90%); α-Pinene (5.80%); β-Caryophyllene (5.00%); Caryophyllene oxide (5.00%); β-Pinene (3.70%); Linalool (3.60%); Carvacrol (3.30%) CHR2Eo: Terpinyl acetate (18.00%); (trans)-Nerolidol (12.60%); α-Pinene (11.10%); Borneol (9.00%); Bornyl acetate (7.70%); Camphene (5.90%); β-Pinene (3.20%); Limonene (2.80%); Camphor (2.30%) ALASEo: Terpinen-4-ol (10.60%); Camphor (10.10%); p-Cymene (9.90%); α-Pinene (6.50%); 1,8-Cineole (6.50%); γ-Terpinene (5.50%); Caryophyllene oxide 3.90%); trans-Verbenol (3.60%); α-Terpinene (2.80%); Camphene (2.30%); cis-Sabinene hydrate (2.30%) | [135] |
| Khedara/Soukaharas (KH) Fatoum Souda/Soukaharas (FAT) | L | Flo, Mar | (1) KHEo (2) FATEo | HD GC-MS | 54 | KHEo: α-Pinene (27.14%); Camphor (8.77%); 1,8-Cineole (7.69%); Sabinene (5.25%); δ-Cadinene (3.39%); Allo-Aromadendrene (3.12%); trans-Ocimene (2.84%); β-Pinene (2.66%); β-Caryophyllene (2.54%); Limonene (2.41%); Borneol (2.40%) FATEo: α-Pinene (25.52%); Camphor (8.45%); 1,8-Cineole (7.68%); Sabinene (5.61%); Allo-Aromadendrene (3.52%); δ-Cadinene (3.14%); β-Pinene (3.12%); Limonene (2.46%) | [53] |
| Media province | AP | Flo, Ma | Eo | HD GC-MS | 55 | Linalool (47.30%); Thymol (29.20%); p-Cymene (6.80%); β-Caryophyllene (2.90%) | [136] |
| Blida province | n.m | n.m | Eo | HD GC-MS, GC-FID | 25 | Linalool (40.20%); Thymol (33.70%); p-Cymene (5.50%); γ-Terpinene (3.20%); β-Caryophyllene (2.70%) | [137] |
| Tunisia | |||||||
| Mount Orbata/Gafsa | AP | Flo, Apr | Eo | HD GC-MS | 52 | Viridiflorol (9.72%); Cyclo-hexene, 1-(1-butenyl) (9.71%); iso-Pulegol (8.27%); α-Terpinylacetate (4.93%) Camphre (4.89%); Terpinen-4-ol (4.50%); β-Ocimene (4.11%); 6-ethenyl-6,9,9-trimethyl-4-methylidenebicyclo [5,2,0] nonane (3.12%); 1-Borneol (3.07%); β-Phellandrene (2.94%); Camphene (2.90%) | [50] |
| Mount Orbata/Gafsa | AP | n,m | Eo | HD GC-MS, FTIR | n.m | Thymol; (+)-epi-bicyclosesquiphellandrene; Ledol; Camphor; Linalool; 2-Carene; Terpinen-4-ol; Endo-borneol; Eucalyptol; α-Pinene | [153] |
| Mount Orbata/Gafsa | AP | L (Veg, Jan) L, F (Flo, Mar) L (Frui, Apr, Ma) | (1) TeoBF (2) TeoF (3) TeoAF | HD GC-MS | 32 46 43 | TeoBF: Eucalyptol (13.37%); Endo-Borneol (9.45%); α-Pinene (8.13%); Camphor (6.50%); Terpinen-4-ol (3.99%); β-Pinene (2.72%); Linalyl acetate (2.70%); Camphene (2.65%) TeoF: Eucalyptol (9.30%); 2-Carene (6.42%); Linalool (6.08%); Terpinen-4-ol (6.10%); Camphor (5.92%); Viridiflorol (4.52%); Linalool oxide (2.57%); α-Terpineol (3.41%) TeoAF: Eucalyptol (10.34%); Viridiflorol (8.69%); Camphor (8.23%); Terpinen-4-ol (6.14%); endo-Borneol (4.93%); Thymol (4.01%); α-Pinene (3.48%); β-Pinene (2.51%) | [86] |
| Korbous (KOR) Jdidi Jebel Mountain (JDID) Hammem Sousse (HAM) | L, S, R | Veg, n,m | (1) EoR (KOR, JDID, HAM) (2) EoS (KOR, JDID, HAM) (3) EoL (KOR, JDID, HAM) | HD GC-MS | 35 46 48 | EoR: Viridiflorol (tr–39.70%); Caryophyllene oxide (18.50–25.30%); α-Pinene (2.70–15.20%); 1,8-Cineole (1.20–12.80%); p-Eugenol (tr–15.80%); Geraniol (tr–7.10%); cis-α-bisabolene (tr–10.60%) EoS: Caryophyllene oxide (9.70–24.20%); Elemol (8.10–13.10%); Viridiflorol (6.40–9.00%); Camphor (2.00–10.30%); α-Pinene (5.80–8.80%); Linalyl acetate (tr–7.20%); γ-Gurjunene (tr–7.20%) EoL: α-Pinene (13.60–23.20%); 1,8-Cineole (7.40–17.80%); Caryophyllene oxide (4.30–17.80%); Camphor (4.10–14.80%); Linalool (3.20–14.50%); Camphene (2.70–5.90%); p-Eugenol (tr–14.40%). | [152] |
| Mount Orbata/Gafsa | AP | n,m | Eo | SD GC-MS | 13 | Linalool (18.05%); Camphor (13.03%); Terpinen-4-ol (11.20%); Viridiflorol (11.71%); Bornyl acetate (5.41%); 1,8-Cineole (3.45%); p-Cymene (3.22%); Spathulenol (2.80%); γ-Terpinene (2.43%); | [150] |
| Gafsa (MG) Tamerza (MT) Kairouan (MOK) | AP | Flo, Mar | (1) MGEo (2) MTEo (3) MOKEo | HD GC-MS | 25 | MGEo: Terpinen-4-ol (33.34%); 1,8-Cineole (14.12%) MTEo: Linalool (18.05%); Camphor (13.03%) MOKEo: 1,8-Cineole (19.96%); Camphor (19.20%) | [48] |
| Zannouch (ZAN) Oued Om Ali (OUE) Ayaycha (AYA) Sidi Harrath (SID) Dachra (DAC) Djebel Slata (DJE) Haydra (HAY) Kalaat Senan (KAL) | F, L | Veg, Dec Flo, Apr | ZAN (Veg, Flo) OUE (Veg, Flo) AYA (Veg, Flo) SID (Veg, Flo) DAC (Veg, Flo) DJE (Veg, Flo) HAY (Veg, Flo) KAL (Veg, Flo) | HD GC-FID, GC-MS | 63 61 58 59 58 57 49 49 44 48 48 48 43 60 39 48 | ZAN-Veg: 1,8-Cineole (10.91%); α-Pinene (10.49%); Camphor (10.23%); Borneol (4.58%); 4-Terpineol (4.36%); Camphene (3.84%); Viridiflorol (3.62%); Sabinene (3.37%); Linalool (2.95%); cis -Sabinene hydrate (2.83%); β-Pinene (2.78%); Bornyl acetate (2.32%) ZAN-Flo: 1,8-Cineole (15.79%); α-Pinene (9.68%); Camphor (9.40%); Borneol (5.19%); 4-Terpineol (4.57%); Viridiflorol (4.24%); Camphene (3.89%); Bornyl acetate (3.28%); Linalool (2.69%) OUE-Veg: cis-Sabinene hydrate (9.86%); 1,8-Cineole (7.55%); α-Pinene (7.41%); Camphor (6.80%); Viridiflorol (5.69%); 4-Terpineol (5.30%); β-Pinene (4.03%); α-Cadinol (3.58%); Borneol (3.47%); Camphene (3.22%); Sabinene (3.15%); γ-Terpinene (3.15%); Carvacrol (2.55%); γ-Cadinene (2.44%) OUE-Flo: Viridiflorol (11.49%); α-Pinene (9.80%); 1,8-Cineole (8.73%); Camphor (8.17%); Sabinene (4.40%); β-Pinene (4.29%); Camphene (3.51%); α-Cadinol (3.4%); Borneol (3.33%); 4-Terpineol (3.32%); Caryophyllene oxide (2.66%); Bornyl acetate (2.61%); cis-Sabinene hydrate (2.59%); γ-Cadinene (2.58%) AYA-Veg: cis-Sabinene hydrate (12.95%); Camphor (9.93%); 1,8-Cineole (9.00%); α-Pinene (8.97%); 4-Terpineol (8.34%); Borneol (4.09%); Camphene (3.48%); Sabinene (2.90%); β-Pinene (2.86%); γ-Terpinene (2.65%) AYA-Flo: 4-Terpineol (11.86%); Camphor (11.72%); 1,8-Cineole (10.87%); α-Pinene (5.60%); γ-Terpinene (5.42%); p-Cymene (4.2%); Borneol (4.18%); Camphene (4.16%); α-Terpinene (3.48%); Viridiflorol (3.25%); cis-Sabinene hydrate (2.79%); Bornyl acetate (2.60%) SID-Veg: 1,8-Cineole (18.02%); Camphor (12.02%); Terpinyl acetate (8.88%); Borneol (6.86%); α-Pinene (6.58%); Bornyl acetate (4.36%); Camphene (4.11%); Caryophyllene oxide (3.90%); 4-Terpineol (2.87%); Myrtenal (2.40%) SID-Flo: Terpinyl acetate (14.92%); 1.8-Cineole (13.82%); Camphor (8.16%); Bornyl acetate (7.56%); Caryophyllene oxide (5.55%) Borneol (5.40%) DAC-Veg: Camphor (19.39%); 1,8-Cineole (14.44%); α-Pinene (9.18%); Camphene (5.59%); Borneol (5.37%); Terpenyl acetate (3.22%); Myrtenal (3.16%); Caryophyllene oxide (2.96%); 4-Terpineol (2.94%); Bornyl acetate (2.88%); Campholenal (2.76%); Verbenone (2.55%) DAC-Flo: 1,8-Cineole (14.73%); Camphor (14.37%); α-Pinene (13.25%); Borneol (4.69%); Camphene (4.01%); β-Pinene (3.40%); β-Eudesmol (2.67%); Caryophyllene oxide (2.30%) DJE-Veg: Camphor (19.93%); 1,8-Cineole (17.90%); α-Pinene (11.74%); Borneol (6.21%); Camphene (6.06%); Caryophyllene oxide (3.32%); β-Pinene (2.72%); 4-Terpineol (2.70%); Myrtenal (2.62%) DJE-Flo: 1,8-Cineole (18.46%); Camphor (15.69%); α-Pinene (10.34%); Borneol (6.14%); Camphene (5.43%); Caryophyllene oxide (3.87%); β-Pinene (3.00%); 4-Terpineol (2.38%); Myrtenal (2.30%) HAY-Veg: 1,8-Cineole (22.07%); Camphor (17.49%); α-Pinene (13.44%); Camphene (5.58%); Borneol (5.04%); β-Pinene (2.37%); 4-Terpineol (2.36%) HAY-Flo: Camphor (13.64%); 1,8-Cineole (12.45%); 4-Terpineol (8.56%); α-Pinene (6.38%); Borneol (4.60%); Camphene (4.38%); p-Cymene (3.68%); γ-Terpinene (3.63%); Bornyl acetate (3.00%) KAL-Veg: 1,8-Cineole (20.48%); Camphor (18.59%); α-Pinene (13.94%); Camphene (6.35%); Borneol (5.94%); Caryophyllene oxide (2.72%); Myrtenal (2.69%); Pinocarveol (2.42%); β-Pinene (2.41%); Verbenone (2.39%) KAL-Flo: 1,8-Cineole (15.36%); Camphor (14.00%); α-Pinene (12.40%); Borneol (4.98%); Camphene (4.94%); Caryophyllene oxide (4.42%); β-Pinene (3.22%); β-Eudesmol (2.65%); Linalool (2.42%); Myrtenal (2.42%) | [143] |
| Sabbah Jebel Mountain (SJM) Bahra (BAH) Mansour Jebel Mountain (MJM) Chaambi Jebel Mountain (CHJM) Chrechira Jebel Mountain (CJM) Toujene Matmata (TME) Ouled Bou Saad (OBS) Douaou Jebel Mountain (DJM) | AP | n.m | (1) SJMEo (2) BAHEo (3) MJMEo (4) CHJMEo (5) CJMEo (6) TMEo (7) OBSEo (8) DJMEo | HD GC-MS | 25 30 32 38 35 18 32 32 | SJMEo: Caryophyllene oxide (18.80%); 1,8-Cineole (15.80%); α-Pinene (14.30%); Camphor (9.20%); allo-Aromadendrene (5.40%); α-Humulene (4.10%) BAHEo: 1.8-Cineole (23.40%); α-Pinene (14.30%); Camphor (9.10%); Allo-Aromadendrene (4.50%); γ-Terpinene (4.80%); α-Humulene (4.20%); Camphene (3.30%); Linalool (2.60%) MJMEo: 1,8-Cineole (20.90%); α-Pinene (11.30%); Camphor (7.40%); Methyl eugenol (6.90%); Linalyl acetate (6.40%); allo-Aromadendrene (5.60%); α-Humulene (5.50%); Camphene (3.90%); β-Pinene (2.50%) CHJMEo: 1,8-Cineole (24.10%); α-Pinene (16.90%); Camphor (10.60%); Linalyl acetate (6.40%); Borneol (5.00%); allo-Aromadendrene (3.9%); Camphene (3.40%); γ-Terpinene (2.50%); Linalool (2.40%); α-Humulene (2.40%); α-Phellandrene (2.30%) CJMEo: 1,8-Cineole (24.10%); α-Pinene (18.40%); Camphor (12.70%); Methyl eugenol (2.50%); Linalyl acetate (6.40%); Borneol (5.00%); allo-Aromadendrene (4.20%); Camphene (5.60%); γ-Terpinene (2.90%); β-Pinene (2.40%); α-Humulene (3.20%); α-Phellandrene (2.30%) TMEo: Thymol (54.90%); p-Cymene (6.60%); Germacrene B (6.10%); γ-Terpinene (6.23%); 1,8-Cineole (4.30%); α-Humulene (3.50%); β-Caryophyllene (3.10%); α-Pinene (2.40%) OBSEo: Linalool (22.40%); 1,8-Cineole (10.10%); α-Pinene (9.30%); α-Copaene (7.60%); γ-Terpinene (6.50%); Camphor (6.00%); Viridiflorol (5.50%) DJMEo: α-Pinene (21.50%); 1,8-Cineole (21.20%); Camphor (9.20%); Camphene (4.80%); Viridiflorol (3.40%); α-Gurjunene (3.30%); β-Pinene (3.2%); Borneol (3%); allo-Aromadendrene (3.10%); Sabinene (2.70%); γ-Terpinene (2.70%); α-Humulene (2.40%) | [85] |
| Ayaycha mountain/Gafsa | AP | Flo, Apr | Eo | HD GC-MS, FID | 57 | Camphor (7.82%); 4-Terpineol (7.36%); 1,8-Cineole (5.54%); cis-Sabinene hydrate (5.29%); Viridiflorol (3.94%); Linalool (3.65%); γ-Terpinene (3.50%); Borneol (3.49%), Camphene (2.88%); p-Cymene (2.57%); Sabinene (2.49%); α-Terpinene (2.46%); trans-β-Ocimene (2.40%) | [49] |
| 3end/Gafsa | AP | n.m, Ma | Eo | HD GC-MS; FID | 39 | Linalool (17.62%); Camphor (13.82%); Terpinen-4-ol (6.80%); α-Terpineol (6.41%); α-Terpenyl acetate (6.27%); Borneol (5.71%); Linalyl acetate (4.63%); Sabinene hydrate (4.15%); 1,8-Cineole (4.12%); Epiglobulol (3.98%); o-Cymene (3.44%); Bornyl acetate (2.61%) | [151] |
| Morocco | |||||||
| Al Hoceima province | S, L | Flo, Mar/Apr | Eo | HD GC-MS | 18 | Thymol (33.24%); γ-Terpinene (25.23%); p-Cymene (13.89%); Carvacrol (7.96%); (+)−4-Carene (4.50%); α-Caryophyllene (3.66%); β-Myrcene (2.53%); Linalool (2.41%) | [155] |
| Imizar-Azilal region | S, L, F | Flo, Mar | Eo | HD GC-MS; FID | 21 | Thymol (46.03%); Borneol (20.38%); Carvacrol (5.86%); δ-3-Carene (3.10%); β-Ocimene (E) (2.80%); 1,8-Cineole (2.63%); α-Terpinene (2.30%) | [63] |
| Al Hoceima National Park | S, L, F | Flo, Jun | Eo | HD GC-MS | 10 | Geranyl acetate (80.00%); Geraniol (7.30%); β-Caryophyllene (2.40%) | [156] |
| Oujda | AP | Flo, Mar | Eo | HD GC-MS | 41 | Borneol (18.30%); Camphene (11.80%); Camphor (10.00%); Geranyl acetate (6.90%); Myrcene (8.60%); α-Pinene (6.00%); 1,8-Cineole (4.90%); β-Pinene (3.00%); Limonene (3.10%); p-Cymene (2.50%) | [157] |
| Rchida, | S, L, F | Flo, Apr | Eo | HD GC-MS | 48 | Camphor (27.7%); α-Pinene (20.5%); α-thujene (9.64%); β-Pinene (8.02%); 1,8-Cineole (7.69%); Limonene (4.85%); Sabinene (3.84%) | [52] |
| Mergchoum Mountain (Taourirt City) | AP | NI | Eo | HD GC-MS | 65 | Borneol (23.48%); Linalool (8.99%); Camphene (6.90%); Carvacrol (7.76%). β-Caryophyllene (6.39%) | [158] |
| Imizar- Azilal (IAZ) Ait AatabAzilal (AAZ) | L, F | Flo, Jun | (1) IAZEo (2) AAZEo | HD GC-MS | 18 10 | IAZEo: Carvacrol (80.40%); p-Cymene (4.98%); Thymol (3.39%) AZZEo: Carvacrol (49.33%); p-Cymene (2.61%) | [56] |
4.4.2. Phenolic Compounds
- Phenolic acids
| * R/P | PP | SA | EX | TA | NC | MC | Ref. |
|---|---|---|---|---|---|---|---|
| Algeria | |||||||
| Chelia mountain/Batna | AP | CH n-Bu | Mac: 1200 g in MEOH–H2O (80:20) followed by LLEx (CHCl3; n-BuOH) | ESI-MS; NMR | 10 | CH and n-Bu: Salvigenin; Cirsimaritin; Santin; Apigenin; Vanillic acid; p-hydroxybenzoic acid; Gallic acid; Rosmarinic acid; Oleanolic acid; β-sitosterol. | [58] |
| Bellezma National Park/Batna | S, L | MEH | Mac: 2.5 g/25 mL MEOH–H2O (80:20) RT | HPLC/UV | 15 | 3-hydroxy-4-methoxycinnamic acid (1.5%); Ferulic acid (0.1%); Anisic acid (26.4%); Salicylic acid (0.2%); Syringic acid (1%); Trans-2,3-dimethoxycinnamic acid (0.9%); Trans-cinnamic acid (5.4%); Vanillic acid (0.2%); Catechin (0.5%); Epicatechin (0.1%); Europetin (6.1%); Kaempferol (1.5%); Myricetin (0.2%); Quercetin (17.3%); Rutin (0.2%) | [55] |
| Taglait/Bordj Bou Arreridj | AP | MEH | (1) 1st Mac: 100 g Pow in 1 L MEOH-H2O (85:15, v/v) for 24 h and 2nd Mac: MEOH-H2O (50:50, v/v) for 24 h (2) Purification: the extract was suspended in water/acetic acid (97.5:2.5, v/v) at a ratio of 1:5 (w/v) and centrifuged at 20,000 × g, followed by solid-phase extraction | UHPLC-DAD-ESI-MSn | 23 | Apigenin di-C-hexoside; Apigenin di-O-hexuronide; Apigenin-O-hexuronide; Caffeoyl rosmarinic acid (isomer 1); Caffeoyl rosmarinic acid (isomer 2); Eriodictyol-O-hexoside (isomer 1); Eriodictyol-O-hexoside (isomer 2); Kaempferol-O-hexuronide (isomer 2); Kaempferol-O-hexuronide (isomer 1); Luteolin di-O-glucuronide; Luteolin-O-hexoside; Luteolin-O-hexuronide; Naringenin-O-hexoside; Quercetin-O-hexoside; Rosmarinic acid; Rosmarinic acid hexoside; Sagerinic acid; Salvianolic acid B; Salvianolic acid E isomer; Salvianolic acid K isomer; Yunnaneic acid E. | [188] |
| Ain Demin/Ain Defla | L | MEH | Mac: 250 g/3 L MEOH–H2O (80:20) RT | HPLC-PDA-ESI-MS/MS. | 35 | 12-Hydroxyjasmonic acid 12-O-hexoside; Apigenin 6,8-di-C-hexosides; Caffeic acid glucoside; Caffeoyl ethylrosmarinate; Carnosol; Taxifolin; Eriodictyol glucoside; Eriodictyol; Feruloyl ethylrosmarinate; Gallocatechin; Genkwanin; Isorhamnetin pentosyl; glucuronide; Luteolin feruloyl glucuronide; Luteolin feruloyl glucuronide; Luteolin glucoside; Luteolin glucuronide; Luteolin pentoside; Luteolin pentosyl-glucoside; Malic acid; Naringenin; Phloretic acid; Phloretic acid caffeoyl 3-hydroxy-3-methylglutaroyl; Quinic acid; Rosmarinic acid glucoside; Rosmarinic acid; Salvianolic Acid A; Salvianolic acid K; Schizotenuin F; Xanthomicrol. | [209] |
| Laghouat province | L | ET | Mac: 15 g/100 mL 100% ETOH; Wb 55 °C/6 h | HPLC | 15 | 2,5-dihydroxybenzoic acid (778.76 µg/g); 3,4-dihydroxybenzoic acid (1.42 µg/g); 4-hydroxybenzoic acid (10.03 µg/g); Caffeic acid (33.3 µg/g); Chlorogenic acid (22.68 µg/g); Cinnamic acid (20.51 µg/g); Ellagic (374.58 µg/g); Epicatechin (824.79 µg/g); Ferulic acid (34.30 µg/g); Gallic acid (10.49 µg/g); Naringin (120.67 µg/g); p-coumaric acid (83.80 µg/g); Quercetin (2.84 µg/g); Rutin (280.39 µg/g); Vanillic acid (182.67 µg/g). | [132] |
| n.m | AP | n-Bu | n.m | HPLC-TOF/MS | 22 | 4-Hydroxybenzoic acid (326.67 ng/mL); Apigenin (69.96 ng/mL); Baicalin (608.37 ng/mL); Caffeic acid (52.79 ng/mL); Catechin (tr); Chlorogenic acid (71.09 ng/mL); Diosmin (750.94 ng/mL); Fumaric acid (191.39 ng/mL); Gentisic acid (94.91 ng/mL); Hesperidin (627.14); Morin (52.35 ng/mL); Naringin (328.31 ng/mL); Neohesperidin (406.48 ng/mL); Polydatin (tr); Protocatechuic acid (77.80 ng/mL); Quercetin-3-β-D-glucoside (30.81 ng/mL); Rutin (11.77 ng/mL); Salicylic acid (96.57 ng/mL); Scutellarin (2725.67 ng/mL); Syringic acid (89.56 ng/mL); Vanillic acid (50.08 ng/mL). | [203] |
| Tebessa province | AP | INF DEC ETH | INF: 1 g/H2O (1:100 m/v); 100 °C; 5 mn RT Deco: 1 g/100 mL H2O; Boilling 5 mn Mac: 1 g/30 mL ETOH -H2O (80:20 v/v); RT; 150 rpm/1 h | LC-DAD-ESI/MS | 70 | Apigenin-6,8-C-dihexoside: INF (20.70 ± 0.10 mg/g); DEC (18.80 ± 0.10 mg/g); ETH (10.0 ± 0.50 mg/g) Apigenin-7-O-glucuronide: INF (12.60 ± 0.50 mg/g); DEC (11.60 ± 0.40 mg/g); ETH (5.75 ± 0.03 mg/g) Apigenin-8-C-glucoside: INF (7.60 ± 0.20 mg/g); DEC (6.80 ± 0.10 mg/g); ETH (3.99 ± 0.02 mg/g) Erydictiol-O-hexoside isomer: INF (tr); DEC (tr); ETH (tr) Kaempferol-O-glucuronide: INF (65.0 ± 0.40 mg/g); DEC (62.20 ± 0.90 mg/g); ETH (16.7 ± 0.20 mg/g) Lithospermic acid A isomer I: INF (12.90 ± 0.20 mg/g); DEC (12.10 ± 0.10 mg/g); ETH (4.54 ± 0.02 mg/g) Lithospermic acid A isomer II: INF (15.80 ± 0.20 mg/g); DEC (16.30 ± 0.50 mg/g); ETH (8.00 ± 0.30 mg/g) Luteolin-7-O-glucuronide: INF (8.90 ± 0.10 mg/g); DEC (7.80 ± 0.30 mg/g); ETH (3.06 ± 0.04 mg/g) Naringenin-O-hexoside: INF (tr); DEC (tr); ETH (tr) Quercetin-3-O-glucoside: INF (4.60 ± 0.20 mg/g); DEC (4.30 ± 0.20 mg/g); ETH (1.73 ± 0.01 mg/g) Quercetin-3-O-glucuronide: INF (4.59 ± 0.01 mg/g); DEC (4.40 ± 0.10 mg/g); ETH (1.44 ± 0.02 mg/g) Quercetin-O-malonyhexoside: INF (3.44 ± 0.02 mg/g); DEC (3.23 ± 0.04 mg/g); ETH (1.20 ± 0.02 mg/g) Rosmarinic acid hexoside: INF (6.60 ± 0.10 mg/g); DEC (7.06 ± 0.05 mg/g); ETH (2.80 ± 0.10 mg/g) Rosmarinic acid: INF (58.20 ± 0.30 mg/g); DEC (54.40 ± 0.90 mg/g); ETH (29.70 ± 0.70 mg/g) Salvianolic acid K: INF (27.20 ± 0.10 mg/g); DEC (28.60 ± 0.40 mg/g) ETH (13.30 ± 0.30 mg/g) Salvianolic acid B: INF (7.70 ± 0.20 mg/g0); DEC (7.10 ± 0.30 mg/g); ETH (n.d) | [183] |
| M’Sila province | L; F | EAE, CH, n-Bu CH fractions (F1-F31) n-Bu fractions (F1-F23) SFE/MAE ext | Mac: ETOH–H2O (70:30 v/v) (15 L) 24 h followed by LLEx SFE, MAE | HPLC-PDA | 21 | F16 (CH): Catechin (1.12 ± 0.01 µg/g); Vanillic acid (5.17 ± 0.11 µg/g); Rutin (0.57 ± 0.02 µg/g); t-Ferulic acid (0.20 ± 0.01 µg/g); 2;3-Dimethoxybenzoic acid (6.51 ± 0.59 µg/g); Naringenin (8.97 ± 0.74 µg/g); Carvacrol (0.43 ± 0.01 µg/g) F24 (CH): Vanillic acid (0.23 ± 0.01 µg/g); t-Ferulic acid (0.23 ± 0.01 µg/g); Naringin (0.16 ± 0.01 µg/g); Benzoic acid (10.92 ± 1.21 µg/g); Naringenin (0.90 ± 0.03 µg/g) F30 (CH): Epicatechin (6.78 ± 0.12 µg/g) F13 (EAext): Epicatechin (0.55 ± 0.01 µg/g); p-Coumaric acid (1.26 ± 0.81 µg/g); Naringin (4.02 ± 0.39 µg/g); Benzoic acid (5.71 ± 0.47 µg/g) F22 (EAE): 4-Hydroxybenzoic acid (16.31 ± 0.91) µg/g; Vanillic acid (0.22 ± 0.01 µg/g); p-Coumaric acid (40.62 ± 3.01 µg/g); t-Ferulic acid (1.46 ± 0.13 µg/g); Naringin (0.46 ± 0.01 µg/g); 2;3-Dimethoxybenzoic acid (7.51 ± 0.47 µg/g) F27 (EAE): Catechin (6.23 ± 0.05 µg/g); 4-Hydroxybenzoic acid (3.61 ± 0.30 µg/g); p-Coumaric acid (1.63 ± 0.88 µg/g); t-Ferulic acid (0.84 ± 0.01 µg/g); o-Coumaric acid (1.03 ± 0.09 µg/g) n-Bu: 4-Hydroxybenzoic acid (0.66 ± 0.02 µg/g); Epicatechin (48.03 ± 2.98 µg/g); Syringic acid (1.93 ± 0.11 µg/g); p-Coumaric acid (1.70 ± 0.58 µg/g); Rutin (4.52 ± 0.41 µg/g); t-Ferulic acid (0.55 ± 0.01 µg/g); 2;3-Dimethoxybenzoic acid (3.52 ± 0.20 µg/g); o-Coumaric acid (9.83 ± 0.87 µg/g); Naringenin (0.47 ± 0.01 µg/g) MAE ext: Gallic acid (37.97 ± 0.25 µg/g); Catechin (359.80 ± 1.98 µg/g); Chlorogenic acid (1745.98 ± 5.65 µg/g); Vanillic acid (23.92 ± 0.66 µg/g); Epicatechin (2462.75 ± 2.00 µg/g); Syringic acid (615.20 ± 4.03 µg/g); 3-Hydroxybenzoic acid (166.73 ± 1.02 µg/g); Isovanillin (40.42 ± 0.78 µg/g); p-Coumaric acid (106.99 ± 0.77 µg/g); Rutin (196.89 ± 1.00 µg/g); Sinapinic acid (46.20 ± 0.63 µg/g); t-Ferulic acid (140.64 ± 0.73 µg/g); Naringin (376.60 ± 2.77 µg/g); Benzoic acid (4157.75 ± 4.67 µg/g); o-Coumaric acid (341.55 ± 1.17 µg/g); Quercetin (180.72 ± 0.77 µg/g) | [184] |
| Jijel province | AP | MEH n-Bu | 85 g in MEOH-H2O (70:30 v/v); extraction with solvents with increasing polarities (EA; n-BuOH) | UV-visible; NMR | 3 | 5-hydroxy-6,7,3′,4′-tetramethoxyflavone (5-desmethylsinensetin); Quercetin-3-O-rutinoside; Luteolin-7-O-rhamnoside | [88] |
| Morocco | |||||||
| Oujda province | AP | AQ | Deco: 50 g/1 L water; 15 mn | HPLC | 7 | Apigenin; Cinnamic acid; Coumaric acid; Luteolin; Quercetin; Rutin; Syringic acid | [59] |
| Ta1: Imizar- Azilal/high Atlas of Morocco Ta2: Ait AatabAzilal/high Atlas of Morocco | L, F | EA ET PEE | n.m | GC-MS | 18 13 18 20 12 24 | Ta1 (EA): Carvacrol (72.69%); p-Cymene (6.82%); γ-Terpinene (3.24%); Bornyl acetate (2.02%); Thymol (2.02%) Ta2 (EA): p-Cymene (7.53%); Thymol (1.06%) Ta1 (EtOHext): Carvacrol (76.03%); Thymol (3.36%); Camphene (1.21%) Ta2 (EtOHext): Carvacrol (69.54%); trans-Caryophyllene (1.65%); Carvacrol methyl ether (1.48%); Borneol (1.46%); Thymol (1.39%) Ta1 (PEext): Carvacrol (69.09%); Thymol (2.54%); trans-Caryophyllene (1.16%) Ta2 (PEext): Carvacrol (48.76%); Camphene (5.78%); 1-Octen-3-ol (2.54%); p-Cymene (2.19%); β-Linalool (2.15%); γ-Terpinene (1.92%); Epoxylinalol (1.59%); Borneol (1.38%); 4-Isopropyl-1M-2cyclohexane-1-ol (1.20%); Thymol (1.08%) | [56] |
| Tunisia | |||||||
| Orbata Gafsa Mount | AP | AQ | Deco: 250 g/2 L H2O, 4 h | UHPLC-HRMS/MS | 18 | 12-hydroxyjasmonic acid; 12-hydroxyjasmonic acid sulphate; Apigenin diglucuronide; Apigenin glucoside glucuronide; Apigenin-7-O-β-glucuronide; Citric acid; Luteolin; Luteolin glucoside derivative; Luteolin glucuronide derivative; Luteolin-7-O-β-glucuronide; Quinic acid; Rosmarinic acid; Scutellarin; Succinic acid; Trihydroxyoctadecedienoic acid isomer; Trihydroxyoctadecenoic acid; Vicenin-2 | [50] |
| Orbata Gafsa Mount | AP | ME | Mac: in MEOH 24 h | HPLC | 9 | Caffeic acid (26.00 ± 14.00 μg/g); Catechin (16.00 ± 5.00 μg/g); Cinnamic acid (0.00 ± 0.00 μg/g); Coumaric acid (124.00 ± 11.00 μg/g); Epicatechin (136.00 ± 11.00 μg/g); Ferulic acid (42.00 ± 6.00 μg/g); Flavone (0.00 ± 0.00 μg/g); Gallic acid (745.00 ± 12.00 μg/g); Quercetin (126.00 ± 16.00 μg/g); Rutin (89.00 ± 3.00 μg/g); Vanillic acid (615.00 ± 4.00 μg/g) | [57] |
| Korbous (Ta1) Essabahia (Ta2) Dj Mansour (Ta3) Jendouba (Ta4) Dj chahid (Ta5) Makther (Ta6) Kesra (Ta7) Siliana (Ta8) Sers (Ta9) Sousse (Ta10) Toujene (Ta11) Matmata (Ta12) | L | ME | Mac: 1 g/10 mL MEOH, 24 h | UHPLC-DAD-ESI/MSn | 18 | Apigenin-di-C-hexoside; Apigenin-O-hexuronide; Caffeoyl rosmarinic acid; Cirsimaritin; Eriodictyol; Eriodictyol-O-hexoside; Kaempferol-O-hexoside; Kaempferol-O-hexuronide; Luteolin-O-hexuronide; Monomethyl lithospermate; Naringenin; Rosmarinic acid; Salvianolic acid E; Salvianolic acid K; Scutellarein-O-hexoside-hexuronide; Tetramethyl-scutellarein | [62] |
| Phenolic acids Rosmarinic acid: Ta1 (531.30 ± 0.50 µg/mL); Ta2 (383.80 ± 0.50 µg/mL); Ta3 (410.40 ± 0.70 µg/mL); Ta4 (1157.80 ± 2.70 µg/mL); Ta5 (593.60 ± 2.10 µg/mL); Ta6 (410.90 ± 0.70 µg/mL); Ta7 (756.30 ± 0.70 µg/mL); Ta8 (391.30 ± 0.50 µg/mL); Ta9 (596.40 ± 0.30 µg/mL); Ta10 (1083.20 ± 3.50 µg/mL); Ta11 (957.00 ± 1.00); Ta12 (807.20 ± 3.00 µg/mL) Caffeoyl rosmarinic acid: Ta1 (39.20 ± 0.10 µg/mL); Ta2 (64.90 ± 0.10 µg/mL); Ta3 (78.90 ± 0.10 µg/mL); Ta4 (85.50 ± 0.10 µg/mL); Ta5 (73.90 ± 0.20 µg/mL); Ta6 (45.80 ± 0.00 µg/mL); Ta7 (232.20 ± 0.20 µg/mL); Ta8 (74.30 ± 0.10 µg/mL); Ta9 (101.60 ± 0.11 µg/mL); Ta10 (86.10 ± 0.30 µg/mL); Ta11 (206.60 ± 1.10 µg/mL); Ta12 (183.00 ± 0.50 µg/mL) Flavanones Eriodictyol hexoside: Ta2 (6.30 ± 0.10 µg/mL); Ta3 (31.90 ± 0.10 µg/mL); Ta4 (40.00 ± 0.10 µg/mL); Ta5 (39.10 ± 0.10 µg/mL); Ta 6 (3.50 ± 0.20 µg/mL); Ta7(5.70 ± 0.10 µg/mL); Ta8 (28.70 ± 0.10 µg/mL); Ta9 (52.80 ± 0.10 µg/mL); Ta11 (9.10 ± 0.20 µg/mL); Ta12 (6.00 ± 1.10 µg/mL) Eriodictyol: Ta1 (4.10 ± 0.10 µg/mL); Ta2 (16.90 ± 0.10 µg/mL); Ta3 (4.40 ± 0.30 µg/mL); Ta4 (12.40 ± 0.70 µg/mL); Ta5 (4.40 ± 0.80 µg/mL); Ta6 (8.20 ± 0.10 µg/mL); Ta7 (11.40 ± 0.10 µg/mL); Ta8 (1.10 ± 0.10 µg/mL); Ta9 (5.50 ± 0.20 µg/mL); Ta10 (9.90 ± 0.40 µg/mL); Ta11 (14.70 ± 0.10 µg/mL); Ta12 (42.00 ± 0.10 µg/mL) Kaempferol-O-hexoside: Ta2 (83.90 ± 0.30 µg/mL); Ta3 (228.10 ± 0.10 µg/mL); Ta4 (326.30 ± 0.20 µg/mL); Ta5 (360.40 ± 0.50 µg/mL); Ta7(118.40 ± 0.20 µg/mL); Ta8 (95.30 ± 0.10 µg/mL); Ta9 (439.60 ± 0.30 µg/mL); Ta10 (10.0 ± 0.20 µg/mL); Ta12 (108.40 ± 0.10 µg/mL) Kaempferol-O-hexuronide: Ta1 (256.30 ± 0.30 µg/mL); Ta2 (363.20 ± 1.90 µg/mL); Ta3 (202.90 ± 1.70 µg/mL); Ta4 (552.00 ± 0.60 µg/mL); Ta5 (213.20 ± 12.70 µg/mL); Ta6 (216.50 ± 0.70 µg/mL); Ta7 (526.40 ± 0.50 µg/mL); Ta8 (225.20 ± 0.40 µg/mL); Ta9 (446.60 ± 9.40 µg/mL); Ta10 (297.10 ± 0.30 µg/mL); Ta11 (862.80 ± 1.20 µg/mL); Ta12 (655.70 ± 2.60 µg/mL) Flavones Luteolin-O-hexuronide: Ta4 (25.30 ± 0.10 µg/mL); Ta5 (20.6 ± 0.10 µg/mL); Ta7 (12.70 ± 0.10 µg/mL); Ta8 (3.00 ± 0.10 µg/mL); Ta9 (27.90 ± 0.10 µg/mL); Ta10 (5.70 ± 0.10 µg/mL) Apigenin-C-di-hexoside: Ta1 (18.40 ± 2.70 µg/mL); Ta2 (10.70 ± 0.10 µg/mL); Ta3 (21.70 ± 0.10 µg/mL); Ta4 (54.10 ± 0.10 µg/mL); Ta5 (53.30 ± 0.10 µg/mL); Ta6 (10.40 ± 0.10 µg/mL); Ta7 (55.30 ± 0.30 µg/mL); Ta8 (53.40 ± 0.10 µg/mL); Ta9 (62.60 ± 0.10 µg/mL); Ta10 (37.70 ± 0.20 µg/mL); Ta11 (40.20 ± 0.10 µg/mL); Ta12 (54.20 ± 0.10 µg/mL) Apigenin-O-hexuronide: Ta1 (112.80 ± 0.10 µg/mL); Ta2 (6.80 ± 0.10 µg/mL); Ta3 (3.80 ± 0.10 µg/mL); Ta4 (6.10 ± 0.10 µg/mL); Ta5 (3.50 ± 0.10 µg/mL); Ta6 (9.80 ± 0.10 µg/mL); Ta7 (3.80 ± 0.10 µg/mL); Ta8 (6.10 ± 0.10 µg/mL); Ta9 (3.10 ± 0.10 µg/mL); Ta10 (1.40 ± 0.10 µg/mL) Phenolic terpene Carvacrol: Ta11 (2221.60 ± 2.50 µg/mL); Ta12 (1374.70 ± 5.00 µg/mL) | |||||||
| Gafsa (S1) Tamerza (S2) Kairouan (S3) | AP | ME (S1, S2, S3) | Mac: 9 g powdered plant MEOH, 8 h (Soxhlet apparatus) | HPLC | 12 | S1: Hydroxyphenylacetic acid (914.26 ± 3.42 μg/g); Gallic acid (723.19 ± 4.10 μg/g); Syringic acid (119.31 ± 4.20 μg/g); Ferulic acid (250.18 ± 3.20 μg/g); Vanillic acid (1189.39 ± 973.30 μg/g); Tyrosin (5013.06 ± 934.10 μg/g); Flavone (128.6 ± 0.40 μg/g); Vanillin (1079.26 ± 57.10 μg/g); (+)-Catechin hydrate (18.01 ± 0.22 μg/g); Rutin (609.62 ± 0.60 μg/g) S2: Hydroxyphenylacetic acid (2053.42 ± 532.20 μg/g); Gallic acid (744.72 ± 12.10 μg/g); Syringic acid (148.45 ± 33.30 μg/g); Ferulic acid (41.64 ± 6.20 μg/g); Methyl gallate (229.84 ± 99.20 μg/g); Vanillic acid (614.72 ± 41.20 μg/g); Tyrosin (59.48 ± 3.90 μg/g); Flavone (65.65 ± 9.60 μg/g); Vanillin (126.08 ± 5.80 μg/g); (+)- Catechin hydrate (4.9 ± 0.70 μg/g); Rutin (88.54 ± 2.80 μg/g) S3: Gallic acid (2780.57 ± 492.10 μg/g); Ferulic acid (4657.94 ± 840.10 μg/g); Flavone (5512.01 ± 372.20 μg/g) | [48] |
- Flavonoids
- Other compounds
4.5. Pharmacological Reports
4.5.1. Antibacterial Effects
| SA | TA | Bacteria Strains | Results | Ref. | ||
|---|---|---|---|---|---|---|
| IZD (mm) | MIC | MBC/MFC | ||||
| Algeria | ||||||
| L/Eo | DDM | Escherichia coli ATCC10536 Micrococcus luteus Staphylococcus aureus CIP7625 Candida albicans IPA200 C. tropicalis C. glabrata Saccharomyces cerevisiae ATCC4226 | 13.0 ± 0.90 18.0 ± 0.60 18.0 ± 0.70 13.0 ± 0.40 2.04 ± 0.80 18.0 ± 0.60 17.0 ± 0.50 | n.m n.m n.m n.m n.m n.m n.m | n.m n.m n.m n.m n.m n.m n.m | [61] |
| AP/Eo | DDM MBS | E. coli SB3 (ESBL) Enterobacter xiangfangensis SB2 Hafnia paralvei SB1 Klebsiella pneumoniae SB4 (ESBL) K. pneumoniae SB5 (ESBL) K. pneumoniae SB6 (ESBL) | 13.54 ± 1.30 13.27 ± 0.12 16.11 ± 1.00 10.26 ± 0.17 12.39 ± 1.46 16.22 ± 2.46 | 12.50 mg/mL 12.50 mg/mL 6.25 mg/mL 12.50 mg/mL 3.12 mg/mL 1.56 mg/mL | 25.00 mg/mL 25.00 mg/mL 25.00 mg/mL 25.00 mg/mL 25.00 mg/mL 12.50 mg/mL | [54] |
| AP/PEE CH n-Bu | DDM | Enterococcus faecalis ATCC29212 E. coli ATCC25922 Pseudomonas aeruginosa DMS1117 S. aureus ATCC29213 | PEE (12.00 ± 0.30); CHCl3ext (n.i); n-Bu (15.0 ± 0.10) PEE (9.50 ± 0.30); CH (8.00 ± 0.30); n-Bu (8.00 ± 0.30) n.i n.i | PEE (12.50); CH (12.50); n-Bu (6,25) µg/mL PEE (25.00); CH (25.00); n-Bu (25.00) µg/mL n.i n.i | n.m n.m n.m n.m | [58] |
| AP/ME PS | DDM | Bacillus cereus ATCC10876 E. coli ATCC25922 M. luteus NRLL B-4375 Proteus mirabilis ATCC35659 Salmonella typhimurium ATCC13311 | n.m n.m n.m n.m n.m | MEH (n.i); PS (2.34 ± 0.00) mg/mL MEH (n.i); PS (9.37 ± 0.00) mg/mL MEH (n.i); PS (7.03 ± 3.30) mg/mL MEH (37.50 ± 0.00); PS (4.68 ± 0.00) mg/mL MEH (n.i); PS (7.06 ± 3.27) mg/mL | MeH2Oext (n.i); PS (18.75 mg/mL) MeH2Oext (n.i); PS (9.37 mg/mL) MeH2Oext (n.i); PS (9.38 mg/mL) MeH2Oext (>37.5); PS (4.68 mg/mL) MeH2Oext (n.i); PS (9.38 mg/mL) | [188] |
| L/Eo ET | MDM | B. subtilis ATCC11562 E. coli ATCC29425 K. pneumoniae ATCC43816 P. aeruginosa ATCC15442 S. aureus ATCC25923 S. epidermidis ATCC12228 C. albicans ATCC10231 C. glabrata ATCC22553 | n.m n.m n.m n.m n.m n.m n.m n.m | ET (64.00); Eo (32.00) µg/mL ET (256.00); Eo (64.00) µg/mL ET (256.00); Eo (256.00) µg/mL ET (512.00); Eo (512.00) µg/mL ET (64.00); Eo (32.00) µg/mL ET (128.00); Eo (32.00) µg/mL ET (128.00); Eo (64.00) µg/mL ET (128.00); Eo (32.00) µg/mL | n.m n.m n.m n.m n.m n.m n.m n.m | [132] |
| AP/ET ME | DDM | E. cloacae ATCC49452 E. faecalis ATCC49452 E. coli ATCC25922 K. pneumonia ATCC4352 P. aeruginosa ATCC27853 S. typhimurium ATCC13311 S. aureus ATCC25923 | ET (7.00); ME (n.i) ET (12.50); ME (17.00) ET (13.00); ME (10.00) n.i ET (16.50); ME (14.00) ET (9.00); ME (12.00) ET (19.00); ME (15.50) | ET (n.i); ME (160.00) µg/mL ET (105.00); ME (80.00) µg/mL ET (270.00); ME (220.00) µg/mL n.i ET (150.00); ME (185.00) µg/mL ET (130.00); ME (110.00) µg/mL ET (165.00); ME (40.00) µg/mL | n.m n.m n.m n.m n.m n.m n.m | [189] |
| L, F/Eo1 Eo2 | DDM | Acinetobacter spp E. faecalis ATCC29212 E. coli ATCC25922 P. aeruginosa ATCC27853 Salmonella spp. S. aureus ATCC43300 | Eo1 (10.12 ± 0.11); Eo2 (12.41 ± 0.08) Eo1 (9.48 ± 0.81); Eo2 (12.24 ± 0.20) Eo1 (15.14 ± 3.25); Eo2 (12.72 ± 0.59) Eo1 (6.00 ± 0.00); Eo2 (6.77 ± 0.25) Eo1 (10.29 ± 0.46); Eo2 (12.31 ± 1.20) Eo1(19.46 ± 3.22); Eo2 (33.28 ± 0.74) | Eo1 (0.05%); Eo2 (0.10%) Eo1 (0.05%); Eo2 (0.10%) Eo1 (0.05%); Eo2 (0.05%) Eo1 (0.80%); Eo2 (0.40%) Eo1 (0.05%); Eo2 (0.05%) Eo1 (0.05%); Eo2 (0.05%) | Eo1 (0.05); Eo2 (0.10) Eo1 (0.05); Eo2 (0.10) Eo1 (0.05); Eo2 (0.05) Eo1 (1.00); Eo2 (0.08) Eo1 (0.05); Eo2 (0.05) Eo1 (0.05); Eo2 (0.10) | [245] |
| n.m/n-Bu | DDM | E. faecalis ATCC29212 E. coli ATCC25922 P. aeruginosa ATCC27853 S. aureus ATCC25923 | 7.00 7.00 6.50 ± 0.70 8.00 | n.m n.m n.m n.m | n.m n.m n.m n.m | [203] |
| AP/INF AP/DEC ETH | MPM | E. faecalis E. coli E. coli ESBL K. pneumoniae K. pneumoniae ESBL Listeria monocytogenes Morganella morganii P. aeruginosa S. aureus MSSA S. aureus MRSA | INF (10.00); DEC (10.00); ETH (10.00) INF (5.00); DEC (10.00); ETH (5.00) INF (5.00); DEC (10.00); ETH (5.00) INF (10.00); DEC (10.00); ETH (5.00) INF (10.00); DEC (10.00); ETH (5.00) INF (10.00); DEC (10.00); ETH(10.00) INF (10.00); DEC (10.00); ETH (5.00) INF (20.00); DEC (20.00); ETH (20.00) INF (5.00); DEC (10.00); ETH (2,50) INF (5.00); DEC (10.00); ETH (2,50) | n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m | n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m | [183] |
| S, L, F, Fr/Eo | DDM | P. aeruginosa ATCC27853 S. aureus ATCC 25923 E. coli ATCC25922 | 10.00 ± 0.50 17.30 ± 0.58 15.00 ± 0.00 | 1.66 mg/mL 0.20 mg/mL 2.50 mg/mL | n.m n.m n.m | [139] |
| AP/Eo1 Eo2 Eo3 | DDM | B. cereus C1060 Helicobacter pyllori J99 H. pylori 26695 L. monocytogenes EGD Salmonella sp S. aureus CFSA2 C. albicans | Eo1 (17.00 ± 1.00), Eo2 (9.00 ± 1.00), Eo3 (n.i) Eo1 (14.33 ± 1.15), Eo2 (13.00 ± 1.00), Eo3 (24.33 ± 0.57) Eo1 (17.00 ± 3.00), Eo2 (15.00 ± 2.00), Eo3 (30.00 ± 0.00) Eo1 (11.66 ± 1.15), Eo2 (n.i), Eo3 (n.i) Eo1 (7.00 ± 0.00), Eo2 (8.33 ± 0.57), Eo3 (n.i) Eo1 (9.33 ± 0.57), Eo2 (n.i), Eo3 (n.i) Eo1 (9.33 ± 0.57), Eo2 (9.00 ± 1.00), Eo3 (9.66 ± 0.57) | n.m n.m n.m n.m n.m n.m n.m | n.m n.m n.m n.m n.m n.m n.m | [135] |
| n.m/Eo1 Eo2 | MDM | C. albican | - | Eo1 (11.379 µg/mL) Eo2 (18.037 µg/mL) | n.m n.m | [53] |
| AP/Eo | DDM | B. subtilis ATCC6633 E. coli CIP 54.8 P. aeruginosa CIPA22 S. aureus CIP 7625 C. albican S. cerevisiae Fusarium oxysporum F. ssp. albedinis. Mucor ramanniamus NRRL6606 | 42.00 n.i n.i n.i 32.00 46.00 34.00 28.00 | 0.50 µL/mL 5.00 µL/mL 2.00 µL/mL 2.00 µL/mL 1.00 µL/mL 1.00 µL/mL 1.00 µL/mL 0.50 µL/mL | n.m n.m n.m n.m n.m n.m n.m n.m | [136] |
| Libya | ||||||
| AP/Eo | MDM | B. cereus E. cloacae E. coli ATCC35210 L. monocytogenes NCTC7973 M. flavus ATCC10240 P. aeruginosa ATCC27853 S. Typhimurium ATCC13311 S. aureus ATCC6538 Aspergillus fumigates A. versicolor ATCC11730 A. ochraceus ATCC12066 A. niger ATCC6275 Trichoderma viride IAM5061 Penicillium funiculosum ATCC36839 P. ochrochloron ATCC9112 P. aurantiogriseum | n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m | 0.04 ± 0.01 mg/mL 0.05 ± 0.04 mg/mL 0.08 ± 0.03 mg/mL 0.04 ± 0.00 mg/mL 0.03 ± 0.00 mg/mL 0.05 ± 0.00 mg/mL 0.09 ± 0.04 mg/mL 0.08 ± 0.03 mg/mL 0.01 ± 0.00 mg/mL 0.04 ± 0.03 mg/mL 0.01 ± 0.00 mg/mL 0.01 ± 0.00 mg/mL 0.01 ± 0.00 mg/mL 0.01 ± 0.00 mg/mL 0.01 ± 0.02 mg/mL 0.02 ± 0.01 mg/mL | 0.08 ± 0.02 mg/mL 0.11 ± 0.07 mg/mL 0.11 ± 0.07 mg/mL 0.09 ± 0.02 mg/mL 0.05 ± 0.00 mg/mL 0.11 ± 0.01 mg/mL 0.18 ± 0.07 mg/mL 0.15 ± 0.05 mg/mL 0.03 ± 0.00 mg/mL 0.03 ± 0.01 mg/mL 0.03 ± 0.00 mg/mL 0.01 ± 0.00 mg/mL 0.01 ± 0.00 mg/mL 0.03 ± 0.02 mg/mL 0.03 ± 0.02 mg/mL 0.04 ± 0.01 mg/mL | [246] |
| AP/Eo | MDM | E. feacalis (IBR E001) P. aeruginosa (IBR P001), Lactobacillus acidophilus (IBR L001) S. aureus (ATCC 25923) Streptococcus mutans (IBR S001) S. pyogenes (IBR S004) S. salivarius (IBR S006) S. sanguinis (IBR S002) | n.m n.m n.m n.m n.m n.m n.m n.m | 20.00 ± 3.40 µL/mL 80.00 ± 2.25 µL/mL 40.00 ± 0.00 µL/mL 80.00 ± 2,25 µL/mL 40.00 ± 1.15 µL/mL 40.00 ± 0.00 µL/mL 40.00 ± 3.00 µL/mL 40.00 ± 0.00 µL/mL | 40.00 ± 6.75 µL/mL 160.00 ± 4.61 µL/mL 80.00 ± 0.00 µL/mL 160.00 ± 4,50 µL/mL 80.00 ± 2.25 µL/mL 80.00 ± 0.00 µL/mL 80.00 ± 4.64 µL/mL 80.00 ± 0.00 µL/mL | [245] |
| Morocco | ||||||
| S, L/Eo | WDA | E. faecalis E. coli K. pneumonia S. enterica S. aureus S. pneumonia C. albicans C. parapsilosis C. glabrata Trichophyton violaceum T. mentagrophytes Microsporum canis | n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m n.m | 0.07% 0.03% 0.07% 0.03% 0.15% 0.07% 0.03% 0.03% 0.07% n.m n.m n.m | 0.30% 0.07% 0.30% 0.07% 0.30% 0.30% 0.07% 0.07% 0.30% n.m n.m n.m | [155] |
| L/Eo | DDM | E. faecalis E. coli O157H7 L. monocytogenes EGD-e L. monocytogenes 4b P. aeruginosa S. enteritidis S. aureus | 14.70 ± 1.20 17.80 ± 1.70 33.70 ± 0.40 26.70 ± 2.30 15.20 ± 1.00 15.60 ± 2.40 51.00 ± 3.40 | < 0.50 µL/mL 1.00 µL/mL < 0.50 µL/mL < 0.50 µL/mL 10.00 µL/mL 1.00 µL/mL < 0.50 µL/mL | < 0.50 µL/mL 2.00 µL/mL < 0.50 µL/mL < 0.50 µL/mL 30.00 µL/mL 1.00 µL/mL < 0.50 µL/mL | [158] |
| Tunisia | ||||||
| AP/Eo AQ | MDM | E. cloacae ABC291 E. faecalis ABC3 E. coli ABC5 K. pneumoniae ABC42 P. aeruginosa ABC4 S. aureus ABC1 Acinetobacter baumannii ABC14 | n.m n.m n.m n.m n.m n.m n.m | Eo (>140.00); AQ (>166.00) µg/mL Eo (140.00); AQ (>83.00) µg/mL Eo (140.00); AQ (>166.00) µg/mL Eo (>140.00); AQ (>166.00) µg/mL Eo (>140.00); AQ (>166.00) µg/mL Eo (70.00); AQ (83.00) µg/mL Eo (>140.00); AQ (>166.00) µg/mL | n.m n.m n.m n.m n.m n.m n.m | [50] |
| AP/ME | n.m | B. subtilis E. coli K oxycota K. pneumonia S. aureus | 24.00 7.00 n.i 10.00 10.00 | n.m n.m n.m n.m n.m | n.m n.m n.m n.m n.m | [57] |
| AP/Eo1 Eo2 Eo3 | DDM | B. subtilis 166 E. coli GM109 L. monocytogynes P. aeruginosa S. enteridis ATCC 502 S. aureus ATCC 25923 | Eo1 (36.00); Eo2 (63.00); Eo3 (63.00) Eo1 (30.00); Eo2 (50.00); Eo3 (30.00) Eo1 (20.00); Eo2 (45.00); Eo3 (32.00) Eo1 (9.00); Eo2 (74.00); Eo3 (30.00) Eo1 (9.00); Eo2 (43.00); Eo3 (65.00) Eo1 (22.00); Eo2 (63.00); Eo3 (63.00) | Eo1 (5.50); Eo2 (4.00); Eo3 (4.50) mg/mL Eo1 (4.00); Eo2 (1.80); Eo3 (4.00) mg/mL Eo1 (7.50); Eo2 (2.00); Eo3 (4.00) mg/mL Eo1 (22.00); Eo2 (9.00); Eo3 (4.50) mg/mL Eo1 (22.00); Eo2 (2.00); Eo3 (1.50) mg/mL Eo1 (4.50); Eo2 (1.50); Eo3 (1.70) mg/mL | n.m n.m n.m n.m n.m n.m | [48] |
| R, S, L/Eo1 Eo2 Eo3 | DDM | B. cereus ATCC11778 E. coli ATCC25922 L. monocytogynes ATCC7644 P. aeruginosa ATCC9027 S. aureus ATTCC25923 | Eo1 (20.90 ± 0.60); Eo2 (25.50 ± 0.50); Eo3 (18.20 ± 0.30) Eo1 (13.70 ± 0.30), Eo2 (16.00 ± 0.50), Eo3 (12.10 ± 0.40) Eo1 (9.10 ± 0.50), Eo2 (12.50 ± 0.50), Eo3 (8.80 ± 0.30); Eo1 (14.40 ± 0.30), Eo2 (16.80 ± 1.00), Eo3 (13.60 ± 0.50) Eo1 (17.20 ± 0.20), Eo2 (19.40 ± 0.50), Eo3 (14.8 ± 0.50) | Eo1 (2.00), Eo2 (1.00), Eo3 (2.50) µL/mL Eo1 (4.50), Eo2 (3.25), Eo3 (5.00) µL/mL Eo1 (4.50), Eo2 (1.75), Eo3 (4.50) µL/mL Eo1 (3.50), Eo2 (2.25), Eo3 (5.00) µL/mL Eo1 (2.00), Eo2 (1.25), Eo3 (2.50) µL/mL | n.m n.m n.m n.m n.m | [152] |
| AP/Eo | DDM | B. cereus ATCC11778 E. faecalis ATCC29212 E. coli ATCC25922 K. pneumoniae ATCC13883 P. aeruginosa ATCC27853 S. typhimurium NRRLB4420 Aspergillus niger F. solani | 30.00 ± 2.00 18.50 ± 0.50 14.00 ± 1.00 13.50 ± 0.50 14.50 ± 0.50 15.00 ± 0.50 64.00 ± 3.00 31.00 ± 1.50 | 1.00 µL/mL 3.00 µL/mL 6.00 µL/mL 6.00 µL/mL 5.00 µL/mL 6.00 µL/mL 2.00 µL/mL 1.00 µL/mL | n.m n.m n.m n.m n.m n.m n.m n.m | [49] |
4.5.2. Antifungal Effects
4.5.3. Antioxidant Activity
| Part of Plant | Product | Antioxidant Assay | Antioxidant Activities | Ref. |
|---|---|---|---|---|
| Algeria | ||||
| n.m | Eo | DPPH BCB | IC50 = 3 7.68 ± 0.245 mg/mL IC50 = 3 8.86 ± 1.13 mg/mL | [130] |
| L | Eo | DPPH ABTS | IC50 = 8.37 mg/mL IC50 = 10.84 mg/mL | [61] |
| S, L | ME | DPPH ABTS FRAP CUPRAC | IC50 = 18.40 ± 0.42 μg/mL IC50 = 11.73 ± 0.20 μg/mL A0.5 = 147.44 ± 0.191 μg/mL A0.5 = 25.04 ± 0.86 μg/mL | [55] |
| AP | PEE CH n-Bu | DPPH CUPRAC RP TAC LPAF | PEE (IC50 = 69.50 ± 0.68), CHCl3ext (IC50 = 79.92 ± 0.30), n-Bu (IC50 = 5.05 ± 0.12) µg/mL PEE (A0.5 = 22.28 ± 0.24), CH (A0.5 = 27.81 ± 3.06), n-Bu (A0.5 = 0.94 ± 0.06) μg/mL PEE (A0.5 = 25.25 ± 0.08), CH (A0.5 = 24.5 ± 0.52), n-Bu (A0.5 = 4.98 ± 0.48) μg/mL PEE (15.69 ± 0.001), CH (16.21 ± 0.02), n-Bu (20.79 ± 0.19) μg EAA/mg DE PEE (27.80 ± 0.37), CH (24.25 ± 0.45), n-Bu (47.43 ± 0.58)% | [58] |
| n.m | ME | DPPH BCB | IC50 = 1.60 ± 0.13 μg/mL AA = 64.31 ± 1.90% | [60] |
| AP | CH EAE ET AQ | DPPH ABTS | CHT (n.a); EAE (n.a); ET (0.052 ± 0.004 mg/mL); AQ (n.a) CH (n.a); EAE (n.a); ET(42.00 ± 0.99); AQ (152.00 ± 31.00) µg/mL | [262] |
| L | ME | TAC | IC50 = 39.27± 3.47 U/L | [209] |
| AP | MEH | DPPH ABTS BCB TAC FRAP RP | IC50 = 7.40 ± 0.30 µg/mL IC50 = 207.00 ± 3.00 µg/mL AA = 90.00 ± 2.00% TAA = 268.00 ± 4.00 µg EAA/mg FRA= 5.3 ± 0.0 mM FeSO4/mg IC50 = 512.00 ± 0.00 µg/mL | [188] |
| L | Eo ET | DPPH ABTS FRAP PM | Eo (IC50 = 1.437 ± 4.51 E-05 mg/mL); ET (IC50 = 1.56 ± 0.01 mg/mL) Eo (IC50 = 0.8960 ± 0.20); ET (IC50 = 1.743 ± 0.195 mg/mL) Eo (IC50 = 1.39 ± 0.26); ET (IC50 = 0.90 ± 0.06) μg/mL) Eo (IC50 = 0.43 ± 0.001); ET (IC50 = 0.007 ± 0.0006) mg/mL) | [132] |
| AP | INF DEC ETH | DPPH RP BCB TBARS | INF (IC50 = 64.80 ± 0.70); DEC (IC50 = 48.00 ± 2.00); ETH (IC50 = 131.00 ± 3.00) µg/mL INF (IC50 = 54.00 ± 0.50); DEC (IC50 = 49.80 ± 0.40); ETH (IC50 = 100.20 ± 0.50) µg/mL INF (IC50 = 139.00 ± 4.00); DEC (IC50 = 149.00 ± 3.00); ETH (IC50 = 85.00 ± 3.00) µg/mL INF (IC50 = 26.30 ± 0.20); DEC (IC50 = 22.70 ± 0.30); ETH (IC50 = 40.30 ± 0.30) µg/mL | [183] |
| S, L, F | EAE n-Bu | DPPH | EAE (IC50 = 0.30 mg/mL) n-Bu (IC50 = 1.45 mg/mL) | [190] |
| AP | Eo | DPPH | IC50 = 8379.03 ± 15.00 µg/mL | [138] |
| AP | HAext | DPPH ABTS TBARS ORAC RP MC HR LIPO SAS | 0.235 ± 0.018 mg/mL 0.150 ± 0.002 mg/mL n.a 38.47 ± 39.71 TE/g DW 0.025 ± 0.006 mg/mL n.a n.d 0.083 ± 0.005 mg/mL n.d | [191] |
| AP | Eo1 Eo2 Eo3 | DPPH RP HR TBARS | At 1 mg/mL Eo1 (53.40 ± 0.20); Eo2 (6.30 ± 0.30); Eo3 (7.80 ± 0.20)% n.m Eo1 (IC50 = 8.50 ±0.10); Eo2 (IC50 = 2.20 ± 0.03); Eo3 (IC50 = 3.30 ± 0.08) µg/mL Eo1 (IC50 = 106.70 ± 8.40); Eo2 (IC50 = n.a); Eo3 (IC50 = 911.60 ± 7.40) µg/mL | [135] |
| Libya | ||||
| AP | Eo | DPPH | IC50 = 0.132 mg/mL | [244] |
| AP | Eo | DPPH RP BCB TBARS assay | IC50 = 1.64 ± 0.05 mg/mL IC50 = 0.68 ± 0.01 mg/mL IC50 = 1.56 ± 0.12 mg/mL IC50 = 0.31 ± 0.01 mg/mL | [245] |
| Morocco | ||||
| S, L | Eo | DPPH ABTS | IC50 = 6.88 ± 0.05 µg/mL IC50 = 6.96 ± 0.02 µg/mL | [155] |
| S, L, F | Eo | DPPH | IC50 = 67.85 ± 1.21 µg/mL | [63] |
| AP | AQ | DPPH | IC50 = 32.40 µg/mL | [156] |
| L | Eo | DPPH | IC50 = 1800 μg/mL | [157] |
| Tunisia | ||||
| AP | Eo AQ | DPPH FRAP | AQ (IC50 = 0.04 μg/mL); Eo (IC50 = 0.06 μg/mL) AQ (IC50 = 0.04 μg/mL); Eo (IC50 = 0.06 μg/mL) | [50] |
| L | ME (1–12) | DPPH BCB FRAP | IC50 (μg/mL): Ta1 (42.70 ± 2.50); Ta2 (54.50 ± 2.10); Ta3 (52.30 ± 1.40); Ta4 (22.70 ± 0.90); Ta5 (37.80 ± 0.60); Ta6 (40.70 ± 1.00); Ta7 (26.60 ± 1.40); Ta8 (68.80 ± 1.00); Ta9 (32.40 ± 1.00); Ta10 (19.90 ± 1.10); Ta11 (8.90 ± 0.10); Ta12 (10.30 ± 0.40) IC50 (mg/mL): Ta1 (1.43 ± 0.00); Ta2 (1.50 ± 0.10); Ta3 (1.81± 0.00); Ta4 (1.04 ± 0.00); Ta5 (1.35 ± 0.30); Ta6 (1.60 ± 0.00); Ta7 (1.13 ± 0.00); Ta8 (1.60 ± 0.00); Ta9 (1.53 ± 0.10); Ta10 (0.40 ± 0.00); Ta11 (0.03 ± 0.00); Ta12 (0.06 ± 0.00) IC50 (mmolFe2+/L): Ta1 (2.00 ± 0.00); Ta2 (1.20 ± 0.00); Ta3 (0.30 ± 0.01); Ta4 (4.80 ± 0.00); Ta5 (6.80 ± 0.00); Ta6 (1.80 ± 0.00); Ta7 (5.10 ± 0.00); Ta8 (1.00 ± 0.00); Ta9 (4.00 ± 0.00); Ta10 (6.50 ± 0.05); Ta11 (16.70 ± 0.10); Ta12 (20.60 ± 0.20) | [62] |
| AP | MEt1, ME2, ME3 Eo1, Eo2, Eo3 | DPPH ABTS BCB | IC50 (%): ME1 (93.00 ± 0.06); ME2 (84.00 ± 0.034); ME3 (81.00 ± 0.26) IC50 (%): Eo1 (85.00 ± 0.57); Eo2 (82.00 ± 0.52); Eo3 (83.00 ± 0.10) IC50 (%): ME1 (75.00 ± 0.72); ME2 (50.00 ± 0.96); MEt3 (22.00 ± 0.90) IC50 (%): Eo1 (16.00 ± 0.12); Eo2 (8.00 ± 0.70); Eo3 (19.00 ± 0.33) IC50 (%): ME1 (31 ± 0.91); ME2 (25 ± 0.08); ME3 (50 ± 0.12) IC50 (%): Eo1 (10.00 ± 0.52); Eo2 (4.00 ± 0.44); Eo3 (5.00 ± 0.71) | [150] |
| R, S, L | Eo1, Eo2, Eo3 | DPPH | Eo1 (IC50 = 9.23 mg/mL); Eo2 (IC50 = 4.31 mg/mL); Eo3 (IC50 = 6.54 mg/mL) | [152] |
| AP | Eo | DPPH BCB | IC50 = 0.8 mg/mL IC50 = 0.5 mg/mL | [49] |
| AP | Eo | DPPH | Radical scavenging activity values (0.6–5.61%) at 200 µg/ml | [151] |
4.5.4. Anti-Inflammatory Activity
4.5.5. Anti-Pyretic and Anti-Nociceptive Activity
4.5.6. Cytotoxic Activities
4.5.7. Neuroprotective Effect
4.5.8. Effect on the Gastrointestinal Tract
4.5.9. Insecticide and Phytotoxic Effects
4.5.10. Other Effects
4.5.11. Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Biological activities of three essential oils of the Lamiaceae family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; De Tok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In VII Flowering Plants Dicotyledons, Lamiales (Except Acanthaceae Including Avicenniaceae); Kadereit, J., Ed.; The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2004; Volume 6, pp. 167–275. [Google Scholar]
- Wordfloraonline Lamiaceae Martinov. Available online: http://www.worldfloraonline.org/taxon/wfo-7000000318 (accessed on 25 June 2022).
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Stankovic, M. (Ed.) Lamiaceae Species: Biology, Ecology and Practical Uses; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Hedge, I. A global survey of the biogeography of the Labiatae. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; The Royal Botanical Garden, Kew: London, UK, 1992; pp. 7–17. [Google Scholar]
- Kaufmann, M.; Wink, M. Molecular systematics of the Nepetoideae (Family Labiatae): Phylogenetic implications from RbcL Gene Sequences. Z. Naturforsch. C J. Biosci. 1994, 49, 635–645. [Google Scholar] [CrossRef]
- Damerdji, A. Malacological diversity on Some Lamiaceae in the region of Tlemcen (Northwest Algeria). J. Life Sci. 2013, 7, 856–861. [Google Scholar] [CrossRef][Green Version]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Par-dossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Chang, T.L.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Food and Drug Administration Code of Federal Regulations (CFR). Title 21: Food and Drugs Chapter I—Food and Drug Administration, Department of Health and Human Services, Subchapter B—Food for Human Consumption, Part182—Substances Generally Recognized as Safe (GRAS). Available online: https://www.zotero.org/didalalou/search/FDA/titleCreatorYear/items/3PIJLFG6/item-list (accessed on 25 June 2022).
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A Review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Gholamnezhad, Z.; Rezaee, R.; Boskabady, M.H. Immuno-modulatory and anti-inflammatory effects of Thymus Vulgaris, Zataria multiflora, and Portulaca oleracea and their constituents. Pharmacol. Res.—Mod. Chin. Med. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef] [PubMed]
- Mouhid, L.; Gómez De Cedrón, M.; Vargas, T.; García-Carrascosa, E.; Herranz, N.; García-Risco, M.; Reglero, G.; Fornari, T.; de Molina, A.R. Identification of antitumoral agents against human pancreatic cancer cells from Asteraceae and Lamiaceae plant extracts. BMC Complement. Altern. Med. 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Dantas, F.G.; de Castilho, P.F.; de Almeida-Apolonio, A.A.; de Araújo, R.P.; Oliveira, K.M.P. Mutagenic potential of medicinal plants evaluated by the Ames Salmonella/microsome assay: A systematic review. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108338. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A.; Ożarowski, M. Radioprotective effects of plants from the Lamiaceae family. Anticancer. Agents Med. Chem. 2022, 22, 4–19. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; dos Reis Gomes, A.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in an-ti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Koulivand, P.H.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the nervous system. Evid.-Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Mohammed, A.; Tajuddeen, N. Antidiabetic compounds from medicinal plants traditionally used for the treatment of diabetes in Africa: A review update (2015–2020). S. Afr. J. Bot. 2022, 146, 585–602. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
- The Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: https://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameId=111505&PTRefFk=8000000 (accessed on 25 June 2022).
- WCSP World Checklist of Selected Plant of Lamiaceae Familly: Royal Botanic Gardens, Kew. Available online: https://wcsp.science.kew.org/reportbuilder.do (accessed on 17 June 2022).
- Fakchich, J.; Elachouri, M. An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: Systematic review (part 1). J. Ethnopharmacol. 2021, 267, 113200. [Google Scholar] [CrossRef]
- Bouafia, M.; Amamou, F.; Gherib, M.; Benaissa, M.; Azzi, R.; Nemmiche, S. Ethnobotanical and ethnomedicinal analysis of wild medicinal plants traditionally used in Naâma, Southwest Algeria. Vegetos 2021, 34, 654–662. [Google Scholar] [CrossRef]
- Karous, O.; Jilani, I.B.H.; Ghrabi-Gammar, Z. Ethnobotanical study on plant used by semi-nomad descendants’ community in Ouled Dabbeb-Southern Tunisia. Plants 2021, 10, 642. [Google Scholar] [CrossRef]
- Kachmar, M.R.; Naceiri Mrabti, H.; Bellahmar, M.; Ouahbi, A.; Haloui, Z.; el Badaoui, K.; Bouyahya, A.; Chakir, S. Traditional knowledge of medicinal plants used in the northeastern part of Morocco. Evid.-Based Complement. Altern. Med. 2021, 2021, 6002949. [Google Scholar] [CrossRef]
- El-Gharbaoui, A.; Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J.; Merzouki, A. Comparison of Lamiaceae medicinal uses in eastern Morocco and Eastern Andalusia and in Ibn Al-Baytar’s compendium of simple medicaments. (13th Century CE). J. Ethnopharmacol. 2017, 202, 208–224. [Google Scholar] [CrossRef]
- Nafis, A.; Iriti, M.; Ouchari, L.; el Otmani, F.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Mezrioui, N.; Hassani, L.; Custódio, L. New insight into the chemical composition, antimicrobial and synergistic effects of the Moroccan endemic Thymus atlanticus (Ball) Roussine essential oil in combination with conventional antibiotics. Molecules 2021, 26, 5850. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis Subsp. Zygis an endemic Portuguese plant: Phytochemical profiling, antioxidant, anti-proliferative and anti-inflammatory activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V.; Ghannouchi, G.N.; Mondelli, A.; Logrieco, D.; Perrino, A.F.; et al. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and biological properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop wild relatives (CWRs) threatened and endemic to Italy: Urgent actions for protection and use. Biology 2022, 11, 193. [Google Scholar] [CrossRef]
- African Plant Database. Available online: https://africanplantdatabase.ch/en/nomen/145270 (accessed on 21 June 2022).
- EMPB. The Euro+Med Plantbase Project, the Information Resource for Euro-Mediterranean Plant Diversity: Lamiaceae Occurring in Algeria. Available online: https://www.emplantbase.org/home.html (accessed on 17 June 2022).
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; CNRS: France, Paris, 1962; Volume 2, pp. 565–1170. [Google Scholar]
- Kew Science Thymus, L. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002942-2 (accessed on 16 June 2022).
- Miladi, H.; Mili, D.; ben Slama, R.; Zouari, S.; Ammar, E.; Bakhrouf, A. Antibiofilm formation and anti-adhesive property of three Mediterranean essential oils against a foodborne pathogen Salmonella Strain. Microb. Pathog. 2016, 93, 22–31. [Google Scholar] [CrossRef]
- Fatma, G.; Mouna, B.F.; Mondher, M.; Ahmed, L. In-Vitro Assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis. Lipids Health Dis. 2014, 13, 114. [Google Scholar] [CrossRef]
- Zouari, N.; Fakhfakh, N.; Zouari, S.; Bougatef, A.; Karray, A.; Neffati, M.; Ayadi, M.A. Chemical composition, Angiotensin I-Converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. & Reut. (Lamiaceae). Food Bioprod. Process. 2011, 89, 257–265. [Google Scholar] [CrossRef]
- Ghorbel, A.; Fakhfakh, J.; Brieudes, V.; Halabalaki, M.; Fontanay, S.; Duval, R.E.; Mliki, K.; Sayadi, S.; Allouche, N. Chemical composition, antibacterial activity using micro-broth dilution method and antioxidant activity of essential oil and water extract from aerial part of Tunisian Thymus algeriensis Boiss. & Reut. J. Essent. Oil-Bear. Plants 2022, 24, 1349–1364. [Google Scholar] [CrossRef]
- Adouane, S.; Mehaoua, M.S.; Bouatrous, Y.; Tudela, J.; Flamini, G.; Mechaala, S. Natural insecticides from native plants of the mediterranean basin and their activity for the control of the date moth Ectomyelois ceratoniae (Zeller) (Lepidoptera: Pyralidae). J. Plant Dis. Prot. 2022, 129, 775–782. [Google Scholar] [CrossRef]
- Amarti, F.; El Ajjouri, M.; Ghanmi, M.; Satrani, B.; Aafi, A.; Farah, A.; Khia, A.; Guedira, A.; Rahouti, M.; Chaouch, A. Composition chimique et activité antimicrobienne des huiles essentielles de Thymus algeriensis Boiss. & Reut. et Thymus ciliatus (Desf.) Benth. du Maroc. Biotechnol. Agron. Soc. Environ. 2010, 14, 141–148. [Google Scholar]
- Giordani, R.; Hadef, Y.; Kaloustian, J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia 2008, 79, 199–203. [Google Scholar] [CrossRef]
- Sara, M.; Yamina, B.; Ramazan, E.; Mesut, G.; Selma, A. Dietary risk of BlaESBL producing multidrug resistant enterobacteriaceae and their inhibition by Artemisia herba-alba and Thymus Algeriensis essential oils. J. Essent. Oil-Bear. Plants 2021, 24, 658–670. [Google Scholar] [CrossRef]
- Zohra, B.F.; Amar, Z.; Chawki, B. Chemical analysis, antioxidant, anti-alzheimer and anti-diabetic effect of two endemic plants from Algeria: Lavandula Antineae and Thymus Algeriensis. Jordan J. Biol. Sci. 2021, 14, 551–558. [Google Scholar] [CrossRef]
- Jaafari, A.; Mouse, H.A.; Rakib, E.M.; M’Barek, L.A.; Tilaoui, M.; Benbakhta, C.; Boulli, A.; Abbad, A.; Zyad, A. Chemical composition and antitumor activity of different Wild varieties of Moroccan Thyme. Rev. Bras. Farmacogn. 2007, 17, 477–491. [Google Scholar] [CrossRef]
- Fatma, G.; Issam, S.; Rawya, S.; Najla, H.; Ahmed, L. Antioxidant potential of four species of natural product and therapeutic strategies for cancer through suppression of viability in the human multiple myeloma cell line U266. Biomed. Environ. Sci. 2019, 32, 22–33. [Google Scholar] [CrossRef]
- Mokhtari, M.; Chabani, S.; Mouffouk, S.; Aberkane, M.C.; Dibi, A.; Benkhaled, M.; Haba, H. Phytochemicals, antihemolytic, anti-inflammatory, antioxidant, and antibacterial activities from Thymus algeriensis. J. Herbs Spices Med. Plants 2021, 27, 253–266. [Google Scholar] [CrossRef]
- Beyi, L.; Zrouri, H.; Makrane, H.; Mekhfi, H.; Ziyyat, A.; Bnouham, M.; Aziz, M. Relaxant and antispasmodic activities of aqueous extract from Thymus algeriensis Boiss. And Reut. J. Nat. Rem. 2021, 21, 165–171. [Google Scholar] [CrossRef]
- Ouakouak, H.; Benarfa, A.; Messaoudi, M.; Begaa, S.; Sawicka, B.; Benchikha, N.; Simal-Gandara, J. Biological properties of essential oils from Thymus Algeriensis Boiss. Plants 2021, 10, 786. [Google Scholar] [CrossRef]
- Jaouadi, R.; Silva, A.M.S.; Boussaid, M.; Yahia, I.B.H.; Cardoso, S.M.; Zaouali, Y. Differentiation of phenolic composition among Tunisian Thymus Algeriensis Boiss. et Reut. (Lamiaceae) populations: Correlation to bioactive activities. Antioxidants 2019, 8, 515. [Google Scholar] [CrossRef]
- El Ouahdani, K.; Es-Safi, I.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bari, A.; Bousta, D. Thymus Algeriensis and Artemisia herba-Alba essential oils: Chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, A.; Djebili, S.; Zouaoui, N.; Barkat, M. Evaluation of phenolic contents and antioxidant activities of some medicinal plants growing in Algerian Aurès Mountains. Acta Nat. Sci. 2020, 7, 15–30. [Google Scholar] [CrossRef]
- Rey, C. Selection of Thyme for extreme areas (of Switzerland). Acta Hortic. 1992, 306, 66–70. [Google Scholar] [CrossRef]
- Morales, R. The History, Botany and Taxonomy of the Genus Thymus. In Thyme the Genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; CRC Press: London, UK, 2002; pp. 1–43. [Google Scholar]
- Anne, M. Healing with Flowers: The Power of Floral Medicine; Aeon Books: London, UK, 2022; p. 488. ISBN 9781913504793. [Google Scholar]
- Evelyn, K. Thyme & Oregano, Healing and Cooking Herbs, and More than 30 Ways to Use Them; Kindle: Athens, Greece, 2014; ISBN 9781312662186. [Google Scholar]
- Richard, H.; Benjilali, B.; Banquour, N.; Baritaux, O. Etude de diverses huiles essentielles de Thym du Maroc—Institut National de Recherche En Agriculture, Alimentation et Environnement. Lebensm-Wiss.+ Technol. 1985, 18, 105–110. [Google Scholar]
- Beloued, A. Plantes Médicinales d’Algérie; Offices Des Publications Universitaires: Ben Aknoun, Algeria, 2005; ISBN 9789961003046. [Google Scholar]
- Morales, R. Synopsis of the Genus Thymus L. in Mediterranean area. Lagascalia 1997, 19, 249–262. [Google Scholar]
- Karaca, M.; İnce, A.G.; Aydin, A.; Elmasulu, S.Y.; Turgut, K. Microsatellites for genetic and taxonomic research on Thyme (Thymus L.). Turk. J. Biol. 2015, 39, 147–159. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Passalacqua, N. Typification of names and taxonomic notes within the genus Thymus L. (Lamiaceae). Taxon 2013, 62, 1308–1314. [Google Scholar] [CrossRef]
- Bower, A.; Marquez, S.; de Mejia, E.G. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef]
- Amrouche, T.A.; Yang, X.; Capanoglu, E.; Huang, W.; Chen, Q.; Wu, L.; Zhu, Y.; Liu, Y.; Wang, Y.; Lu, B. Contribution of edible flowers to the Mediterranean diet: Phytonutrients, bioactivity evaluation and applications. Front. Nutr. 2022, 9, 1–39. [Google Scholar] [CrossRef]
- Nieto, G. A Review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Sánchez-Mata, D.; Morales, R. Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 15–31. [Google Scholar] [CrossRef]
- Tassin, C. Paysages Végétaux du Domaine Méditerranéen: Bassin Méditerranéen, Californie, Chili Central, Afrique du Sud, Australie Méridionale; IRD (Institut de Recherche pour le Développement): Paris, France, 2012; p. 1135. ISBN -10 2709917319. [Google Scholar] [CrossRef]
- Eflora Maghreb Thymus Algeriensis Boiss. & Reut. Available online: https://efloramaghreb.org/specie/145270 (accessed on 21 June 2022).
- El Ajjouri, M.; Ghanmi, M.; Satrani, B.; Amarti, F.; Rahouti, M.; Aafi, A.; Rachid Ismaili, M.; Farah, A. Composition chimique et activité antifongique des huiles essentielles de Thymus Algeriensis Boiss. & Reut. et Thymus Ciliatus (Desf.) Benth. contre les champignons de pourriture du bois. Acta Bot. Gall. 2010, 157, 285–294. [Google Scholar] [CrossRef][Green Version]
- Morales, R. Studies on the genus Thymus. Lamiales Newsl. 1996, 4, 6–8. [Google Scholar]
- Stahl-Biskup, E.; Sáez, F. Thyme: The Genus Thymus, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780429218651. [Google Scholar] [CrossRef]
- INaturalist Site, Species Observed in Algeria and Tunisia by Larbi Afoutni and Khaled Bessrour, Occurrence 3455238505. Available online: https://www.gbif.org/occurrence/3455238505 (accessed on 22 July 2022).
- Ali, I.B.E.H.; Zaouali, Y.; Bejaoui, A.; Boussaid, M. Variation of the chemical composition of essential oils in Tunisian populations of Thymus Algeriensis Boiss. et Reut. (Lamiaceae) and implication for conservation. Chem. Biodivers. 2010, 7, 1276–1289. [Google Scholar] [CrossRef]
- Tarayre, M.; Thompson, J.D. Population genetic structure of the Gynodioecious Thymus vulgaris L. (Labiatae) in Southern France. J. Evol. Biol. 1997, 10, 157–174. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Guetat, A.; Boussaid, M. Chemical and genetic variability of Thymus Algeriensis Boiss. et Reut. (Lamiaceae), a North African endemic species. Ind. Crops Prod. 2012, 40, 277–284. [Google Scholar] [CrossRef]
- Guesmi, F.; Saidi, I.; Bouzenna, H.; Hfaiedh, N.; Landoulsi, A. Phytocompound variability, antioxidant and an-tibacterial activities, anatomical features of glandular and aglandular hairs of Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut. over developmental stages. S. Afr. J. Bot. 2019, 127, 234–243. [Google Scholar] [CrossRef]
- Baba Aïssa, F. Les Plantes Medicinales en Algerie; Bouchéne-ad Diwan: Algiers, Algeria, 1991. [Google Scholar]
- Benkiniouar, R.; Rhouati, S.; Touil, A.; Seguin, E.; Chosson, E. Flavonoids from Thymus algeriensis. Chem. Nat. Compd. 2007, 43, 321–322. [Google Scholar] [CrossRef]
- Mechaala, S.; Bouatrous, Y.; Adouane, S. Traditional knowledge and diversity of Wild medicinal plants in El Kantara’s Area (Algerian Sahara Gate): An ethnobotany survey. Acta Ecol. Sin. 2022, 42, 33–45. [Google Scholar] [CrossRef]
- Hachi, M.; Ouafae, B.; Hachi, T.; Mohamed, E.B.; Imane, B.; Atmane, R.; Zidane, L. Contribution to ethnobotanical study of antidiabetic medicinal plants of the central middle Atlas region (Morocco). Lazaroa 2016, 37, 135–144. [Google Scholar] [CrossRef]
- Sijelmassi, A. Les Plantes Médicinales du Maroc; Le Fennec: Casablanca, Maroco, 1993; Volume 1, p. 285. ISBN -10 9981838020. [Google Scholar]
- Bellakhdar, J. Pharmacopée Marocaine traditionnelle: Médecine Arabe Ancienne et Savoirs Populaires; Paris Ibiss Press: Paris, France, 1997; p. 766. [Google Scholar]
- Ismaili, H.; Milella, L.; Fkih-Tetouani, S.; Ilidrissi, A.; Camporese, A.; Sosa, S.; Altinier, G.; della Loggia, R.; Aquino, R. In Vivo topical anti-inflammatory and in vitro antioxidant activities of two extracts of Thymus satureioides Leaves. J. Ethnopharmacol. 2004, 91, 31–36. [Google Scholar] [CrossRef]
- Ismaili, H.; Sosa, S.; Brkic, D.; Fkih-Tetouani, S.; Ilidrissi, A.; Touati, D.; Aquino, R.P.; Tubaro, A. Topical antiinflammatory activity of extracts and compounds from Thymus broussonettii. J. Pharm. Pharmacol. 2002, 54, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, H.; Tortora, S.; Sosa, S.; Fkih-Tetouani, S.; Ilidrissi, A.; Della Loggia, R.; Tubaro, A.; Aquino, R. Topical Anti-inflammatory activity of Thymus willdenowii. J. Pharm. Pharmacol. 2001, 53, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Pottier-Alapetite, G.A. Flore de la Tunisie: Angiosperme–Dicotylédones: Gamopétales; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture: Tunis, Tunisia, 1981; Volume 2. [Google Scholar]
- Le Floc’h, É. Contribution à une Étude Ethnobotanique de la Flore Tunisienne; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique: Tunis, Tunisia, 1983. [Google Scholar]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant Sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Milevskaya, V.V.; Prasad, S.; Temerdashev, Z.A. Extraction and chromatographic determination of phenolic compounds from medicinal herbs in the Lamiaceae and Hypericaceae Families: A review. Microchem. J. 2019, 145, 1036–1049. [Google Scholar] [CrossRef]
- Lichman, B.R.; Godden, G.T.; Buell, C.R. Gene and genome duplications in the evolution of chemodiversity: Perspectives from studies of Lamiaceae. Curr. Opin. Plant Biol. 2020, 55, 74–83. [Google Scholar] [CrossRef]
- Boachon, B.; Buell, C.R.; Crisovan, E.; Dudareva, N.; Garcia, N.; Godden, G.; Henry, L.; Kamileen, M.O.; Kates, H.R.; Kilgore, M.B.; et al. Phylogenomic mining of the mints reveals multiple mechanisms contributing to the evolution of chemical diversity in Lamiaceae. Mol. Plant 2018, 11, 1084–1096. [Google Scholar] [CrossRef]
- Trindade, H.; Pedro, L.G.; Figueiredo, A.C.; Barroso, J.G. Chemotypes and terpene synthase genes in Thymus genus: State of the art. Ind. Crops Prod. 2018, 124, 530–547. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild officinal species: A Pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef]
- Pelvan, E.; Karaoğlu, Ö.; Önder Fırat, E.; Betül Kalyon, K.; Ros, E.; Alasalvar, C. Immunomodulatory effects of selected medicinal herbs and their essential oils: A comprehensive review. J. Funct. Foods 2022, 94, 105108. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; bin Emran, T.; Nainu, F.; Si-mal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in hu-man health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Wangchuk, P. Essential oils and their bioactive molecules in healthcare. herbal biomolecules in healthcare applications. In Herbal Biomolecules in Healthcare Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–648. [Google Scholar] [CrossRef]
- Hüsnü, K.; Baśer, C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis Subsp. Aristatus (Godr.) Nyman from Central Italy. Ind. Crops Prod. 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Delaney, K.J.; Breza-Boruta, B.; Grzegorz, L.; Bocianowski, J. Maize. VOC Induction after infection by the bacterial pathogen, Pantoea ananatis, alters neighbouring plant voc emissions. J. Plant Dis. Prot. 2015, 122, 125–132. [Google Scholar] [CrossRef]
- Kong, H.G.; Song, G.C.; Sim, H.J.; Ryu, C.M. Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J. 2020, 15, 397–408. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Dicke, M.; Bruin, J. Chemical Information Transfer between Plants: Back to the Future. Biochem. Syst. Ecol. 2001, 29, 981–994. [Google Scholar] [CrossRef]
- Agrawal, A.A. Mechanisms, Ecological Consequences and Agricultural Implications of Tri-Trophic Interactions. Curr. Opin. Plant Biol. 2000, 3, 329–335. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T.; Baxter, K.J.; Laue, G.; Felton, G.W. Communication between plants: Induced resistance in wild Tobacco plants following clipping of neighboring sagebrush. Oecologia 2000, 125, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Ozawa, R.; Shimoda, T.; Nishloka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Fahn, A. Structure and Function of Secretory Cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. A Contemporary introduction to essential oils: Chemistry, bioactivity and prospects for australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef]
- Abu-Asab, M.; Cantino, P.D. Phylogenetic implications of leaf anatomy in sub-tribe Melittidinae (Labiatae) and Related Taxa. J. Arnold Arbor. 1987, 68, 1–34. [Google Scholar] [CrossRef]
- Seyedi, Z.; Salmaki, Y. Trichome Morphology and Its Significance in the Systematics of Phlomoides (Lamiaceae; Lamioideae; Phlomideae). Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 213, 40–48. [Google Scholar] [CrossRef]
- Bhatt, A.; Naidoo, Y.; Nicholas, A. The foliar trichomes of Plectranthus laxiflorus Benth: An important medicinal plant. N. Z. J. Bot. 2010, 48, 55–61. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Ra-jabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. Plants—Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 8, 1147–1154. [Google Scholar] [CrossRef]
- Elhouiti, F.; Benabed, K.H.; Tahri, D.; Ouinten, M.; Yousfi, M. Antioxidant and antifungal activities of essential oils from algerian spontaneous plants against five strains of Fusarium spp. Hell. Plant Prot. J. 2022, 15, 30–39. [Google Scholar] [CrossRef]
- Zouaoui, N.; Chenchouni, H.; Bouguerra, A.; Massouras, T.; Barkat, M. Characterization of volatile organic compounds from six aromatic and medicinal plant species growing wild in North African drylands. NFS J. 2020, 18, 19–28. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Med. Ther. 2019, 19, 146. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Effect of climatic factors on essential oil accumulation in two Lamiaceae species from Algerian semiarid lands. Jordan J. Biol. Sci. 2019, 12, 57–60. [Google Scholar] [CrossRef]
- Kouache, B.; Brada, M.; Saadi, A.; Fauconnier, M.L.; Lognay, G.; Heuskin, S. Chemical composition and acaricidal activity of Thymus Algeriensis essential oil against Varroa destructor. Nat. Prod. Commun. 2017, 12, 135–138. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Benabdelkader, T.; Chelghoum, C. Studies on the essential oil composition and antimicrobial activity of Thymus Algeriensis Boiss. et Reut. Int. J. Aromather. 2006, 16, 95–100. [Google Scholar] [CrossRef]
- Houmani, Z.; Azzoudj, S.; Naxakis, G.; Skoula, M. The essential oil composition of Algerian Zaâtar: Origanum spp. and Thymus spp. J. Herbs Spices Med. Plants 2002, 9, 275–280. [Google Scholar] [CrossRef]
- Hamza, O.; Fahima, A.; Hassani, A.A. Chemical composition, antioxidant activity of the essential oil of Thymus algeriensis Boiss, North Algeria. Int. Lett. Chem. Phys. Astron. 2015, 59, 72–80. [Google Scholar] [CrossRef]
- Mehalaine, S.; Belfadel, O.; Menasria, T.; Messaili, A. Chemical composition and antibacterial activity of essential oils of three medicinal plants from Algerian semi-arid climatic zone. Phytothérapie 2018, 16, S155–S163. [Google Scholar] [CrossRef]
- Kebbi, S.; Fadel, H.; Chalchat, J.; Figueredo, G.; Chalard, P.; Hazmoune, H.; Benayache, F.; Benayache, S.; Seghiri, R. Chemical composition of Algerian Thymus algeriensis Boiss. & Reut. and Marrubium vulgare L. (Lamiaceae) essential oils from the Aures Region. Acta Nat. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Jamali, C.A.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic Thyme (Thymus maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Goyal, S.; Pathak, R.; Pandey, H.K.; Kumari, A.; Tewari, G.; Bhandari, N.S.; Bala, M. Comparative study of the volatile constituents of Thymus Serpyllum L. grown at different altitudes of Western Himalayas. SN Appl. Sci. 2020, 2, 1208. [Google Scholar] [CrossRef]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of chemical composition of essential oils in Wild populations of Thymus Algeriensis Boiss. et Reut., a North African endemic species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Rustaiee, A.R.; Yavari, A.; Nazeri, V.; Shokrpour, M.; Sefidkon, F.; Rasouli, M. Genetic diversity and chemical polymorphism of some Thymus Species. Chem. Biodivers. 2013, 10, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in terpene profiles of Thymus vulgaris in water deficit stress response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Smékalová, K.; Kaffková, K.; Štefelová, N. Influence of post-harvesting period on quality of Thyme and Spearmint essential oils. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100335. [Google Scholar] [CrossRef]
- Rowshan, V.; Bahmanzadegan, A.; Saharkhiz, M.J. Influence of storage conditions on the essential oil composition of Thymus daenensis Celak. Ind. Crops Prod. 2013, 49, 97–101. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Javidnia, K.; Majzoobi, M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from Thymus vulgaris L. Innov. Food Sci. Emerg. Technol. 2012, 14, 85–91. [Google Scholar] [CrossRef]
- Guesmi, F.; ben Ali, M.; Barkaoui, T.; Tahri, W.; Mejri, M.; Ben-Attia, M.; Bellamine, H.; Landoulsi, A. Effects of Thymus Hirtus sp. algeriensis Boiss. & Reut. (Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids Health Dis. 2014, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Ben Hadj Ahmed, S.; Sghaier, R.M.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaali, I. Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the Leishmaniasis-endemic region of Sned (Tunisia). Nat. Prod. Res. 2011, 25, 1195–1201. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus Algeriensis Boiss. & Reut. Ind. Crops Prod. 2015, 77, 631–639. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; ben Ali, M.; Ismail, I.A.; Landoulsi, A. Thymus hirtus sp. algeriensis Boiss. & Reut. volatile oil enhances TRAIL/Apo2L induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging 2021, 13, 21975. [Google Scholar] [CrossRef] [PubMed]
- Touhami, A.; Chefrour, A.; Khellaf, N.; Bukhari, A.; Fadel, I. phytochemical characterization of the essential oils obtained from Mediterranean Thymus spp. (Lamiaceae) harvested at different stages of growth. J. Pharm. Pharmacol. 2017, 5, 37–45. [Google Scholar] [CrossRef]
- Labiad, M.H.; Belmaghraoui, W.; Ghanimi, A.; El-Guezzane, C.; Chahboun, N.; Harhar, H.; Egea-Gilabert, C.; Zarrouk, A.; Tabyaoui, M. Biological properties and chemical profiling of essential oils of Thymus (vulgaris, algeriensis and broussonettii) grown in Morocco. Chem. Data Collect. 2022, 37, 100797. [Google Scholar] [CrossRef]
- Salhi, A.; Bouyanzer, A.; El Mounsi, I.; Bendaha, H.; Hamdani, I.; El Ouariachi, E.; Chetouani, A.; Chahboun, N.; Hammouti, B.; Desjobert, J.M.; et al. Chemical composition, anti-oxidant and anticorrosive activities of Thymus algeriensis. J. Mater. Environ. Sci. 2016, 7, 3949–3960. [Google Scholar]
- El Ouariachi, E.M.; Hamdani, I.; Bouyanzer, A.; Hammouti, B.; Majidi, L.; Costa, J.; Paolini, J.; Chetouani, A. Chemical composition and antioxidant activity of essential oils of Thymus broussonetii Boiss. and Thymus algeriensis Boiss. from Morocco. Asian Pac. J. Trop. Dis 2014, 4, 281–286. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus Algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Croteau, R.; Gershenzon, J. Genetic control of monoterpene biosynthesis in Mints (Mentha: Lamiaceae). In Genetic Engineering of Plant Secondary Metabolism; Ellis, B.E., Kuroki, G.W., Stafford, H.A., Eds.; Recent Advances in Phytochemistry; Springer: Boston, MA, USA, 1994; Volume 28, pp. 193–229. [Google Scholar] [CrossRef]
- Osbourn, A.; Lanzotti, V. Plant-Derived Natural Products, Synthesis, Function, and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; p. 597. ISBN 978-0-387-85497-7 158. [Google Scholar] [CrossRef]
- Ifuku, O. Botanical ingredients. In Cosmetic Science and Technology: Theoretical Principles and Applications; Kazutami, S., Robert, Y.L., Howard, I.M., Yuji, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 305–320. [Google Scholar] [CrossRef]
- Saito, K.; Matsuda, F. Metabolomics for functional genomics, systems biology, and biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Claudiu, T.S. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; de Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the con-text of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef]
- Scossa, F.; Fernie, A.R. The evolution of metabolism: How to test evolutionary hypotheses at the genomic level. Comput. Struct. Biotechnol. J. 2020, 18, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation. In Potential of Essential Oils; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Savitskaya, T.A.; Grinshpan, D.D. Medicinal Herbs: Important Source of Bioactive Compounds for Food Industry. In Herbs and Spices—New Processing Technologies; Ahmad, R.S., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Waqas, S.; Akram, M.; Panda, A.K.; Elbossaty, W.F.; Hegazil, A.G.; Ghasemian, A.; Aharwal, R.P.; Chelladurai, G.; Mandal, S.K.; Mbaye, E.H.S.; et al. Current trends and future prospect of medicinal plants de-rived nutraceuticals: A review. Curr. Trends Pharm. Pharm. Chem. 2022, 4, 30–34. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Rabadi, G.J.; Tranchant, C.C.; Almajwal, A.; Kubow, S.; Alli, I. Occurrence, types, properties and interactions of phenolic compounds with other food constituents in oil-bearing plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 3209–3218. [Google Scholar] [CrossRef]
- Pereira, O.R.; Cardoso, S.M. Overview on Mentha and Thymus polyphenols. Curr. Anal. Chem. 2013, 9, 382–396. [Google Scholar] [CrossRef]
- Leal, F.; Taghouti, M.; Nunes, F.; Silva, A.; Coelho, A.C.; Matos, M. Thymus plants: A review—Micropropagation, molecular and antifungal activity. In Active Ingredients from Aromatic and Medicinal Plants; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, chemotaxonomy, ethnopharmacology, and nutraceutics of Lamiaceae. Stud. Nat. Prod. Chem. 2019, 62, 125–178. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. the potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Gâvan, A.; Iacoviță, C.; Pinela, J.; Barros, L.; Ferreira, I.C.F.R.; Zhang, L.; Lucini, L.; Rocchetti, G.; et al. Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. Ex Griseb. et Schenk (Wild Thyme) and their bioactive potential. Ultrason. Sonochem. 2022, 84, 105954. [Google Scholar] [CrossRef] [PubMed]
- Barbouti, A.; Goulas, V. Dietary antioxidants in the Mediterranean diet. Antioxidants 2021, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- Küçükaydın, S.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. HPLC-DAD Phytochemical profiles of Thymus cariensis and T. cilicicus with antioxidant, cytotoxic, anticholinesterase, anti-urease, anti-tyrosinase, and antidiabetic activities. S. Afr. J. Bot. 2021, 143, 155–163. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G. Polyphenolic and molecular variation in Thymus species using HPLC and SRAP Analyses. Sci. Rep. 2021, 11, 5019. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange Thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. 2021, 12, 100171. [Google Scholar] [CrossRef]
- Boga, M.; Ozkan, E.E.; Ersoy, E.; Tuncay, E.; Canturk, Y.Y.; Cinar, E.; Kara, E.M.; Zengin, G. Identification and quantification of phenolic and volatile constituents in five different Anatolian Thyme species using LC–MS/MS and GC-MS, with biological activities. Food Biosci. 2021, 43, 101141. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus Algeriensis Boiss. & Reut. and Ephedra alata Decne. Int. Food Res. J. 2019, 116, 312–319. [Google Scholar] [CrossRef]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Carradori, S.; Cesa, S.; Giusti, A.M.; Campestre, C.; Menghini, L.; Innosa, D.; et al. Qualitative and quantitative phytochemical analysis of different extracts from Thymus algeriensis aerial parts. Molecules 2018, 23, 463. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a Rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Yin, H.; Zou, L.; Sheng, Y.; Bai, X.; Liu, Q.; Yan, B. Rapid HPLC analytical method development for herbal med-icine formulae based on retention rules acquired from the constituting herbs. Anal. Sci. 2018, 34, 207–214. [Google Scholar] [CrossRef]
- Watson, R. (Ed.) Polyphenols in Plants: Isolation, Purification and Extract Preparation; Academic Press: Cambridge, MA, USA, 2018; p. 442. ISBN 9780128137697. [Google Scholar] [CrossRef]
- Righi, N.; Boumerfeg, S.; Fernandes, P.A.R.; Deghima, A.; Baali, F.; Coelho, E.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Thymus algeriensis Bioss & Reut: Relationship of phenolic compounds composition with in vitro/in vivo antioxidant and antibacterial activity. Int. Food Res. J. 2020, 136, 109500. [Google Scholar] [CrossRef]
- Messaoudi, M.; Benreguieg, M.; Merah, M.; Messaoudi, Z.A. Antibacterial effects of Thymus algeriensis extracts on some pathogenic bacteria. Acta Sci. Biol. Sci. 2019, 41, 48548. [Google Scholar] [CrossRef]
- Benabdallah, F.Z.; Zellagui, A.; Demirtas, I. Chemical composition of essential oils and antioxidant activities of extracts of two endemic plants from Algeria. Int. J. Pharm. Sci. Res. 2017, 13, 244–250. [Google Scholar]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Gross, G.G. Biosynthesis and metabolism of phenolic acids and monolignols. In Biosynthesis and Biodegradation of Wood Components; Takayoshi, H., Ed.; Academic Press: Kyoto, Japan, 1985; pp. 229–271. [Google Scholar] [CrossRef]
- Zampelas, A.; Micha, R. (Eds.) Antioxidants in Health and Disease; CRC Press: Boca Raton, FL, USA, 2015; 341p. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Appl. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Jamshidi-Kia, F.; Hosseini, Z. Analysis of aromatic acids (phenolic acids and hydroxycinnamic acids). In Recent Advances in Natural Products Analysis; Ana, S.S., Seyed, F.N., Mina, S., Seyed, M.N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 199–219. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Chapter 13—Phenolic acids from plants: Extraction and application to human health. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 58, pp. 389–417. [Google Scholar]
- Clifford, M.N. Review Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents—A Review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Ait Kaki, F.; Benkiniouar, R.; Demirtas, I.; Merzoug, A.; Touil, A. Phytochemical study of two Algerian plants Origanum vulgare L. Sbsp. Glandulosum (Desf) Ietswaart and Thymus algeriensis (Boiss. and Reut). Asian J. Chem. 2019, 31, 1105–1109. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Extraction techniques for the determination of phenolic compounds in food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 4, pp. 159–180. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic content of brown Algae (Phe-ophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Seeram, N.P. Development and UFLC-MS/MS characterization of a product-specific standard for phenolic quantification of maple-derived foods. J. Agric. Food Chem. 2016, 64, 3311–3317. [Google Scholar] [CrossRef]
- Michiu, D.; Socaciu, M.I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an analytical method for spectrophotometric evaluation of total phenolic content in essential oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Chen, H.; Song, X.; Huang, X. Development of magnetism-assisted in-tube solid phase microextraction of phenolic acids in fruit juices prior to high-performance liquid chromatography quantification. J. Sep. Sci. 2021, 44, 3418–3428. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Cheurfa, M.; Abdelfattah, M.A.O.; Rashied, R.M.H.; El-Shazly, A.M.; Yasri, A.; Wink, M.; Mahmoud, M.F. Thymus algeriensis and Thymus fontanesii: Chemical composition, in vivo antiinflammatory, pain killing and antipyretic activities: A comprehensive comparison. Biomolecules 2020, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Valentão, P.; Pereira, J.A.P.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in Mda-Mb-231 and Mcf-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Fatma, G.; Sami, B.H.A.; Ahmed, L.; Fatma, G.; Sami, B.H.A.; Ahmed, L. Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic Acids and Human Health: Recent Advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Edraki, N.; Kamat, S.P.; Khoshneviszadeh, M.; Kayani, Z.; Mirzaei, H.H.; Miri, R.; Erfani, N.; Nejati, M.; Cavaleiro, J.A.S.; et al. Long Chain alkyl esters of hydroxycinnamic acids as promising anticancer agents: Selective induction of apoptosis in cancer cells. J. Agric. Food Chem. 2017, 65, 7228–7239. [Google Scholar] [CrossRef]
- Rocha, L.D.; Monteiro, M.C.; Teodoro, A.J. Anticancer Properties of Hydroxycinnamic Acids—A Review. Cancer Clin. Oncol. 2012, 1, 109. [Google Scholar] [CrossRef]
- Markham, K.R.; Andersen, O.M. Flavonoids. Chemistry, Biochemistry and Applications; Markham, K.R., Andersen, O.M., Eds.; CRC Press: Boca Raton, FL, USA, 2006; p. 1256. ISBN 9780849320217. [Google Scholar] [CrossRef]
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Enzymology and molecular biology. In Advances in Enzymology and Related Areas of Molecular Biology; John Wiley & Sons: Hoboken, NJ, USA, 1983; Volume 55, pp. 1–136. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and isoflavonoids: From plant biology to agriculture and neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 1–276. [Google Scholar] [CrossRef]
- Martens, S.; Preuß, A.; Matern, U. Multifunctional flavonoid dioxygenases: Flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of Rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The Effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of Basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Ganie, S.H.; Upadhyay, P.; Das, S.; Prasad Sharma, M. Authentication of medicinal plants by dna markers. Plant Gene 2015, 4, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Int. Food Res. J. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of flavonoids in management of inflammatory disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Ronald, R.W., Victor, R.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 293–322. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Lima, J.L.F.C.; Fernandes, E. Proinflammatory pathways: The modulation by flavonoids. Med. Res. Rev. 2015, 35, 877–936. [Google Scholar] [CrossRef]
- Celik, S.; Cakir, E.; Akyuz, S.; Ozel, A.E. Flavonoids: Their Anticarcinogenic Effects and Molecular Modeling Studies. In Handbook of Research on Natural Products and Their Bioactive Compounds as Cancer Therapeutics; IGI Global: Hershey, PA, USA, 2022; pp. 265–296. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anti-cancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Hoensch, H.P.; Oertel, R. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nutr. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Wright, B.; Spencer, J.P.E.; Lovegrove, J.A.; Gibbins, J.M. Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs. Cardiovasc. Res. 2013, 97, 13–22. [Google Scholar] [CrossRef]
- Gherairia, N.; Boukerche, S.; Chouikh, A.; Khoudir, S.; Chefrour, A. Antibacterial activity of essential oils from two species of genus Thymus growing in different sites of north-eastern Algerian. An. Univ. Din Oradea Fasc. Biol. 2019, 26, 100–104. [Google Scholar]
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Cheese supplemented with thymus algeriensis oil, a potential natural food preservative. Int. J. Dairy Sci. 2018, 101, 3859–3865. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Rezq, S.; Alsemeh, A.E.; D’Elia, L.; El-Shazly, A.M.; Monti, D.M.; Sobeh, M.; Mahmoud, M.F. Thymus algeriensis and Thymus fontanesii exert neuroprotective effect against chronic constriction injury-induced neuropathic pain in rats. Sci. Rep. 2020, 10, 20559. [Google Scholar] [CrossRef] [PubMed]
- Ben Haj Yahia, I.; Jaouadi, R.; Trimech, R.; Boussaid, M.; Zaouali, Y. Variation of chemical composition and antioxidant activity of essential oils of Mentha x rotundifolia (L.) Huds. (Lamiaceae) collected from different bioclimatic areas of Tunisia. Biochem. Syst. Ecol. 2019, 84, 8–16. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Marco, G.J. A Rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimi-crobial activities of Tilia (Tilia argentea Desf Ex DC), Sage (Salvia triloba l.), and Black Tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from Northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Dong, J.-W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.-T. Re-evaluation of ABTS•+ assay for total antioxidant capacity of natural products. Nat. Prod. Commun. 2015, 10, 1934578X1501001239. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. standardized methods for the determination of antioxidant capacity and phe-nolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Yeretzian, C. Methodology for the measurement of antioxidant capacity of coffee: A validated platform composed of three complementary antioxidant assays. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 253–264. [Google Scholar] [CrossRef]
- Ratnavathi, C.V.; Komala, V.V. Sorghum grain quality. In Sorghum Biochemistry: An Industrial Perspective; Ratnavathi, C.V., Patil, J.V., Chavan, U.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–61. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Elasri, O. Phytochemical screening and antioxidant activities of different organic extracts of three Algerian plants. J. Drug Deliv. Ther. 2020, 10, 75–79. [Google Scholar] [CrossRef]
- Halvorsen, B.; Holte, K.; Mari, C.W.; Barikmo, I.; Hvattum, E.; Fagertun, R.S.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 287–333. [Google Scholar] [CrossRef]
- Kumar, S.; Krishna Chaitanya, R.; Preedy, V.R. Assessment of antioxidant potential of dietary components. In HIV/AIDS: Oxidative Stress and Dietary Antioxidants; Preedy, V., Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–253. [Google Scholar] [CrossRef]
- Naghdi Badi, H.; Yazdani, D.; Ali, S.M.; Nazari, F. Effects of spacing and harvesting time on herbage yield and quality/quantity of oil in Thyme, Thymus vulgaris L. Ind. Crops Prod. 2004, 19, 231–236. [Google Scholar] [CrossRef]
- Sarveswaran, R.; Jayasuriya, W.; Suresh, T. In vitro assays to investigate the anti-inflammatory activity of herbal extracts a review. World J. Pharm. Res. 2017, 6, 131. [Google Scholar] [CrossRef]
- Candelise, N.; Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C.; Manganelli, V.; Garofalo, T.; Sorice, M.; Misasi, R. Protein aggregation landscape in neurodegenerative diseases: Clinical relevance and future applications. Int. J. Mol. Sci. 2021, 22, 6016. [Google Scholar] [CrossRef]
- Horwich, A. Protein aggregation in disease: A role for folding intermediates forming specific multimeric interactions. J. Clin. Investig. 2002, 110, 1221–1232. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- Eze, F.I.; Uzor, P.F.; Ikechukwu, P.; Obi, B.C.; Osadebe, P.O. In vitro and in vivo models for anti-inflammation: An evaluative review. INNOSC Theranost. Pharmacol. Sci. 2019, 2, 755. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immuno-pathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5896. [Google Scholar] [CrossRef] [PubMed]
- Garscha, U.; Romp, E.; Pace, S.; Rossi, A.; Temml, V.; Schuster, D.; König, S.; Gerstmeier, J.; Liening, S.; Werner, M.; et al. Pharmacological profile and efficiency in vivo of Diflapolin, the first dual inhibitor of 5-lipoxygenase-activating protein and soluble epoxide hydrolase. Sci. Rep. 2017, 7, 9398. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, J.; Hu, Y.; Lu, W.; Zhang, X.; Wang, R.; Chu, K. Rosmarinic Acid Mitigates lipopolysaccharide induced neuroinflammatory responses through the inhibition of TLR4 and CD14 Expression and NF-ΚB and NLRP3 inflammasome activation. Inflammation 2018, 41, 732–740. [Google Scholar] [CrossRef]
- Opal, S.M.; van der Poll, T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef]
- Kumar, T.; Jain, V. Antinociceptive and anti-inflammatory activities of Bridelia retusa methanolic fruit extract in experimental animals. Sci. World J. 2014, 2014, 890151. [Google Scholar] [CrossRef]
- Do Malvar, D.C.; Aguiar, F.A.; Vaz, A.d.L.L.; Assis, D.C.R.; de Melo, M.C.C.; Jabor, V.A.P.; Kalapothakis, E.; Ferreira, S.H.; Clososki, G.C.; de Souza, G.E.P.; et al. Dipyrone metabolite 4-MAA induces hypothermia and inhibits PGE2-dependent and -independent Fever While 4-AA Only Blocks PGE2-dependent Fever. Br. J. Pharmacol. 2014, 171, 3666–3679. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic Acid for Analgesic Screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Chapman, C.R.; Casey, K.L.; Dubner, R.; Foley, K.M.; Gracely, R.H.; Reading, A.E. Pain measurement: An overview. Pain 1985, 22, 1–31. [Google Scholar] [CrossRef]
- Witkin, L.B.; Heubner, C.F.; Galdi, F.; O’Keefe, E.; Spitaletta, P.; Plummer, A.J. Pharmacology of 2-amino-indane hydrochloride (SU-8629): A potent non-narcotic analgesic. J. Pharmacol. Exp. Ther. 1961, 133, 400–408. [Google Scholar]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.P.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by Zymosan and Acetic Acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Liu, J.A.; Yu, J.; Cheung, C.W. Immune actions on the peripheral nervous system in pain. Int. J. Mol. Sci. 2021, 22, 1448. [Google Scholar] [CrossRef]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic compounds that cross the blood–brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021, 12, 10356–10369. [Google Scholar] [CrossRef]
- Jäger, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; dos Santos Tavares, D.; Guimarães, A.G. Antitumor effects of carvacrol and thymol: A systematic review. Front. Pharmacol. 2021, 12, 1673. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Small, D.H.; Avila, J.; Sáez-Valero, J. Revisiting the role of Acetylcholinesterase in Alzheimer’s disease: Cross-talk with β-Tau and p-Amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef]
- Richbart, S.D.; Merritt, J.C.; Nolan, N.A.; Dasgupta, P. Acetylcholinesterase and human cancers. Adv. Cancer Res. 2021, 152, 1–66. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet 1977, 309, 189. [Google Scholar] [CrossRef]
- Kaduszkiewicz, H.; Zimmermann, T.; Beck-Bornholdt, H.P.; van Bussche, H. Cholinesterase inhibitors for patients with Alzheimer’s disease: Systematic review of randomised clinical trials. BMJ 2005, 331, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Long-term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer’ disease. J. Neural Transm. Suppl. 2002, 62, 181–187. [Google Scholar] [CrossRef]
- Cohen, S.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Bannister, K.; Sachau, J.; Baron, R.; Dickenson, A.H. Neuropathic pain: Mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 257–274. [Google Scholar] [CrossRef]
- Modzelewska, B.; Drygalski, K.; Kleszczewski, T.; Chomentowski, A.; Koryciński, K.; Kiełczewska, A.; Pawłusze-wicz, P.; Razak Hady, H. Quercetin relaxes human gastric smooth muscles directly through ATP-Sensitive potassium channels and not depending on the nitric oxide pathway. Neurogastroenterol. Motil. 2021, 33, e14093. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghanadian, M.; Asghari, G.; Sekhavati, N. Antispasmodic activity of apigenin and luteolin, two components of Dracocephalum kotschyi extract, on rat ileum contractions. J. HerbMed Pharmacol. 2018, 7, 100–105. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Chengala, L.; Singh, N. Botanical pesticides—A Major alternative to chemical pesticides: A Review. Int. J. Life Sci. 2017, 5, 722–729. [Google Scholar]
- Amoabeng, B.W.; Johnson, A.C.; Gurr, G.M. Natural enemy enhancement and botanical insecticide source: A review of dual use companion plants. Appl. Entomol. Zool. 2019, 54, 1–19. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Mehaoua, M.S.; Hadjeb, A.; Lagha, M.; Bensalah, M.K.; Ouakid, M.L. Study of the toxicity of Azadira chtinon larval mortality and fertility of Carob Moth’s Female Ectomyelois ceratoniae (Lepidoptera, Pyralidae) under Controlled. Am.-Eurasian J. Sustain. Agric. 2013, 7, 1–9. [Google Scholar]
- Credland, P.F. The structure of bruchid eggs may explain the ovicidal effect of oils. J. Stored Prod. Res. 1992, 28, 1–9. [Google Scholar] [CrossRef]
- Abdullahi, N.; Majeed, Q.; Oyeyi, T.I. Studies on the efficacy of Vittallaria paradoxa seed oil on the oviposition, hatchability of eggs and emergence of Callasobruchus maculatus (F.) (Coleoptera: Bruchidae) on treated cow-pea seed. J. Entomol. 2011, 8, 391–397. [Google Scholar] [CrossRef][Green Version]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A.; Saritha, V.; Duraipandiyan, V.; Muthukumar, C.; Kim, S.J. Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol. 2013, 13, 105. [Google Scholar] [CrossRef]
- Das Graças Freire De Medeiros, M.; da Silva, A.C.; das Graças Lopes Citó, A.M.; Borges, A.R.; de Lima, S.G.; Lopes, J.A.D.; Figueiredo, R.C.B.Q. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef]
- Karin Lima, R.; das Graças Cardoso, M.; Campos Moraes, J.; Malfitano Carvalho, S.; Gregório Rodrigues, V.; Gustavo Lima Guimarães, L. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham. and monoterpenes against Tenebrio molitor (L.) (Coleoptera: Tenebrionidae). Ciênc. Agrotec. 2011, 35, 664–671. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on Acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef] [PubMed]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 871, 1579. [Google Scholar] [CrossRef]
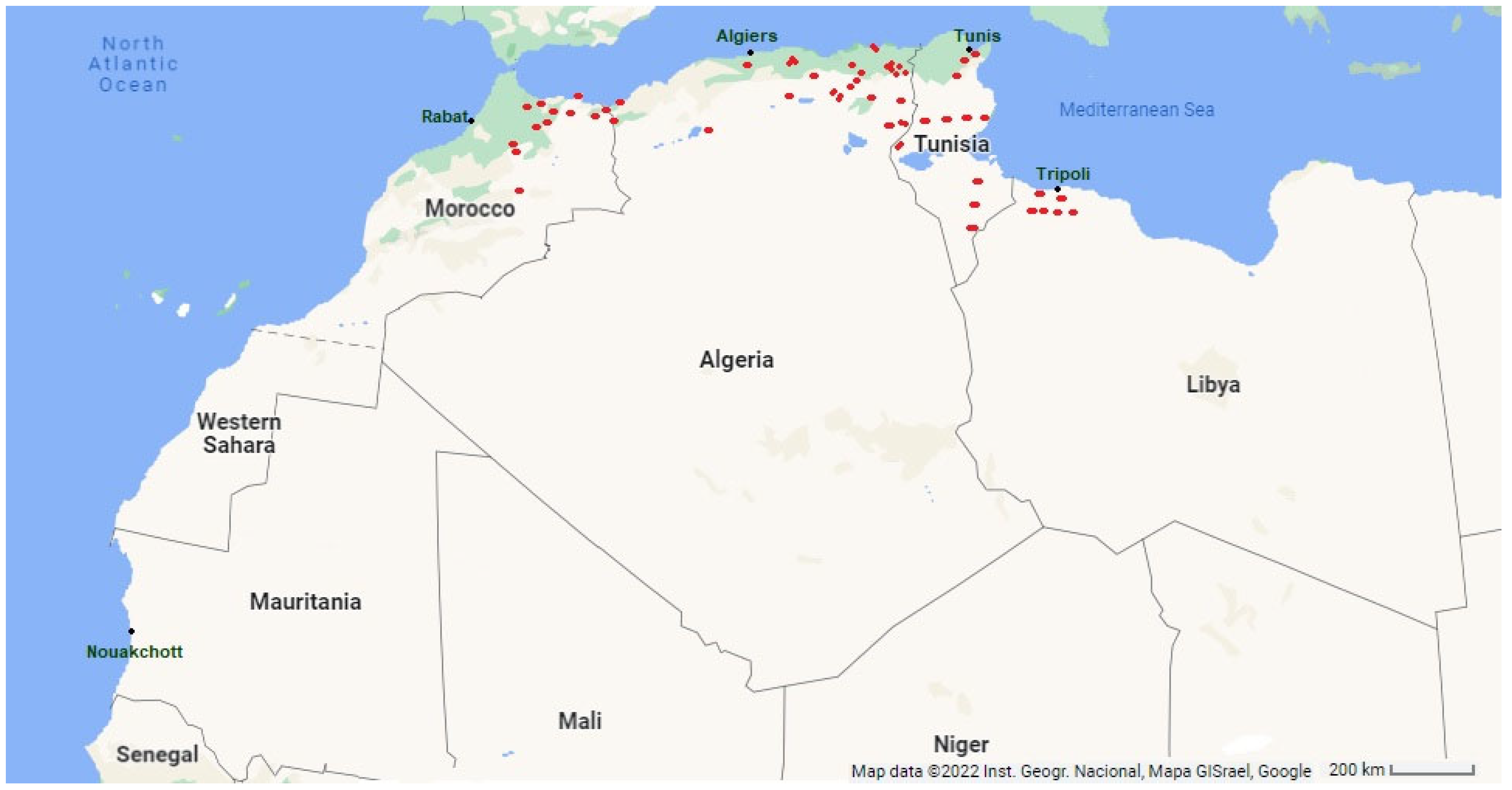


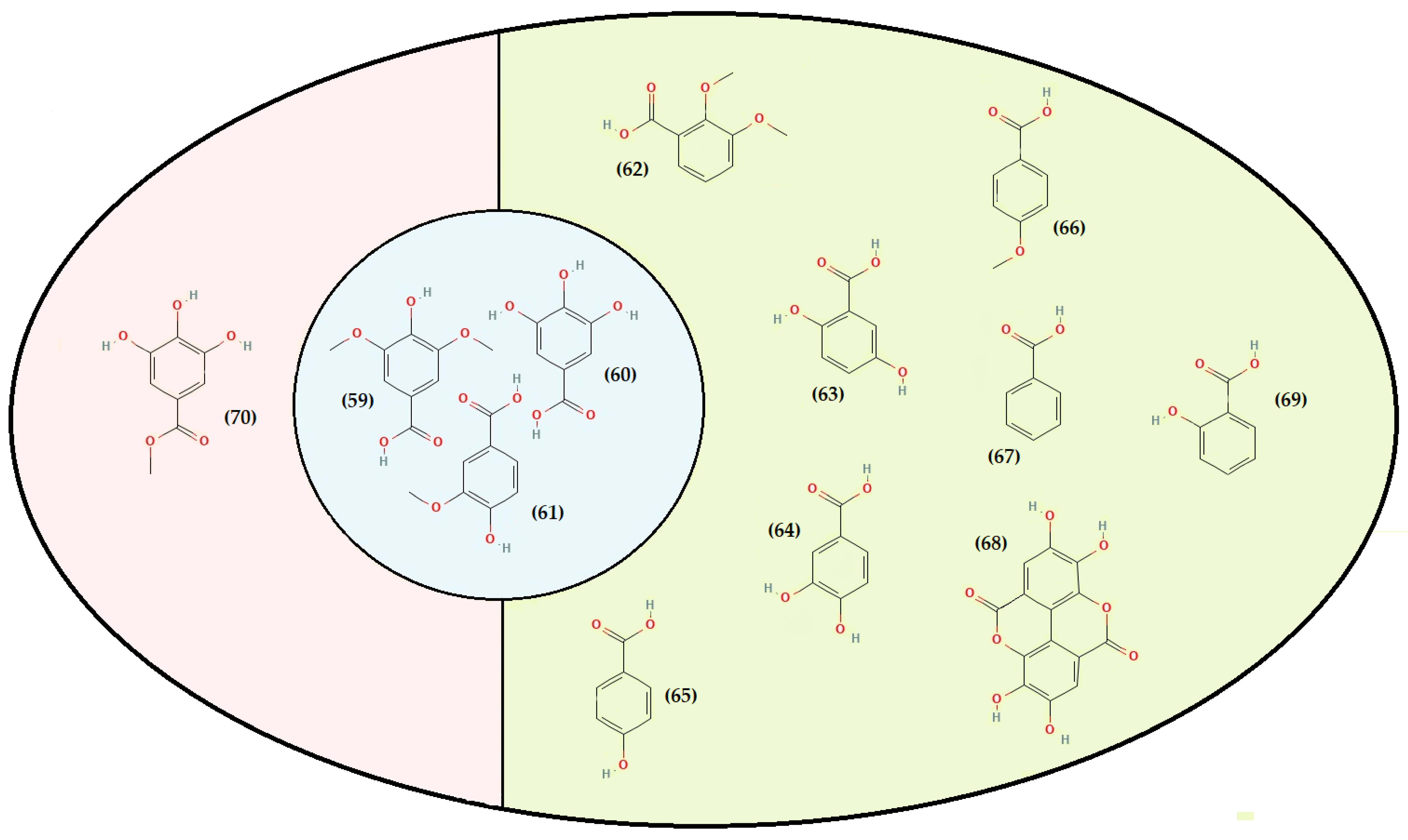
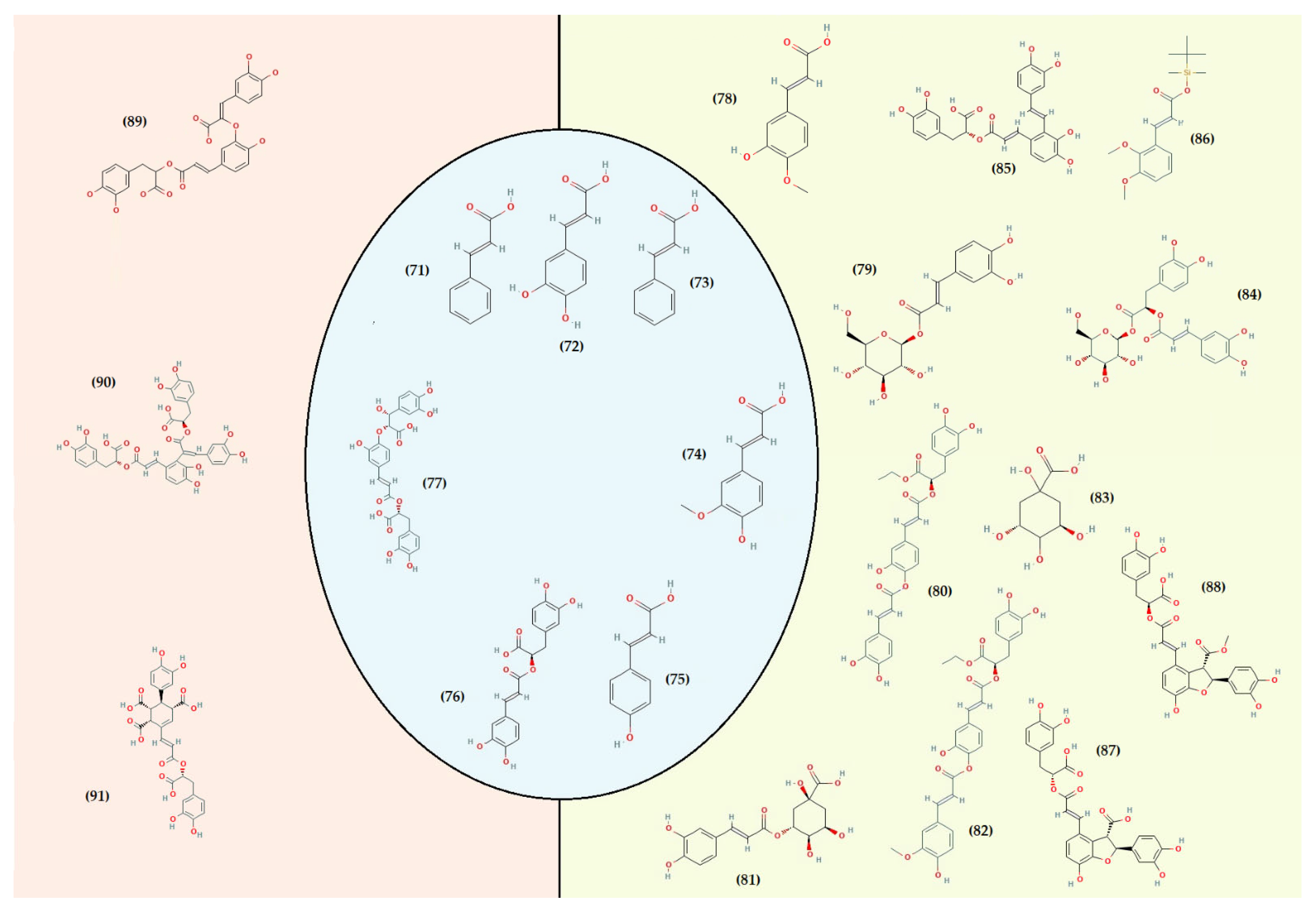
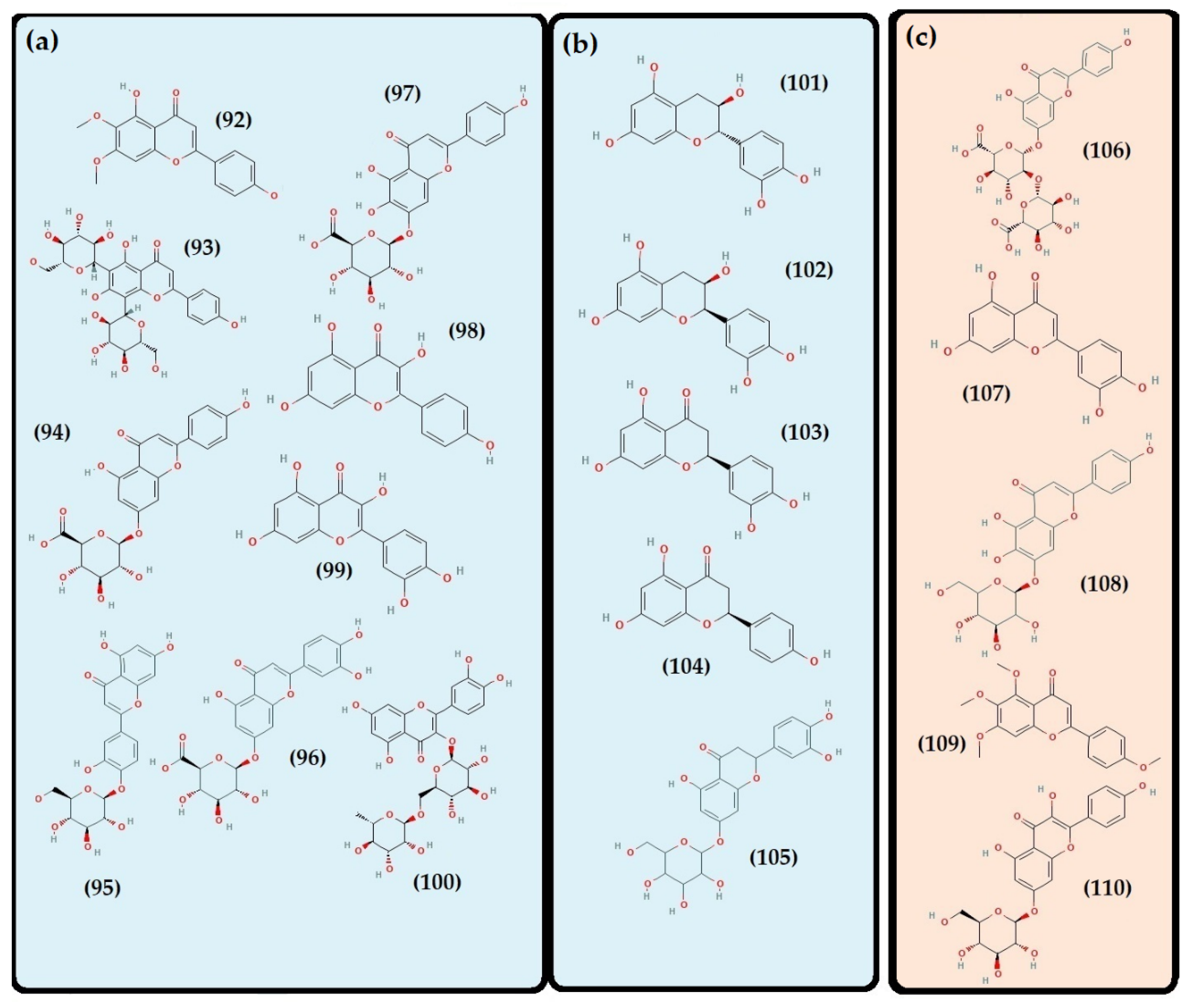
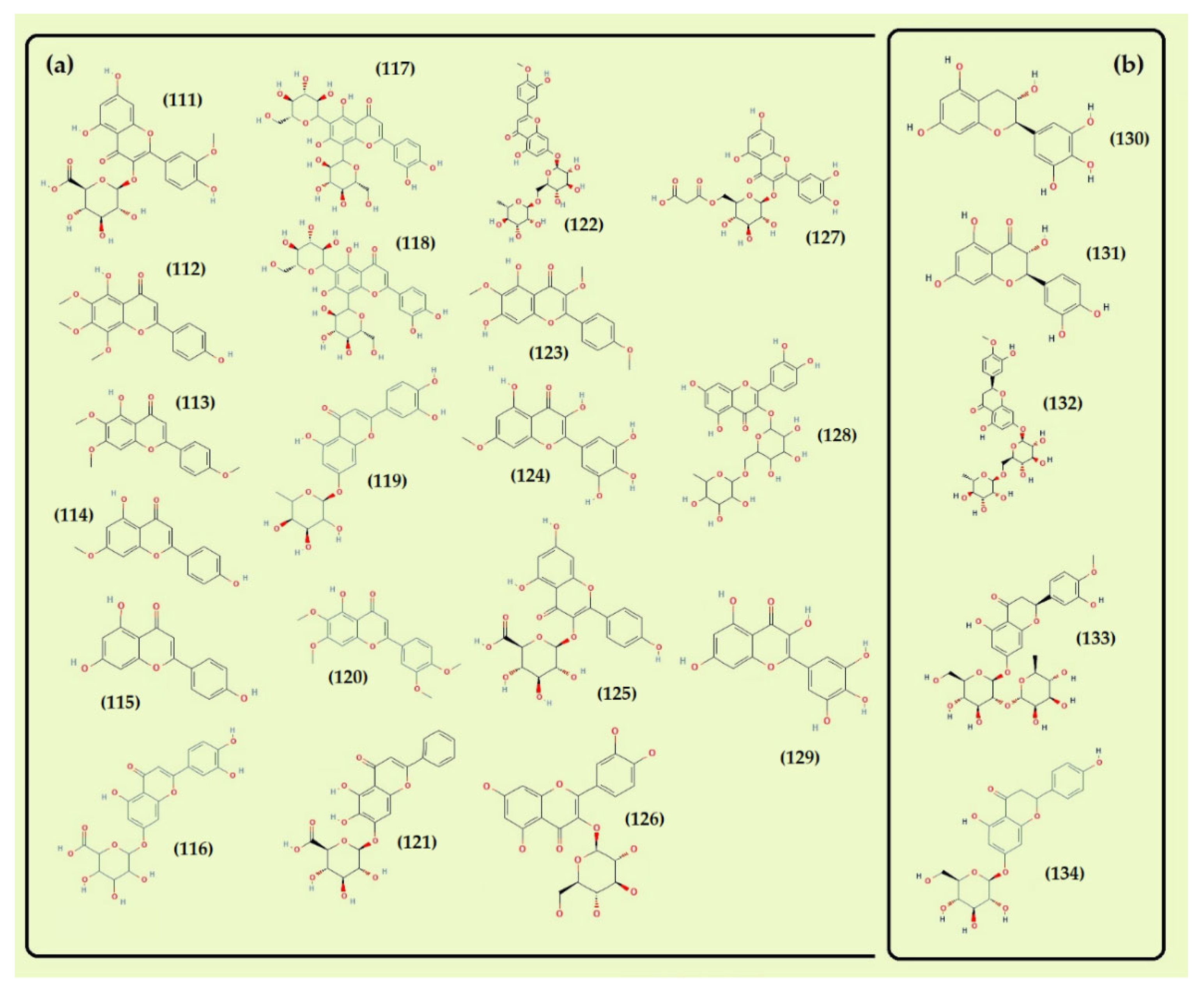
| * PP | Product | Preparation Method | TPC | TFC | TPA | FLC | TAC | TTCs | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Algeria | |||||||||
| AP | PEE CH n-Bu | Mac: 1200 g in MEOH-H2O (80:20 v/v) at RT, Liquid-liquid extraction by PE, CHCl3, n-BuOH | n-Bu (318.07 ± 0.88 µg GAE/mg Ext) CH (161.78 ± 0.09 µg GAE/mg Ext) PEE (62.65 ± 0.56 µg GAE/mg Ext) | n-Bu (198.17 ± 0.12 µg QE/mg Ext) CH (9.77 ± 0.14 µg QE/mg Ext) PEE (8.57 ± 0.27 µg QE/mg Ext) | – | – | – | – | [58] |
| n.m | ME | 3 g in MEOH for 30 mn | 8.33 ± 1.15 mg GAE/g DW | 2.95 ± 0.25 mg QE/g DW | – | – | – | – | [60] |
| AP | MEH PS | (1) 1st Mac: 100 g Pow in 1 L MEOH-H2O (85:15, v/v) for 24 h and 2nd Mac: MEOH-H2O (50:50, v/v) for 24 h (2) Purification: the extract was suspended in water/acetic acid (97.5:2.5, v/v) at a ratio of 1:5 (w/v) and centrifuged at 20,000× g, followed by solid-phase extraction | MEH (304.00 ± 3.00 µg GAE/mg Ext) PS (451.00 ± 4.00 µg GAE/mg Ext) | MEH (16.00 ± 1.00 µg QE/mg Ext) PS (40.00 ± 2.00 µg QE/mg Ext) | – | MEHext (60.00 ± 2.00 (µg RE/mg Ext) PS (107.00 ± 2.00 µg RE/mg Ext) | – | MEH (105.00 ± 2.00 µg TA/mg Ext) PS (71.00 ± 9.00 TA/mg Ext) | [188] |
| L | ET | Mac: 15 g/100 mL ETOH (100%), Incubation in a water bath at 55 °C for 6 h | 125.00 ± 1.00 mg GAE/g DW | 118.00 ± 1.00 mg RE/g DW | – | – | – | – | [132] |
| AP | ET ME | Mac: 50 g/500 mL. ETOH 100% + agit Mac: 50 g/500 mL. MEOH 100% + agit | 67.13 mg GAE/g DW 79.45 mg GAE/g DW | 25.04 mg QE/g DW 36.18 mg QE/g DW | – | – | 8.14 mg C3 G/g DW 6.98 mg C3 G/g DW | [189] | |
| AP | INF DEC ETH | INF: 1 g/H2O 1:100 m/v (100 °C), 5 min at RT DEC: 1 g/100 mL H2O boiling 5 min ETH: Mac 1 g/30 Ml ETOH-H2O (80:20, v/v) at 25 °C (150 rpm 1 h) | INF (256.0 ± 0.2 mg/g Ext) DEC (245 ± 4 mg/g Ext) ETH (102.30 ± 0.50 mg/g Ext) | INF (127.50 ± 0.40 mg/g Ext) DEC (119.00 ± 2.00 mg/g Ext) ETH (43.80 ± 0.80 mg/g Ext) | INF (128.50 ± 0.20 mg/g Ext) DEC (126 ± 2.00 mg/g Ext) ETH (58.50 ± 0.30 mg/g Ext) | – | – | – | [183] |
| L | MEH | Mac (1): 200 g/25 mL MEOH-H2O (80:20, v/v) Mac (2): 2.5 g/2 L of MEOH | ME1 (1.38 ± 0001 mg GAE/g DW) | ME2 (0.34 ± 0.001 mg QE/g DW) | – | – | – | – | [190] |
| n.m | n.m | 1 g Pow in 7 mL of a hydro-alcoholic solution (70%), After sonication, the samples were centrifuged for 5 min, at 2000 g at 20 °C | 18.73 ± 4.59 mg GAE/g DW | – | (A) 8.07 ± 2.68 mg/g DW | (B) 2.10 ± 0.54 mg QE/g DW (C) 3.24 ± 0.60 mg/g DW | – | – | [191] |
| Morocco | |||||||||
| AP | AQ | n.m | 117.50 ± 6.30 mg GAE/g Pow | 17.31 ±0.08 mg QE/g Pow | – | 5.38 ± 0.08 mg QE/g Pow | – | – | [156] |
| Tunisia | |||||||||
| AP | MEH | Mac: 1 g/10 mL MEOH, 24 h | 34.40 ± 0.80 mg GAE/g DW | 10.60 ± 0.20 mg RE/g DW | – | – | – | – | [62] |
| AP | MEH | Mac 24 h | 500.00 ± 11.00 µg GAE/mg Ext | 180.00 ± 12.00 µg QE/mg Ext | – | – | – | 94.00 ± 8.00 µg CE/mg Ext | [57] |
| AP | MEH | 9 g in MEOH, 8 h (Soxhlet apparatus) | ME1 (7.08 ± 0.70 mg GAE/g DW) ME2 (8.70 ± 0.59 mg GAE/g DW) ME3 (8.81 ± 0.12 mg GAE/g DW) | ME1 (1.08 ± 0.80 mg RE/g DW) ME2 (1.95 ± 0.40 mg RE/g DW) ME3 (2.25 ± 0.43) mg RE/g DW) | – | – | – | – | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahlou, R.A.; Samba, N.; Soeiro, P.; Alves, G.; Gonçalves, A.C.; Silva, L.R.; Silvestre, S.; Rodilla, J.; Ismael, M.I. Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects. Foods 2022, 11, 3195. https://doi.org/10.3390/foods11203195
Lahlou RA, Samba N, Soeiro P, Alves G, Gonçalves AC, Silva LR, Silvestre S, Rodilla J, Ismael MI. Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects. Foods. 2022; 11(20):3195. https://doi.org/10.3390/foods11203195
Chicago/Turabian StyleLahlou, Radhia Aitfella, Nsevolo Samba, Pedro Soeiro, Gilberto Alves, Ana Carolina Gonçalves, Luís R. Silva, Samuel Silvestre, Jesus Rodilla, and Maria Isabel Ismael. 2022. "Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects" Foods 11, no. 20: 3195. https://doi.org/10.3390/foods11203195
APA StyleLahlou, R. A., Samba, N., Soeiro, P., Alves, G., Gonçalves, A. C., Silva, L. R., Silvestre, S., Rodilla, J., & Ismael, M. I. (2022). Thymus hirtus Willd. ssp. algeriensis Boiss. and Reut: A Comprehensive Review on Phytochemistry, Bioactivities, and Health-Enhancing Effects. Foods, 11(20), 3195. https://doi.org/10.3390/foods11203195











