Obtaining and Characterizing Andean Multi-Floral Propolis Nanoencapsulates in Polymeric Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
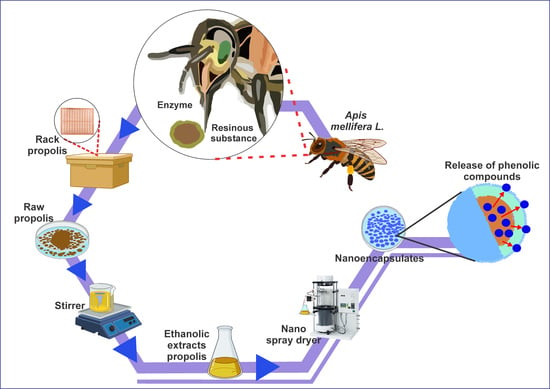
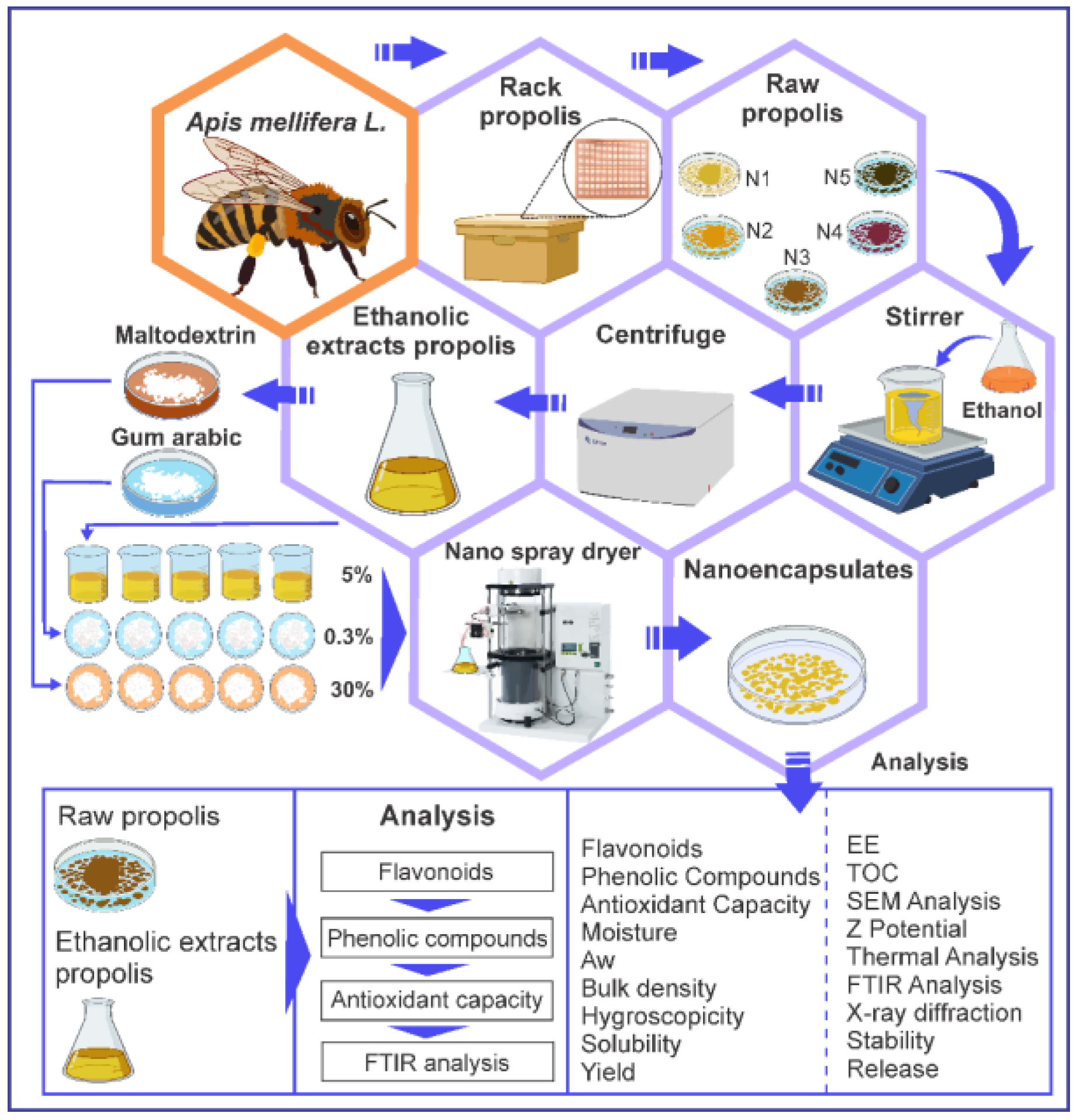
2.2. Ethanolic Extraction of Propolis
2.3. Nanoencapsulation of Ethanolic Extract of Propolis
2.4. Flavonoid Content
2.5. Total Phenolic Compound Content
2.6. Antioxidant Capacity by DPPH and ABTS Assays
2.7. Moisture, Water Activity (Aw), Bulk Density, and Color in Nanoencapsulates
2.8. Hygroscopicity, Solubility, Percentage of Yield, and Encapsulation Efficiency in Nanoencapsulates
2.9. Total Organic Carbon (TOC) in Nanoencapsulates
2.10. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) in Nanoencapsulates
2.11. Particle Size and ζ Potential in Nanoencapsulates
2.12. Thermal Analysis of Nanoencapsulates
2.13. Fourier Transform Infrared Spectroscopy (FTIR)
2.14. X-ray Diffraction (XRD) in Nanoencapsulates
2.15. Stability and Release of Phenolic Compounds in Nanoencapsulates
2.16. Statistical Analysis
3. Results and Discussion
3.1. Flavonoid Content, Phenolic Compounds, and Antioxidant Capacity using ABTS and DPPH
3.2. Characterization of Nanoencapsulates
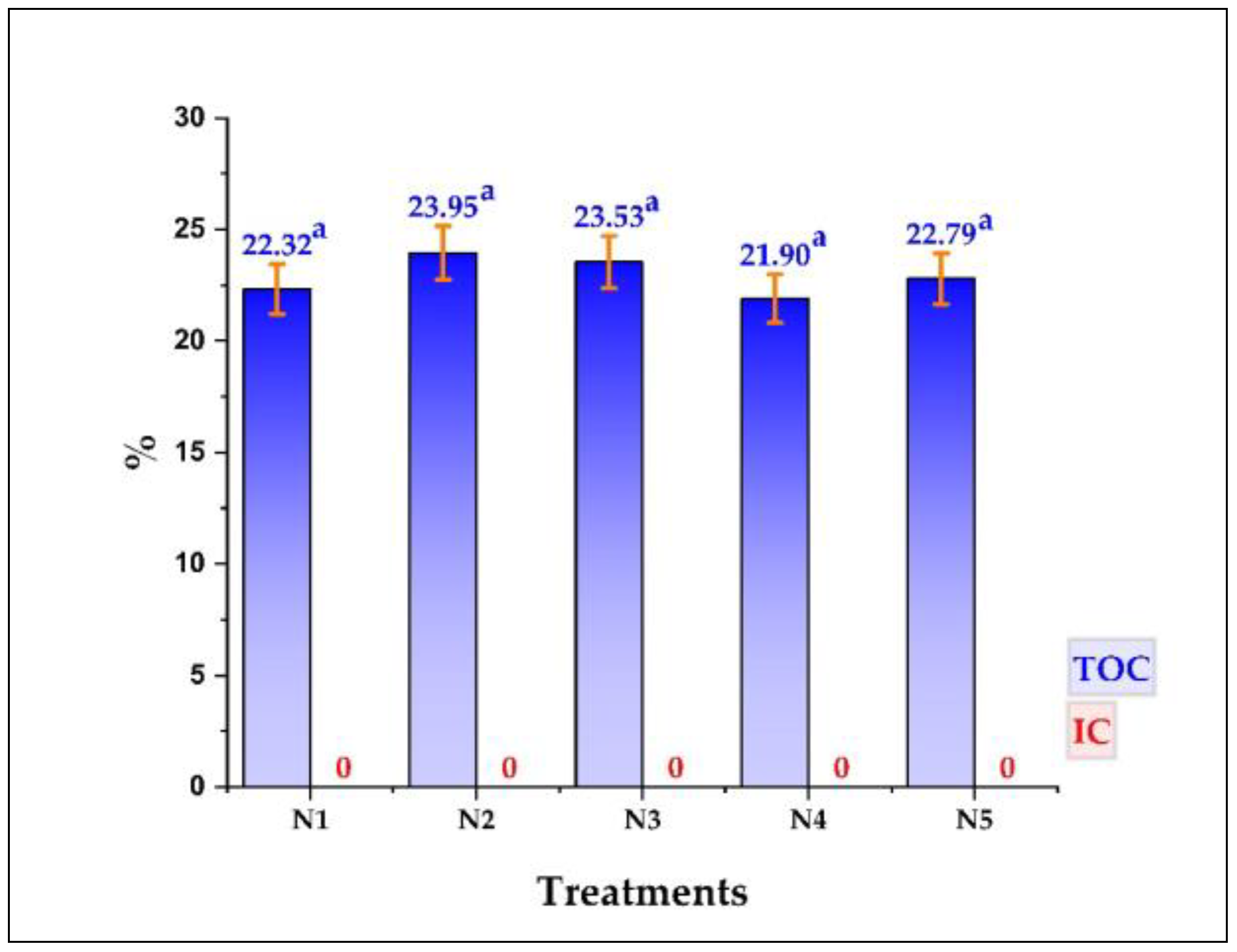
3.3. Total Organic Carbon (TOC) in Nanoencapsulates
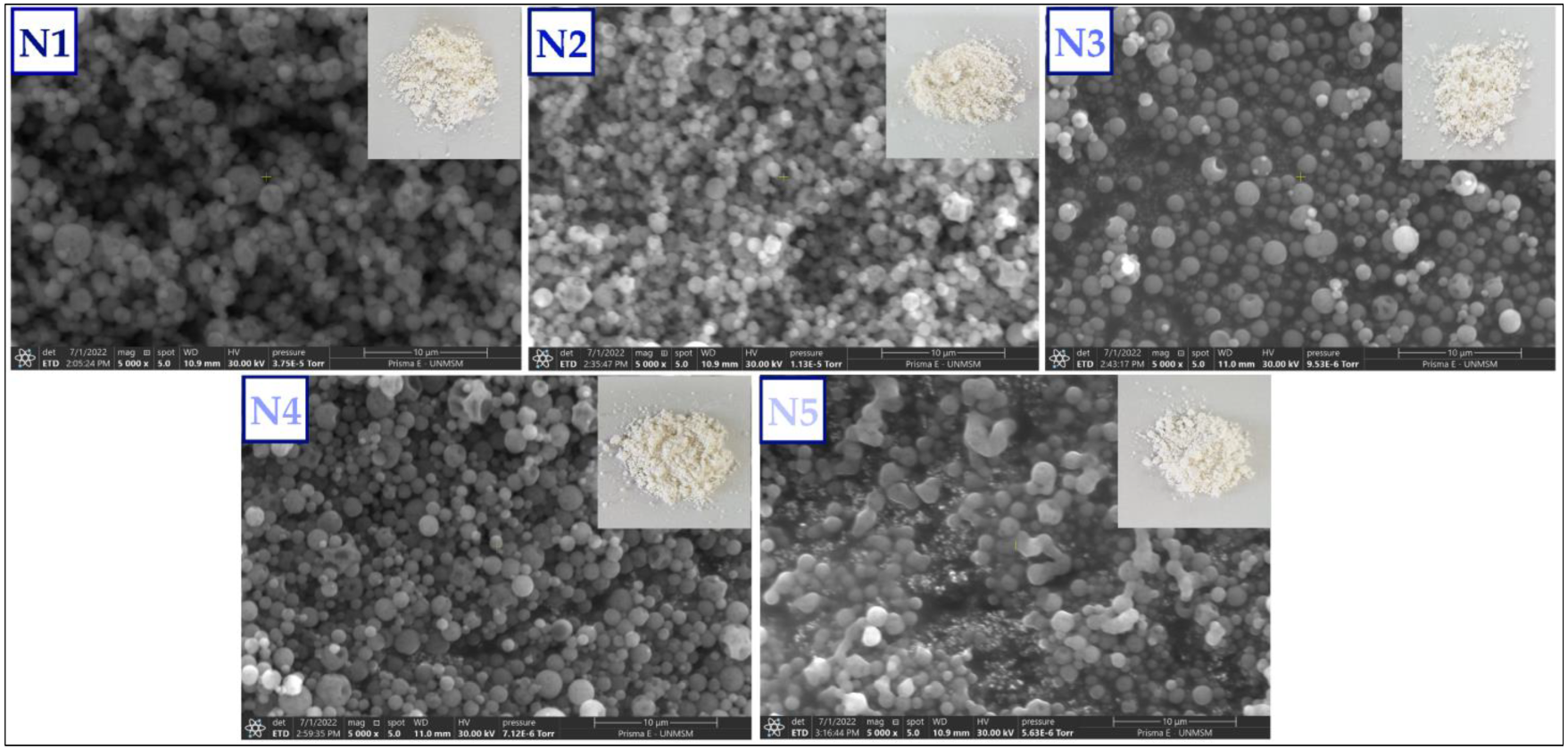
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) in Nanoencapsulates
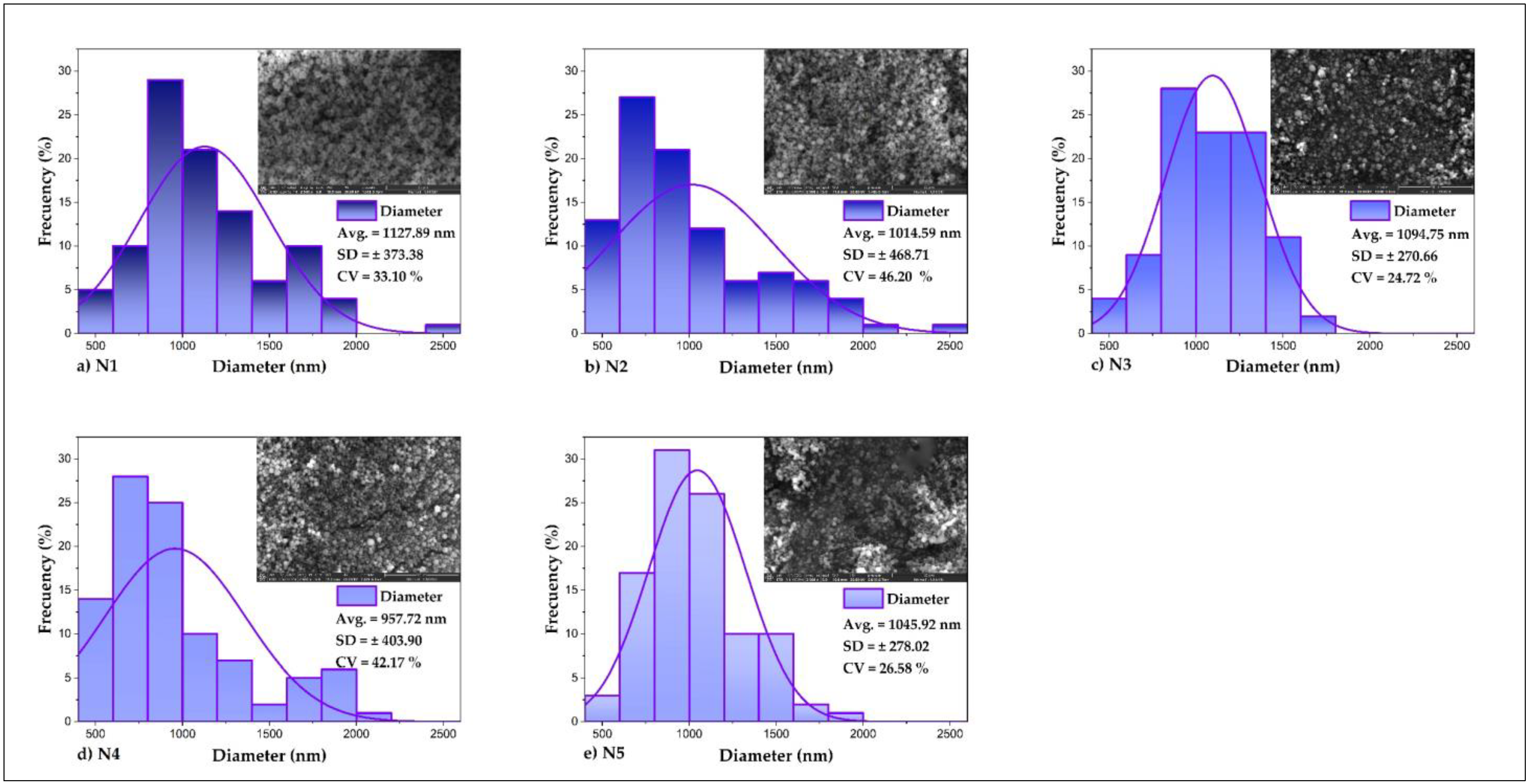
3.5. Particle Size and ζ Potential in Nanoencapsulates
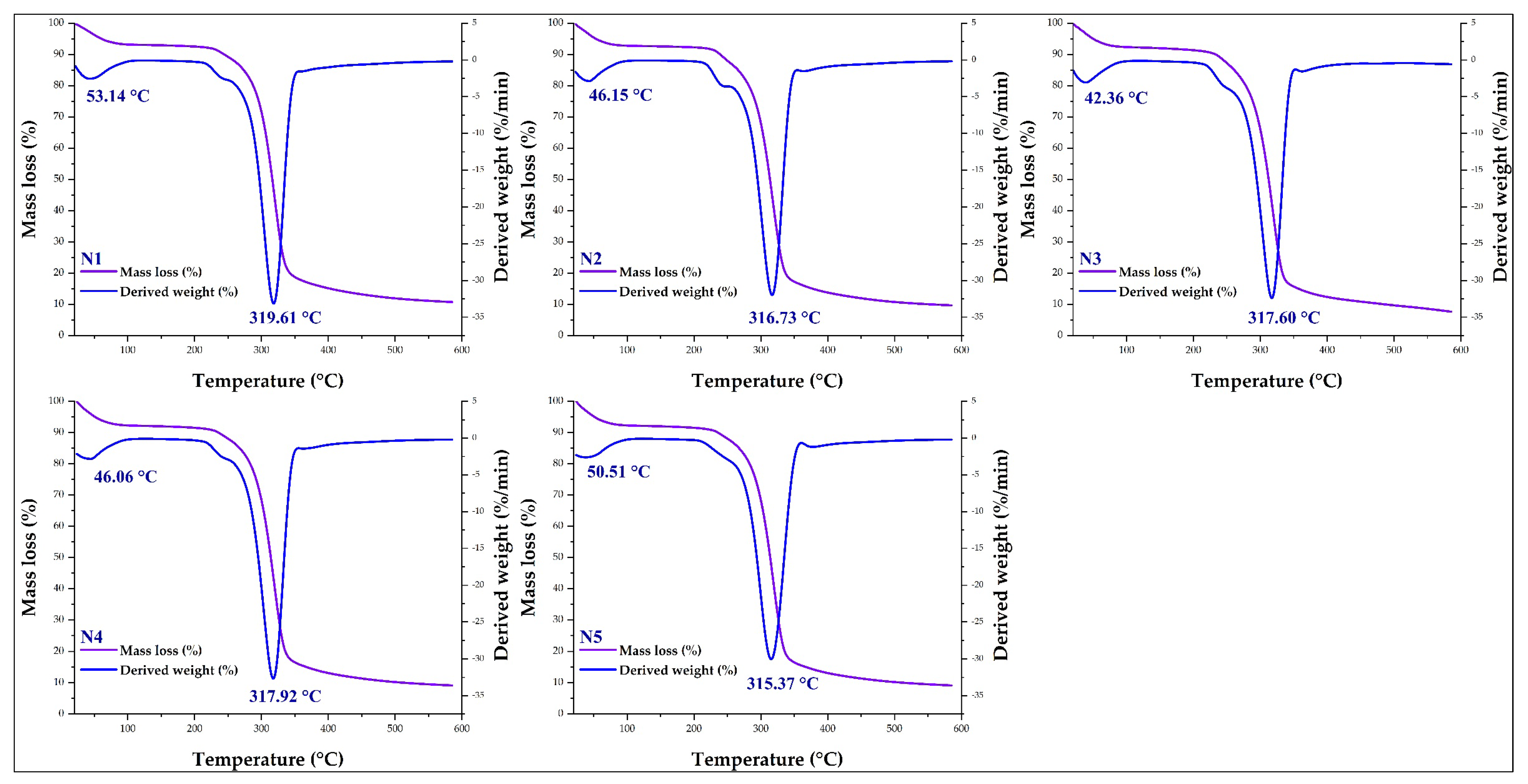
3.6. Thermal Analysis in Nanoencapsulates
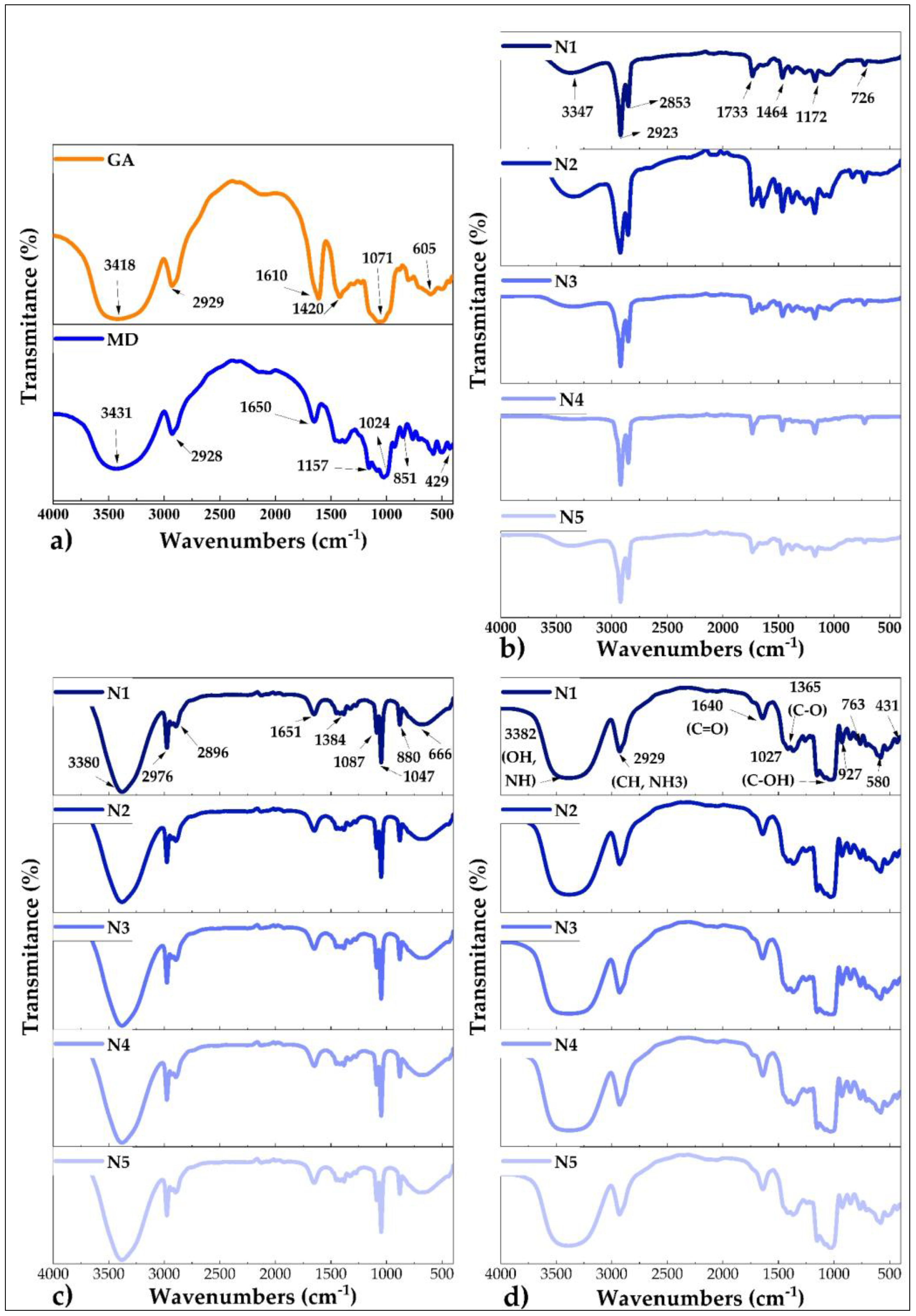
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
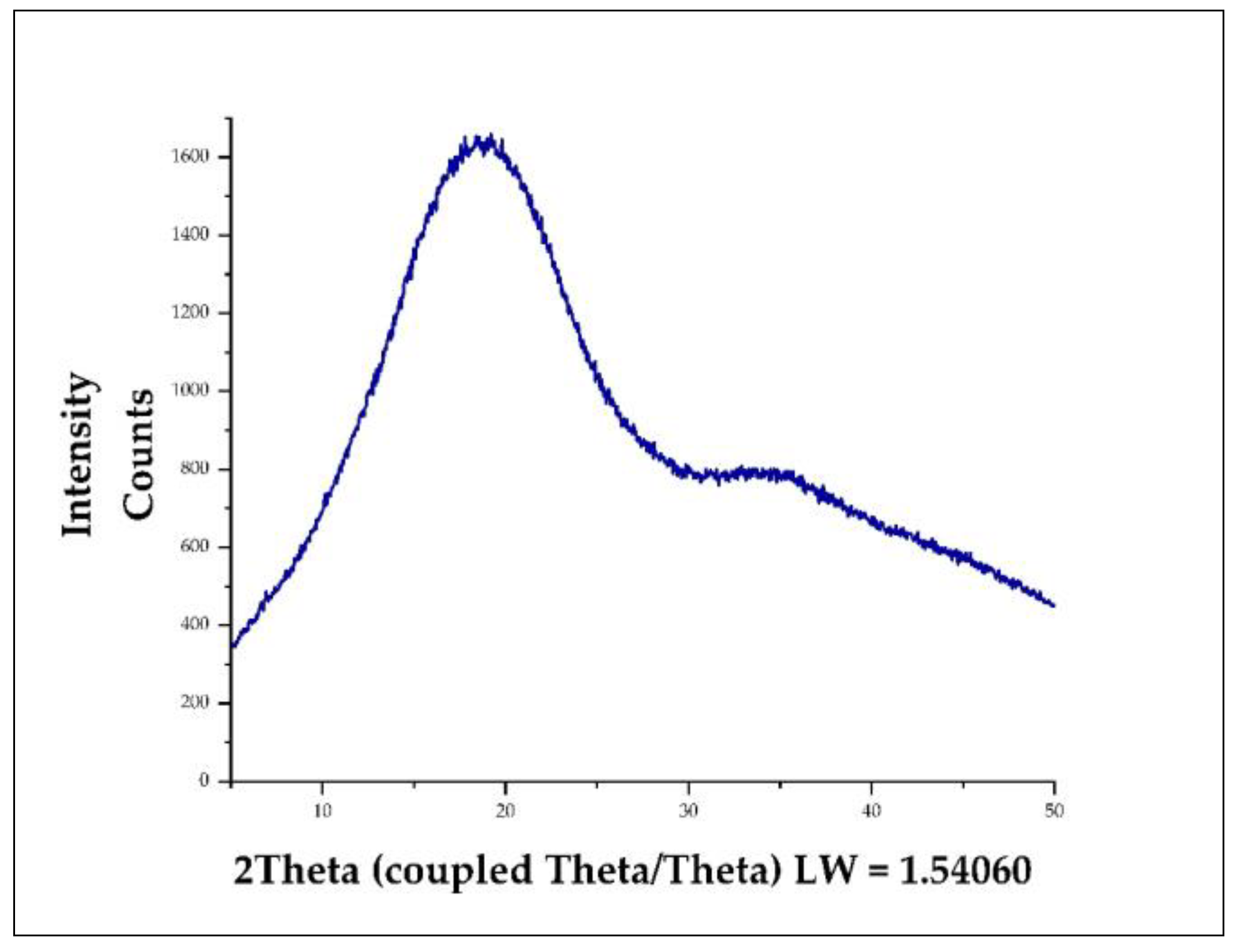
3.8. X-ray Diffraction (XRD) in Nanoencapsulates
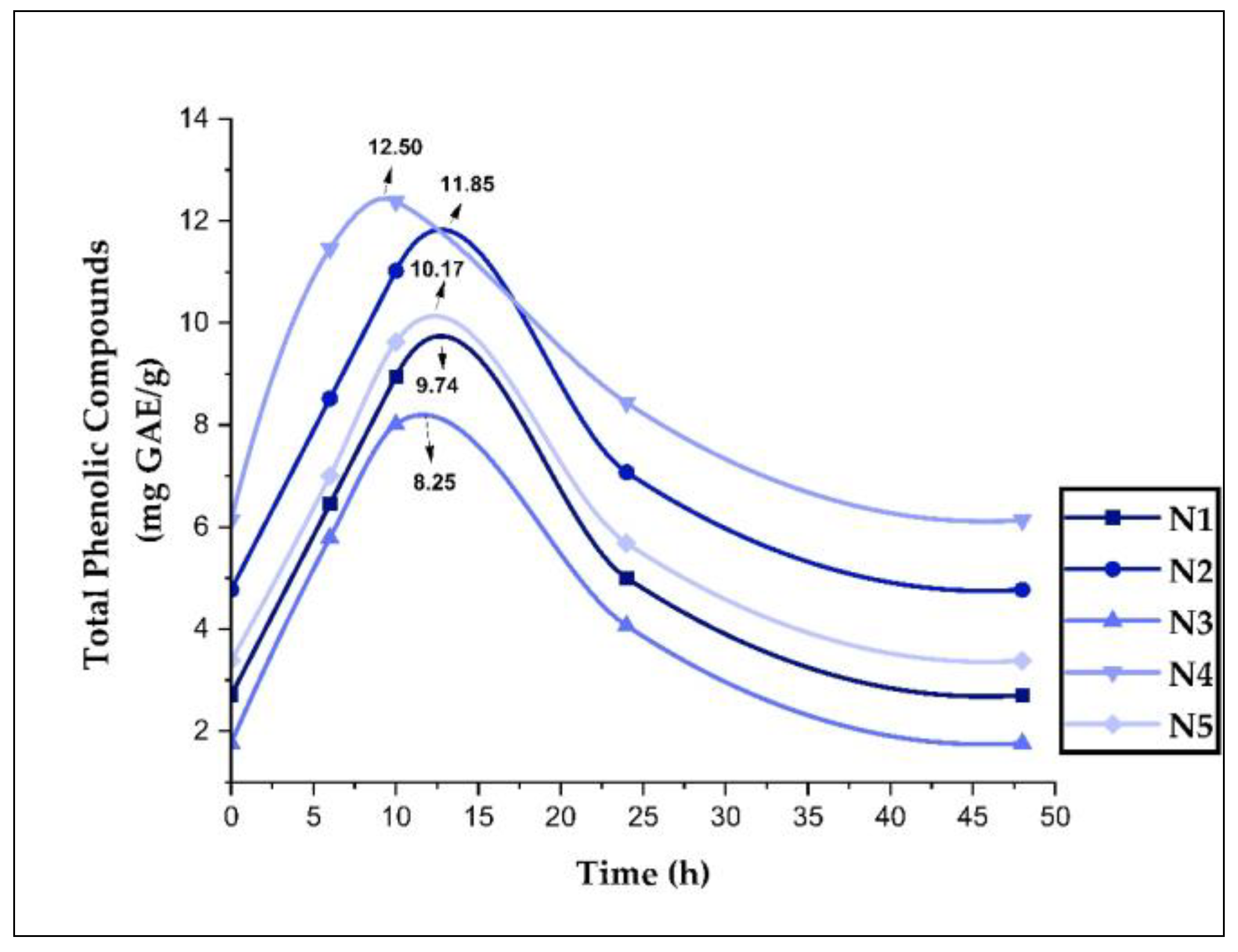
3.9. Stability and Release of Phenolic Compounds in Nanoencapsulates
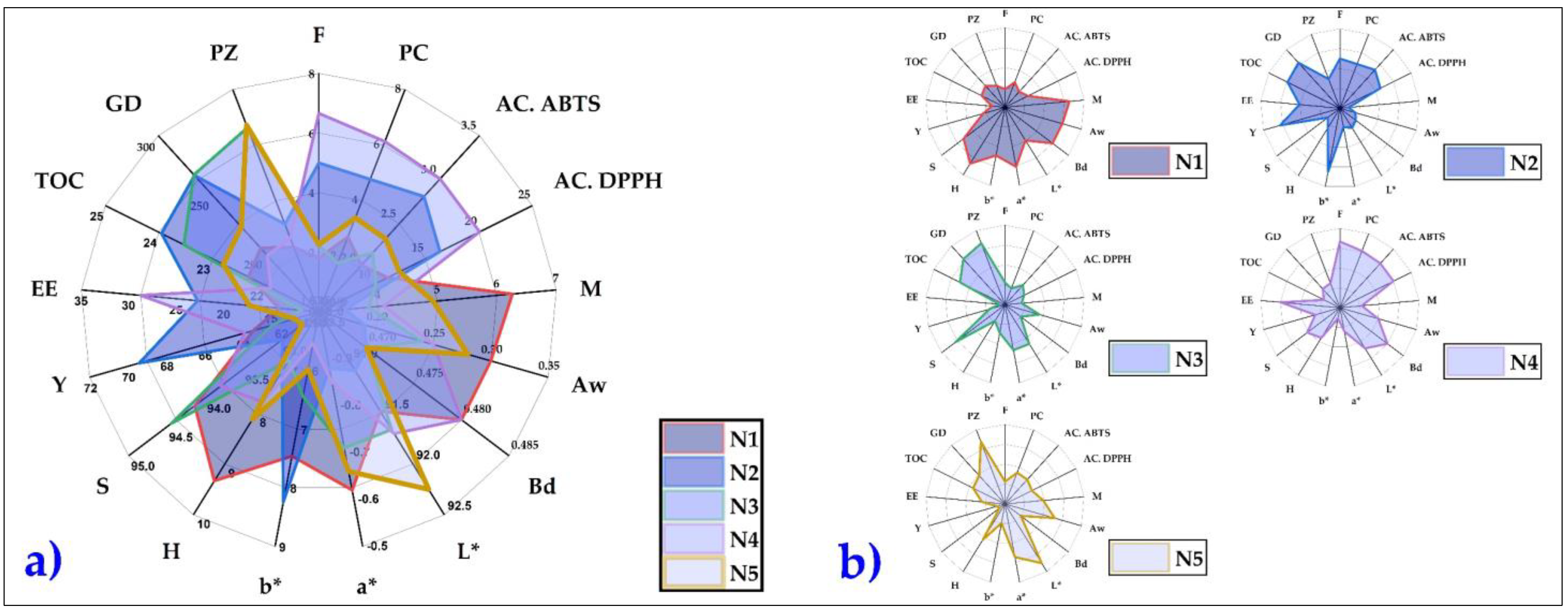
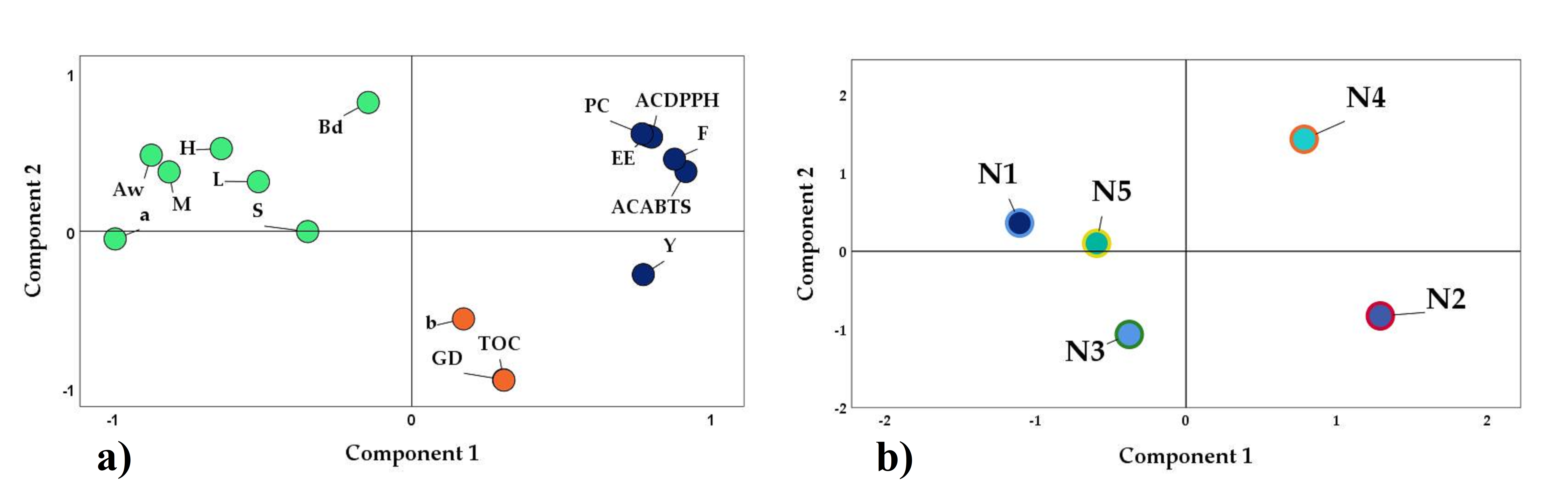
3.10. Overview of the Results Obtained in Nanoencapsulates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis encapsulation by spray drying: Characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Baysan, U.; Zungur Bastıoğlu, A.; Coşkun, N.Ö.; Konuk Takma, D.; Ülkeryıldız Balçık, E.; Sahin-Nadeem, H.; Koç, M. The effect of coating material combination and encapsulation method on propolis powder properties. Powder Technol. 2021, 384, 332–341. [Google Scholar] [CrossRef]
- Shakoury, N.; Aliyari, M.A.; Salami, M.; Emam-Djomeh, Z.; Vardhanabhuti, B.; Moosavi-Movahedi, A.A. Encapsulation of propolis extract in whey protein nanoparticles. LWT 2022, 158, 113138. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and characterization of microencapsules containing spray dried powder obtained from Brazilian brown, green and red propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 127, 227–235. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Nanda, V. Encapsulated bee propolis powder: Drying process optimization and physicochemical characterization. LWT 2022, 155, 112956. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum Arabic, maltodextrin and inulin as coating materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of propolis extract by complex coacervation. LWT—Food Sci. Technol. 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, J.; Bhainsa, K.C. Applicability of Spray Drying Technique to Prepare Nano-Micro Carriers: A Review. Nanoarchitectonics 2022, 3, 33–45. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Malekjani, N.; Jafari, S.M. Innovations in spray drying process for food and pharma industries. J. Food Eng. 2022, 321, 110960. [Google Scholar] [CrossRef]
- Jafari, S.M.; Arpagaus, C.; Cerqueira, M.A.; Samborska, K. Nano spray drying of food ingredients; materials, processing and applications. Trends Food Sci. Technol. 2021, 109, 632–646. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Peres, I.; Rocha, S.; Gomes, J.; Morais, S.; Pereira, M.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, 61, e14059. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 346–401. [Google Scholar] [CrossRef]
- Bordón, M.G.; Alasino, N.P.X.; Villanueva-Lazo, Á.; Carrera-Sánchez, C.; Pedroche-Jiménez, J.; del Carmen Millán-Linares, M.; Ribotta, P.D.; Martínez, M.L. Scale-up and optimization of the spray drying conditions for the development of functional microparticles based on chia oil. Food Bioprod. Process. 2021, 130, 48–67. [Google Scholar] [CrossRef]
- Chopde, S.; Datir, R.; Deshmukh, G.; Dhotre, A.; Patil, M. Nanoparticle formation by nanospray drying & its application in nanoencapsulation of food bioactive ingredients. J. Agric. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Jameel, Q.Y.; Ajeel, M.A.; Mohammed, N.K. Nutritional and anti-gastro ulcerative role of the gum Arabic (Acacia senegal L.) compared to a reference drug. Funct. Foods Health Dis. 2022, 12, 294–307. [Google Scholar] [CrossRef]
- Chranioti, C.; Tzia, C. Arabic gum mixtures as encapsulating agents of freeze-dried fennel oleoresin products. Food Bioprocess Technol. 2014, 7, 1057–1065. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Nishtha. Natural gums as corrosion inhibitor: A review. Mater. Today Proc. 2022, 64, 141–146. [Google Scholar] [CrossRef]
- Almansour, K.; Ali, R.; Alheibshy, F.; Almutairi, T.J.; Alshammari, R.F.; Alhajj, N.; Arpagaus, C.; Elsayed, M.M.A. Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions. Pharmaceutics 2022, 14, 800. [Google Scholar] [CrossRef]
- Plati, F.; Papi, R.; Paraskevopoulou, A. Characterization of Oregano Essential Oil (Origanum vulgare L. subsp hirtum) Particles Produced by the Novel Nano Spray Drying Technique. Foods 2021, 10, 2923. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z. Properties of encapsulated saffron extracts in maltodextrin using the Büchi B-90 nano spray-dryer. Food Chem. 2018, 266, 458–465. [Google Scholar] [CrossRef]
- Machado, M.H.; Almeida, A.d.R.; Maciel, M.V.d.O.B.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Moscoso-Moscoso, E.; Choque-Quispe, D.; Palomino-Rincón, H.; Martínez-Huamán, E.L.; Huamán-Carrión, M.L.; Peralta-Guevara, D.E.; Aroni-Huamán, J.; Arévalo-Quijano, J.C.; Palomino-Rincón, W.; et al. Microencapsulation of Erythrocytes Extracted from Cavia porcellus Blood in Matrices of Tara Gum and Native Potato Starch. Foods 2022, 11, 2107. [Google Scholar] [CrossRef]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.-E.; Maugard, T. Microencapsulation and controlled release of α-tocopherol by complex coacervation between pea protein and tragacanth gum: A comparative study with arabic and tara gums. Innov. Food Sci. Emerg. Technol. 2022, 77, 102951. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Wardana, I.N.; Ulya, H.N.; Cahyono, H.; Kumoro, A.C.; Aryanti, N. The effect of spray-drying inlet conditions on iron encapsulation using hydrolysed glucomannan as a matrix. Food Bioprod. Process. 2020, 123, 72–79. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Trusheva, B.; Popova, M. Propolis extraction methods: A review. J. Apicult. Res. 2021, 60, 734–743. [Google Scholar] [CrossRef]
- Tavares, L.; Santos, L.; Zapata Noreña, C.P. Bioactive compounds of garlic: A comprehensive review of encapsulation technologies, characterization of the encapsulated garlic compounds and their industrial applicability. Trends Food Sci. Technol. 2021, 114, 232–244. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Fernandes, K.F.; Crizel-Cardozo, M.M.; Krumreich, F.D.; Borges, C.D.; Zambiazi, R.C. Microencapsulation of propolis in protein matrix using spray drying for application in food systems. Food Bioprocess Technol. 2018, 11, 1422–1436. [Google Scholar] [CrossRef]
- Piedrahíta Márquez, D.G.; Fuenmayor, C.A.; Suarez Mahecha, H. Effect of chitosan-propolis edible coatings on stability of refrigerated cachama (Piaractus brachypomus) vacuum-packed fish fillets. Packag. Technol. Sci. Total Environ. 2019, 32, 143–153. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Krumreich, F.D.; Zandoná, G.P.; Gularte, M.A.; Borges, C.D.; Zambiazi, R.C. Production of propolis extract microparticles with concentrated pea protein for application in food. Food Bioprocess Technol. 2019, 12, 729–740. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. The Effect of Enriching Honey with Propolis on the Antioxidant Activity, Sensory Characteristics, and Quality Parameters. Molecules 2020, 25, 1176. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Gómez, P.A.M.; Jon, L.Y.T.C.; Torres, D.J.M.; Amaranto, R.E.B.; Díaz, I.E.C.; Medina, C.A.M.; Choque, J.S.C. Antibacterial, antibiofilm, and cytotoxic activities and chemical compositions of Peruvian propolis in an in vitro oral biofilm. F1000Research 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Duan, X.; Ke, L.; Pan, X.; Liu, J.; Song, X.; Ma, W.; Zhang, W.; Liu, Y.; Fan, Y. Extraction, purification, structural character and biological properties of propolis flavonoids: A review. Fitoterapia 2022, 157, 105106. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Ibrahim, M.E.E.-D.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Hiltunen, R. Fe(III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem. 2004, 88, 193–199. [Google Scholar] [CrossRef]
- Horwitz, W. Official methods of analysis of AOAC International. agricultural chemicals, contaminants, drugs; Horwitz, W., Ed.; AOAC International, 1997: Gaithersburg, MD, USA, 2010; Volume I. [Google Scholar]
- Tomé, A.C.; da Silva, F.A. Alginate based encapsulation as a tool for the protection of bioactive compounds from aromatic herbs. Food Hydrocoll. Health 2022, 2, 100051. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A. Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods 2022, 11, 1640. [Google Scholar] [CrossRef]
- Khacha-Ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef]
- Betances-Salcedo, E.; Revilla, I.; Vivar-Quintana, A.M.; González-Martín, M.I. Flavonoid and Antioxidant Capacity of Propolis Prediction Using Near Infrared Spectroscopy. Sensors 2017, 17, 1647. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Liben, T.; Atlabachew, M.; Abebe, A.J.C.F. Total phenolic, flavonoids and some selected metal content in honey and propolis samples from South Wolo zone, Amhara region, Ethiopia. Cogent Food Agric. 2018, 4, 1475925. [Google Scholar] [CrossRef]
- Ding, Q.; Sheikh, A.R.; Gu, X.; Li, J.; Xia, K.; Sun, N.; Wu, R.A.; Luo, L.; Zhang, Y.; Ma, H. Chinese Propolis: Ultrasound-assisted enhanced ethanolic extraction, volatile components analysis, antioxidant and antibacterial activity comparison. Food Sci. Nutr. 2021, 9, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Becerra, T.B.; Calla-Poma, R.D.; Requena-Mendizabal, M.F.; Millones-Gómez, P.A. Antibacterial effect of Peruvian propolis collected during different seasons on the growth of. Open Dent. J. 2019, 13, 327–331. [Google Scholar] [CrossRef]
- Premi, M.; Sharma, H. Effect of different combinations of maltodextrin, gum arabic and whey protein concentrate on the encapsulation behavior and oxidative stability of spray dried drumstick (Moringa oleifera) oil. Int. J. Biol. Macromol. 2017, 105, 1232–1240. [Google Scholar] [CrossRef]
- Ruengdech, A.; Siripatrawan, U. Improving encapsulating efficiency, stability, and antioxidant activity of catechin nanoemulsion using foam mat freeze-drying: The effect of wall material types and concentrations. LWT 2022, 162, 113478. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; da Silva, V.M.; Durigon, A.; Hubinger, M.D.; Laurindo, J.B. Production of mango powder by spray drying and cast-tape drying. Powder Technol. 2017, 305, 447–454. [Google Scholar] [CrossRef]
- Tuyen, C.K.; Nguyen, M.H.; Roach, P.D. Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Ricci, A.; Mejia, J.A.A.; Versari, A.; Chiarello, E.; Bordoni, A.; Parpinello, G.P. Microencapsulation of polyphenolic compounds recovered from red wine lees: Process optimization and nutraceutical study. Food Bioprod. Process. 2022, 132, 1–12. [Google Scholar] [CrossRef]
- Salum, P.; Berktas, S.; Cam, M.; Erbay, Z. Enzyme-modified cheese powder production: Influence of spray drying conditions on the physical properties, free fatty acid content and volatile compounds. Int. Dairy J. 2022, 125, 105241. [Google Scholar] [CrossRef]
- Koç, M.; Baysan, U.; Devseren, E.; Okut, D.; Atak, Z.; Karataş, H.; Kaymak-Ertekin, F. Effects of different cooking methods on the chemical and physical properties of carrots and green peas. Innov. Food Sci. Emerg. Technol. 2017, 42, 109–119. [Google Scholar] [CrossRef]
- Kaul, S.; Kaur, K.; Mehta, N.; Dhaliwal, S.S.; Kennedy, J.F. Characterization and optimization of spray dried iron and zinc nanoencapsules based on potato starch and maltodextrin. Carbohydr. Polym. 2022, 282, 119107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Clardy, A.; Hui, D.; Wu, Y. Physiochemical properties of encapsulated bitter melon juice using spray drying. Bioact. Carbohyd. Diet. Fibre 2021, 26, 100278. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Jó, J.; Oliveira, G.A.d.; Chaimsohn, F.P.; Sierakowski, M.R.; Freitas, R.A.d.; Ribani, R.H. Microencapsulation of juçara (Euterpe edulis M.) pulp by spray drying using different carriers and drying temperatures. Dry. Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Moslemi, M.; Hosseini, H.; Erfan, M.; Mortazavian, A.M.; Fard, R.M.N.; Neyestani, T.R.; Komeyli, R. Characterisation of spray-dried microparticles containing iron coated by pectin/resistant starch. Int. J. Food Sci. Technol. 2014, 49, 1736–1742. [Google Scholar] [CrossRef]
- De Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, T.; Li, J.; Pan, B.; Hu, Q.; Duan, M.; Zhang, X. Study on the Effect of Spray Drying Process on the Quality of Microalgal Biomass: A Comprehensive Biocomposition Analysis of Spray-Dried S. acuminatus Biomass. BioEnergy Res. 2022, 15, 320–333. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Yang, P.; Liu, H.; Kong, L.; Yu, X. Microencapsulation of Fe2+ in Spray-Dried Lactose for Improved Bioavailability. Bioinorg. Chem. Appl. 2021, 2021, 5840852. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.d.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Gu, B.; Linehan, B.; Tseng, Y.-C. Optimization of the Büchi B-90 spray drying process using central composite design for preparation of solid dispersions. Int. J. Pharm. 2015, 491, 208–217. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Ramírez, D.M.N.; Herrera-Valencia, E.E.; Bernad, M.J.B.; Manero, O. Spray drying egg using either maltodextrin or nopal mucilage as stabilizer agents. J. Food Sci. Technol. 2017, 54, 4427–4435. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of spray-drying conditions on the physicochemical properties of blackberry powder. Dry. Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, Suspensions, and Aerosols: Microscience and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Y.; Sabahi, H.; Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016, 211, 700–706. [Google Scholar] [CrossRef]
- Outuki, P.M.; de Francisco, L.M.B.; Hoscheid, J.; Bonifácio, K.L.; Barbosa, D.S.; Cardoso, M.L.C. Development of arabic and xanthan gum microparticles loaded with an extract of Eschweilera nana Miers leaves with antioxidant capacity. Coll. Surf. A Physicochem. Eng. Asp. 2016, 499, 103–112. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Cai, R.; Wang, S.; Meng, Y.; Meng, Q.; Zhao, W. Rapid quantification of flavonoids in propolis and previous study for classification of propolis from different origins by using near infrared spectroscopy. Anal. Methods 2012, 4, 2388–2395. [Google Scholar] [CrossRef]
- Dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. Microencapsulation of Morinda citrifolia L. extract by spray-drying. Chem. Eng. Res. Des. 2012, 90, 622–632. [Google Scholar] [CrossRef]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A. Propolis-based chitosan varnish: Drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complement. Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef]
- Martín Ramos, P.; Ruíz Potosme, N.M.; Fernández Coppel, I.A.; Martín Gil, J. Potential of ATR-FTIR Spectroscopy for the Classification of Natural Resins; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV–vis spectroscopic data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Cardoso, E.d.O.; Conti, B.J.; Santiago, K.B.; Conte, F.L.; Oliveira, L.P.G.; Hernandes, R.T.; Golim, M.d.A.; Sforcin, J.M. Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J. Pharm. Pharmacol. 2017, 69, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.d.M.; Silva, H.R.; Ayres, M.C.C.; Costa, C.L.S.d.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.d.M.; Brandão, M.S.; Chaves, M.H. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quím. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Abramovič, H.; Polak, T.; Bertoncelj, J.; Jamnik, P.; Smole Možina, S.; Jeršek, B. Chemical properties and antioxidant and antimicrobial activities of Slovenian propolis. Chem. Biodivers. 2012, 9, 1545–1558. [Google Scholar] [CrossRef]
- Iliinsky, N.; Steele, J. Designing Data Visualizations: Representing Informational Relationships; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2011. [Google Scholar]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Vítězová, M.; Jančiková, S.; Dordević, D.; Vítěz, T.; Elbl, J.; Hanišáková, N.; Jampílek, J.; Kushkevych, I. The possibility of using spent coffee grounds to improve wastewater treatment due to respiration activity of microorganisms. Appl. Sci. 2019, 9, 3155. [Google Scholar] [CrossRef]
- Kegode, T.M.; Bargul, J.L.; Mokaya, H.O.; Lattorff, H.M.G. Phytochemical composition and bio-functional properties of Apis mellifera propolis from Kenya. R. Soc. Open Sci. 2022, 9, 211214. [Google Scholar] [CrossRef]



| Flavonoids (mg Quercetin/g) | Phenolic Compounds (mg GAE/g) | Antioxidant Capacity ABTS (mg ET/g) | Antioxidant Capacity DPPH (mg ET/g) | |||||
|---|---|---|---|---|---|---|---|---|
| SD | * | SD | * | SD | * | SD | * | |
| Raw propolis | ||||||||
| N1 | 2.91 ± 0.04 | a | 3.40 ± 0.02 | a | 1.30 ± 0.01 | a | 11.59 ± 0.04 | a |
| N2 | 9.31 ± 0.03 | b | 9.66 ± 1.92 | b | 1.74 ± 0.01 | b | 13.70 ± 0.19 | b |
| N3 | 2.92 ± 0.03 | a | 3.02 ± 0.03 | a | 1.29 ± 0.01 | a | 8.98 ± 0.17 | c |
| N4 | 10.97 ± 0.06 | c | 11.50 ± 0.01 | b | 1.83 ± 0.01 | b | 15.80 ± 0.04 | d |
| N5 | 3.12 ± 0.03 | d | 5.29 ± 0.10 | a | 1.36 ± 0.01 | b | 12.09 ± 0.03 | e |
| Ethanolic extracts propolis | ||||||||
| N1 | 13.79 ± 0.25 | a | 19.09 ± 0.01 | a | 20.07 ± 0.34 | a | 94.11 ± 0.18 | a |
| N2 | 20.24 ± 0.07 | b | 20.97 ± 0.01 | b | 25.52 ± 0.16 | b | 95.58 ± 0.21 | b |
| N3 | 13.84 ± 0.07 | a | 14.89 ± 0.08 | c | 18.75 ± 0.25 | c | 81.28 ± 0.66 | c |
| N4 | 21.28 ± 0.10 | c | 21.30 ± 0.01 | d | 31.07 ± 0.80 | d | 106.05 ± 0.27 | d |
| N5 | 19.3 ± 0.05 | d | 19.53 ± 0.01 | e | 22.01 ± 0.31 | e | 81.22 ± 0.21 | c |
| Nanoencapsulates | ||||||||
| N1 | 1.81 ± 0.03 | a | 2.70 ± 0.01 | a | 2.03 ± 0.11 | a | 11.46 ± 0.33 | a |
| N2 | 5.00 ± 0.07 | b | 4.77 ± 0.01 | b | 2.81 ± 0.05 | b | 16.35 ± 0.57 | b |
| N3 | 2.23 ± 0.04 | c | 1.76 ± 0.04 | c | 2.17 ± 0.07 | ac | 10.36 ± 0.41 | a |
| N4 | 6.66 ± 0.07 | d | 6.13 ± 0.01 | d | 3.01 ± 0.04 | d | 20.07 ± 1.26 | c |
| N5 | 2.26 ± 0.04 | c | 3.38 ± 0.04 | e | 2.33 ± 0.05 | c | 12.55 ± 0.21 | d |
| N1 | N2 | N3 | N4 | N5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Property | SD | * | SD | * | SD | * | SD | * | SD | * |
| Moisture (%) | 6.26 ± 0.53 | a | 3.44 ± 0.53 | b | 3.84 ± 0.52 | b | 4.10 ± 0.21 | bc | 4.93 ± 0.19 | c |
| Aw | 0.30 ± 0.00 | a | 0.19 ± 0.53 | b | 0.24 ± 0.00 | c | 0.25 ± 0.00 | c | 0.28 ± 0.01 | d |
| Bulk density | 0.48 ± 0.00 | a | 0.47 ± 0.53 | a | 0.47 ± 0.00 | a | 0.48 ± 0.01 | a | 0.47 ± 0.00 | a |
| L * | 91.48 ± 0.04 | a | 91.08 ± 0.53 | b | 91.66 ± 0.01 | c | 91.70 ± 0.01 | c | 92.25 ± 0.01 | d |
| a * | −0.62 ± 0.01 | a | −0.88 ± 0.53 | b | −0.71 ± 0.01 | c | 0.85 ± 0.01 | d | −0.66 ± 0.01 | e |
| b * | 7.46 ± 0.33 | a | 8.24 ± 0.53 | b | 6.44 ± 0.04 | c | 5.54 ± 0.01 | d | 5.98 ± 0.02 | e |
| Hygroscopicity (%) | 9.33 ± 0.09 | a | 7.17 ± 0.53 | b | 6.96b ± 0.27 | c | 7.78 ± 0.10 | cd | 8.12 ± 0.39 | d |
| Solubility (%) | 94.13 ± 0.02 | a | 92.98 ± 0.53 | ab | 94.44 ± 0.02 | b | 93.79 ± 0.01 | ab | 92.72 ± 1.34 | a |
| Yield (%) | 63.43 ± 1.38 | a | 69.40 ± 0.53 | b | 62.44 ± 3.57 | a | 63.88 ± 2.67 | ab | 61.26 ± 1.96 | a |
| EE (%) | 14.16 ± 0.06 | a | 22.77 ± 0.53 | b | 11.82 ± 0.20 | c | 28.78 ± 0.05 | d | 17.32 ± 0.08 | e |
| Element | Atomic % | Atomic % Error | Weight % | Weight % Error | |
|---|---|---|---|---|---|
| N1 | C | 73.6 | 0.2 | 67.7 | 0.2 |
| O | 26.4 | 0.2 | 32.3 | 0.3 | |
| N2 | C | 50.4 | 0.2 | 43.3 | 0.1 |
| O | 49.6 | 0.3 | 56.7 | 0.3 | |
| N3 | C | 55.1 | 0.2 | 47.9 | 0.1 |
| O | 44.9 | 0.2 | 52.1 | 0.3 | |
| N4 | C | 77.3 | 0.2 | 71.8 | 0.2 |
| O | 22.7 | 0.2 | 28.2 | 0.3 | |
| N5 | C | 80.3 | 0.2 | 75.3 | 0.2 |
| O | 19.7 | 0.2 | 24.7 | 0.3 |
| Treatments | NICOMP Distribution | Gaussian Distribution | ζ Potential (mV) | ||||
|---|---|---|---|---|---|---|---|
| Peak | Size (nm) | % | SD | CV (%) | |||
| N1 | 1 | 123.4 | 48.6 | 204.4 | 129 | 63.11 | −36.66 |
| 2 | 562.6 | 51.4 | |||||
| N2 | 1 | 217.1 | 100 | 266.1 | 158.9 | 59.71 | −28.41 |
| N3 | 1 | 228.8 | 100 | 266.7 | 149.9 | 56.21 | 6.29 |
| N4 | 1 | 188.2 | 100 | 198.1 | 78.3 | 39.53 | −33.45 |
| N5 | 1 | 11.1 | 0.6 | 222.4 | 106.3 | 47.80 | 7.21 |
| 2 | 194.4 | 99.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Ramos-Pacheco, B.S.; Peralta-Guevara, D.E.; Cruz, G.D.l.; Martínez-Huamán, E.L.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; et al. Obtaining and Characterizing Andean Multi-Floral Propolis Nanoencapsulates in Polymeric Matrices. Foods 2022, 11, 3153. https://doi.org/10.3390/foods11203153
Ligarda-Samanez CA, Choque-Quispe D, Moscoso-Moscoso E, Huamán-Carrión ML, Ramos-Pacheco BS, Peralta-Guevara DE, Cruz GDl, Martínez-Huamán EL, Arévalo-Quijano JC, Muñoz-Saenz JC, et al. Obtaining and Characterizing Andean Multi-Floral Propolis Nanoencapsulates in Polymeric Matrices. Foods. 2022; 11(20):3153. https://doi.org/10.3390/foods11203153
Chicago/Turabian StyleLigarda-Samanez, Carlos A., David Choque-Quispe, Elibet Moscoso-Moscoso, Mary L. Huamán-Carrión, Betsy S. Ramos-Pacheco, Diego E. Peralta-Guevara, Germán De la Cruz, Edgar L. Martínez-Huamán, José C. Arévalo-Quijano, Jenny C. Muñoz-Saenz, and et al. 2022. "Obtaining and Characterizing Andean Multi-Floral Propolis Nanoencapsulates in Polymeric Matrices" Foods 11, no. 20: 3153. https://doi.org/10.3390/foods11203153
APA StyleLigarda-Samanez, C. A., Choque-Quispe, D., Moscoso-Moscoso, E., Huamán-Carrión, M. L., Ramos-Pacheco, B. S., Peralta-Guevara, D. E., Cruz, G. D. l., Martínez-Huamán, E. L., Arévalo-Quijano, J. C., Muñoz-Saenz, J. C., Muñoz-Melgarejo, M., Muñoz-Saenz, D. M., & Aroni-Huamán, J. (2022). Obtaining and Characterizing Andean Multi-Floral Propolis Nanoencapsulates in Polymeric Matrices. Foods, 11(20), 3153. https://doi.org/10.3390/foods11203153