Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. SS Processing
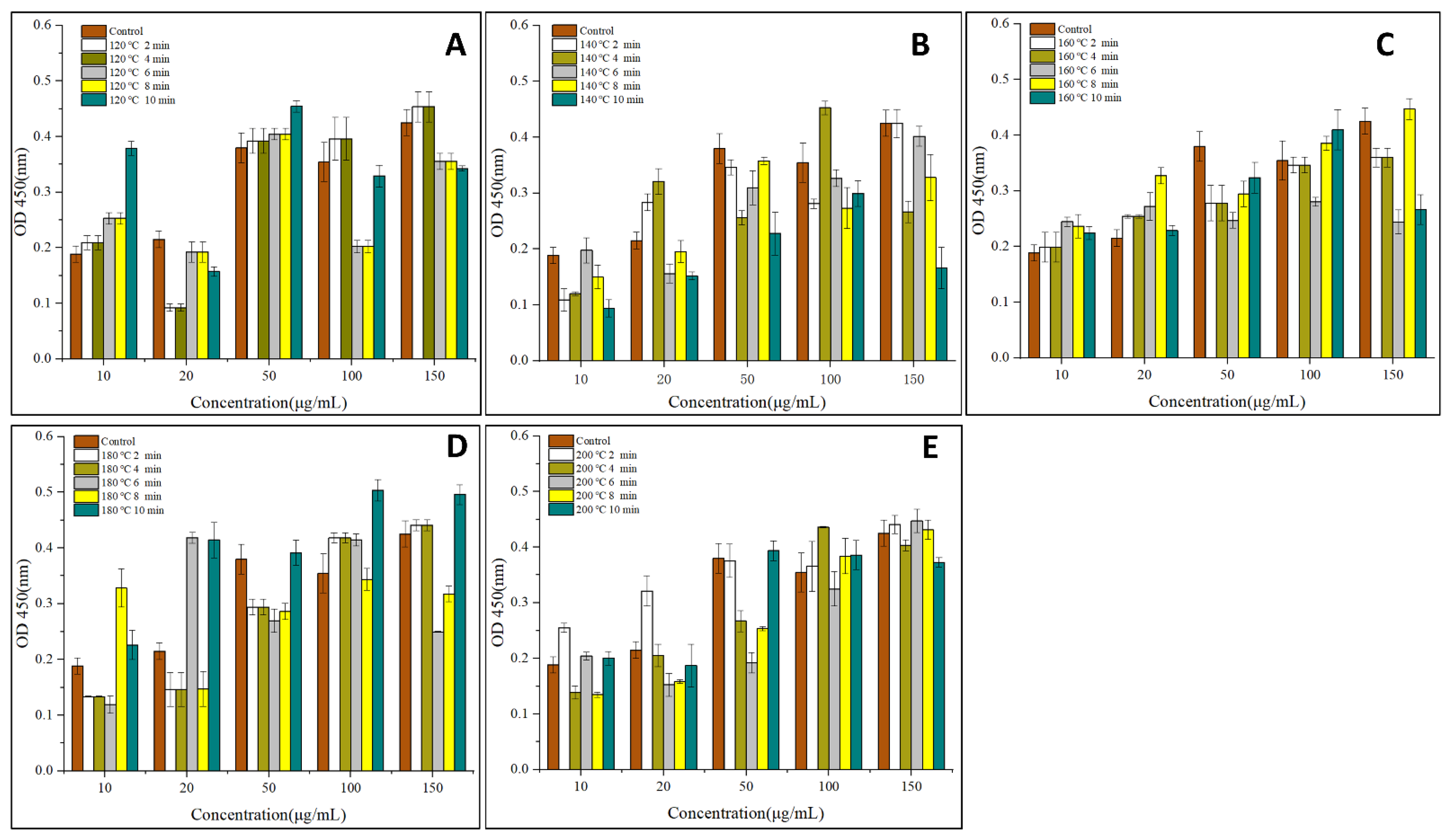
2.3. IgG and IgE-Binding Abilities
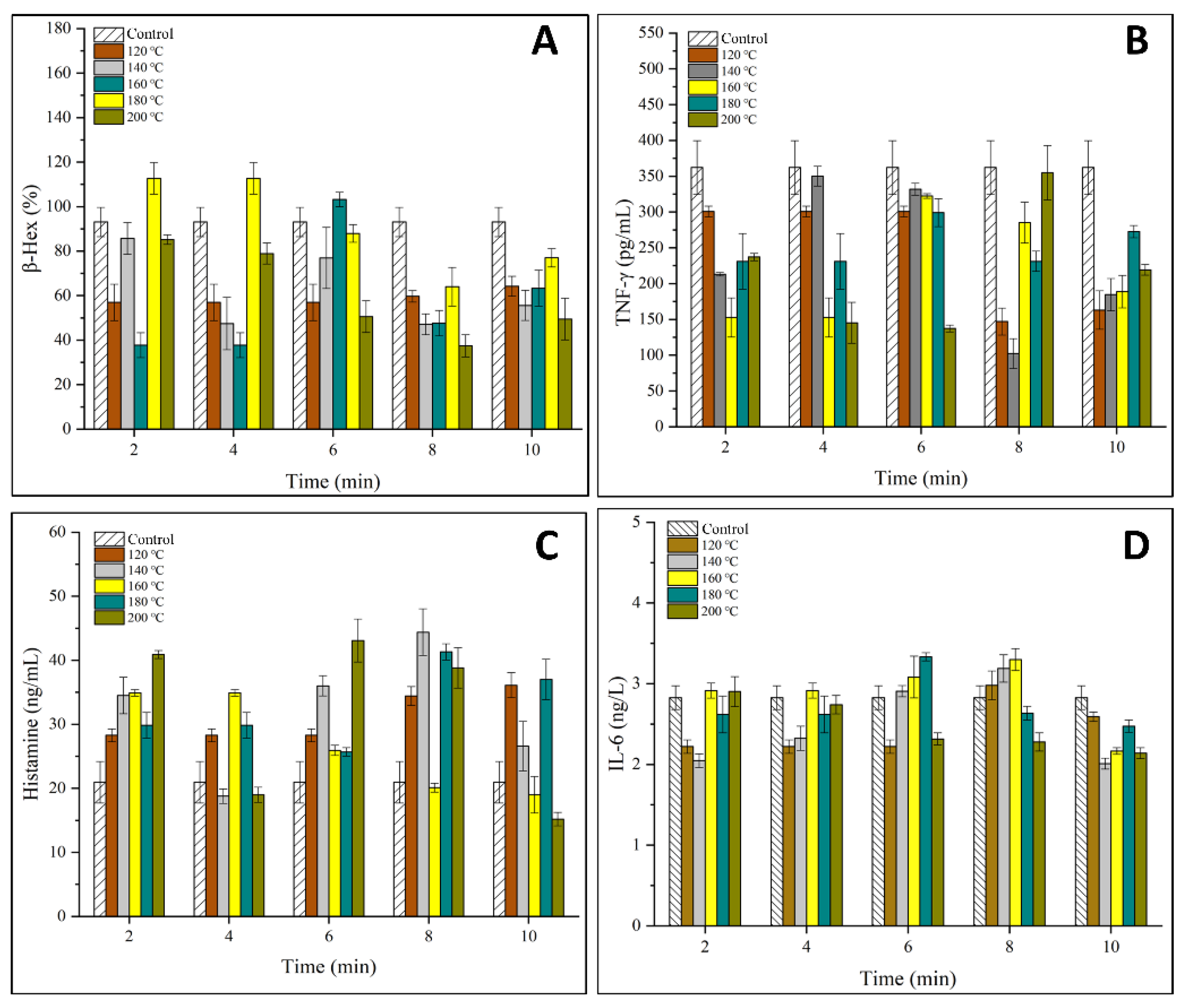
2.4. Human Peripheral Blood Basophilic Leukemia Cells (KU812 Cells) Culture and Degranulation Assay
2.5. Determination of Protein Carbonyl Content
2.6. Determination of Surface Hydrophobicity
2.7. Determination of FREE SH Content
2.8. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI TOF MS) Analysis
2.9. High Performance Liquid Chromatography Orbitrap Tandem Mass Spectrometry (HPLC Orbitrap MS/MS) Analysis
2.10. Statistical Analysis
3. Results and Discussion
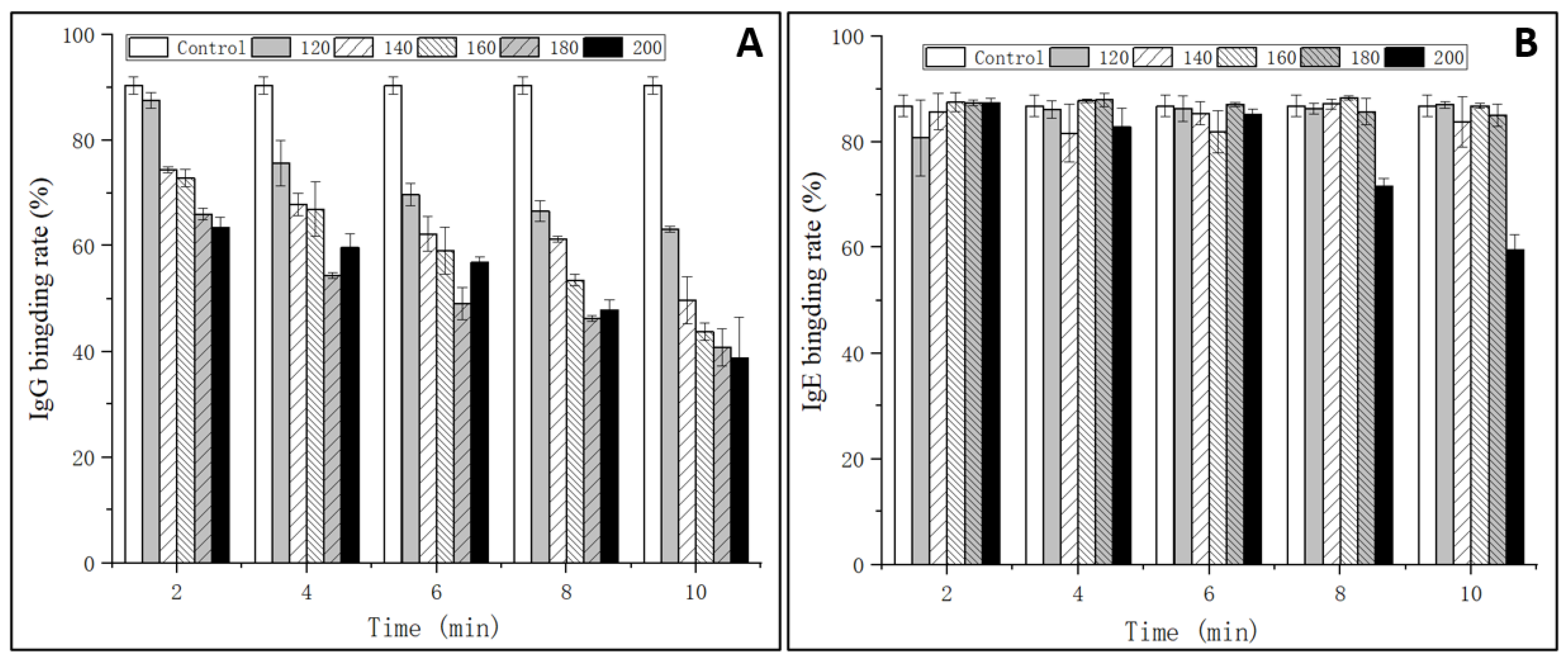
3.1. IgG and IgE Binding Abilities
3.2. Effects of OVM on the Viability and Degranulation of IgE Sensitized KU812 Cells
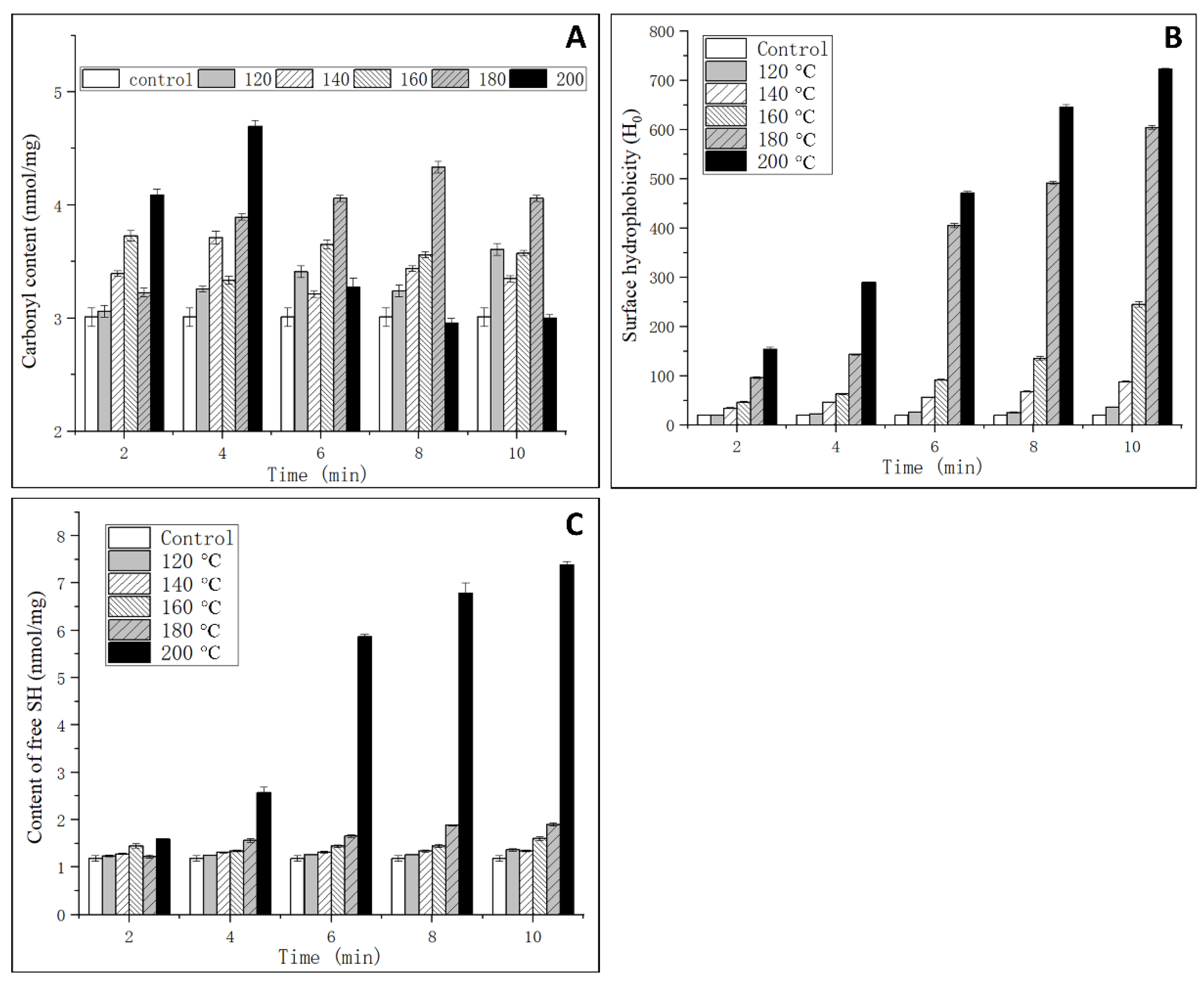
3.3. Analysis of Oxidation Degree
3.4. Analysis of Surface Hydrophobicity
3.5. Analysis of Free SH Content
3.6. Molecular Weight Analysis
3.7. Modification Sites of OVM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | superheated steam |
| IgE | immunoglobulin E |
| IgG | immunoglobulin G |
| KU812 cells | human peripheral blood basophilic leukemia cells |
| β-hex | β-hexosaminidase |
| IL-6 | interleukin-6 |
| DTT | dithiothreitol |
| ANS | 8-aniline-1-naphthalene sulfonic acid |
| SH | sulfhydryl |
| ELISA | enzyme-linked immunosorbent assay |
| MALDI TOF MS | Matrix-assisted laser desorption/ionization time of flight mass spectrometry |
| HPLC Orbitrap MS/MS | high performance liquid chromatography Orbitrap tandem mass spectrometry |
| DNPH | 2,4-dinitrophenylhydrazine |
References
- He, W.; Xu, H.; Lu, Y.; Zhang, T.; Li, S.; Lin, X.; Xu, B.; Wu, X. Function, digestibility and allergenicity assessment of ovalbumin-EGCG conjugates. J. Funct. Foods 2019, 61, 103490. [Google Scholar] [CrossRef]
- Pablos-Tanarro, A.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Assessment of the allergenic potential of the main egg white proteins in BALB/c mice. J. Agric. Food Chem. 2018, 11, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, J.W. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J. Agric. Food Chem. 2002, 50, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Azdad, O.; Mejrhit, N.; Aarab, L. Reduction of the allergenicity of cow’s milk α-lactalbumin under heat-treatment and enzymatic hydrolysis in Moroccan population. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 177–183. [Google Scholar] [CrossRef]
- Li, H.J.; Zhu, K.X.; Zhou, H.M.; Peng, W.; Guo, X.N. Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric. Immunol. 2016, 27, 1–20. [Google Scholar] [CrossRef]
- Fredericq, E.; Deutsch, H.F. Studies on ovomucoid. J. Biol. Chem. 1949, 181, 499–510. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Li, Z. Modification of protein structure and dough rheological properties of wheat flour through superheated steam treatment. J. Cereal Sci. 2017, 76, 222–228. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Hu, X.; Li, Z. Microbial decontamination of wheat grain with superheated steam. Food Control 2016, 62, 264–269. [Google Scholar] [CrossRef]
- Sehrawat, R.; Nema, P.K.; Kaur, B.P. Effect of superheated steam drying on properties of foodstuffs and kinetic modeling. Innov. Food Sci. Emerg. 2016, 34, 285–301. [Google Scholar] [CrossRef]
- Zhang, N.C.; Gao, Y.Q.; Tong, L.T.; Li, Z.G. Superheated steam processing improved the qualities of oats flour and noodles. J. Cereal Sci. 2018, 83, 96–100. [Google Scholar] [CrossRef]
- Ceccanti, C.; Pellegrini, E.; Guidi, L. Effect of superheated steam and conventional steam roasting on nutraceutical quality of several vegetables. LWT-Food Sci. Technol. 2021, 149, 112014. [Google Scholar] [CrossRef]
- Hu, X.; Guo, B.; Liu, C.; Yan, X.; Chen, J.; Luo, S.; Liu, Y.; Wang, H.; Yang, R.; Zhong, Y.; et al. Modification of potato starch by using superheated steam. Carbohyd. Polym. 2018, 198, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, J.; Liu, W.; Liu, C.; Zhong, Y.; Luo, D.; Li, Z.; Huang, Z. Selective peroxidase inactivation of lightly milled rice by superheated steam. J. Cereal Sci. 2014, 60, 623–630. [Google Scholar] [CrossRef]
- Wang, X.M.; Tu, Z.C.; Ye, Y.H.; Liu, G.X.; Wang, H.; Hu, Y.M. Mechanism on the allergenicity changes of α-lactalbumin treated by sonication-assisted glycation during in vitro gastroduodenal digestion. J. Agric. Food Chem. 2021, 69, 6850–6859. [Google Scholar] [CrossRef]
- Wang, X.M.; Ye, Y.H.; Tu, Z.C.; Hu, Y.M.; Wang, H.; Huang, T. Mechanism of the reduced IgG/IgE binding abilities of glycated β-lactoglobulin and its digests through high-resolution mass spectrometry. J. Agric. Food Chem. 2021, 69, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Radinger, M.; Brown, J.M.; Ali, K.; Vanhaesebroeck, B.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced chemotactic responses of mast cells. J. Cell Sci. 2010, 123 Pt 15, 2576–2585. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.T.; Lin, H.; Li, Z.X.; Nayak, B.; Ahmed, I.; Tian, S.L.; Chen, G.Z.; Lin, H.; Zhao, J.X. Structural changes of 2,2’-Azobis (2-amidinopropane) dihydrochloride (AAPH) treated shrimp tropomyosin decrease allergenicity. Food Chem. 2018, 274, 547–557. [Google Scholar] [CrossRef]
- Zhang, J.J.; Tu, Z.C.; Wang, H.; Hu, Y.M.; Du, P.C.; Yang, Y.P. Mechanism of the effect of 2, 2′-azobis (2-amidinopropane) dihydrochloride simulated lipid oxidation on the IgG/IgE binding ability of ovalbumin. Food Chem. 2020, 327, 127037. [Google Scholar] [CrossRef]
- Xi, C.; Kang, N.; Zhao, C.; Liu, Y.; Sun, Z.; Zhang, T. Effects of pH and different sugars on the structures and emulsification properties of whey protein isolate-sugar conjugates. Food Biosci. 2020, 33, 100507. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissues sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Liu, G.X.; Tu, Z.C.; Yang, W.H.; Wang, H.; Zhang, L.; Ma, D.; Huang, T.; Liu, J.; Li, X. Investigation into allergenicity reduction and glycation sites of glycated β-lactoglobulin with ultrasound pretreatment by high-resolution mass spectrometry. Food Chem. 2018, 252, 99–107. [Google Scholar] [CrossRef]
- Martos, G.; Lopez-Exposito, I.; Bencharitiwong, R.; Berin, M.C.; Nowak-Węgrzyn, A. Mechanisms underlying differential food allergy response to heated egg. J. Allergy Clin. Immun. 2011, 127, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Djurtoft, R.; Pedersen, H.S.; Aabin, B.; Barkholt, V. Studies of food allergens: Soybean and egg proteins. Adv. Exp. Med. Biol. 1991, 289, 281–293. [Google Scholar] [PubMed]
- Rupa, P.; Schnarr, L.; Mine, Y. Effect of heat denaturation of egg white proteins ovalbumin and ovomucoid on CD4+ T cell cytokine production and human mast cell histamine production. J. Funct. Foods. 2015, 18, 28–34. [Google Scholar] [CrossRef]
- Cianferoni, A.; Muraro, A. Food-induced anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 165–195. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.B.; Zhao, M.M.; Zhao, H.F.; Sun, W.F.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Zhang, J.W.; Hayakawa, S.; Mine, Y. Immunochemical and structural analysis of pepsin-digested egg white ovomucoid. J. Agric. Food Chem. 2000, 48, 6261–6266. [Google Scholar] [CrossRef]
- Mine, Y.; Yang, M. Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. J. Agric. Food Chem. 2008, 56, 4874–4900. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Xu, D.; Jin, Y.; Xu, X. Wheat flour superheated steam treatment induced changes in molecular rearrangement and polymerization behavior of gluten. Food Hydrocolloid. 2021, 118, 106769. [Google Scholar] [CrossRef]
- Yang, Y.P.; Liu, G.X.; Wang, H. Investigation of the mechanism of conformational alteration in ovalbumin as induced by glycation with different monoses through conventional spectrometry and liquid chromatography high-resolution mass spectrometry. J. Agric. Food Chem. 2019, 67, 3096–3105. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.M.; Clerens, S.; Thomas, A.; Callaghan, C.; Deb-Choudhury, S.; Haines, S. Photo-oxidation of whey proteins: Molecular markers of modification. Int. Dairy J. 2017, 66, 56–60. [Google Scholar] [CrossRef]
- Wang, H.; Zong, B.Z.; Liu, G.X.; Zhang, L.; Chen, Y. Identification and quantification of the phosphorylated ovalbumin by high resolution mass spectrometry under dry-heating treatment. Food Chem. 2016, 210, 141–147. [Google Scholar] [CrossRef] [PubMed]
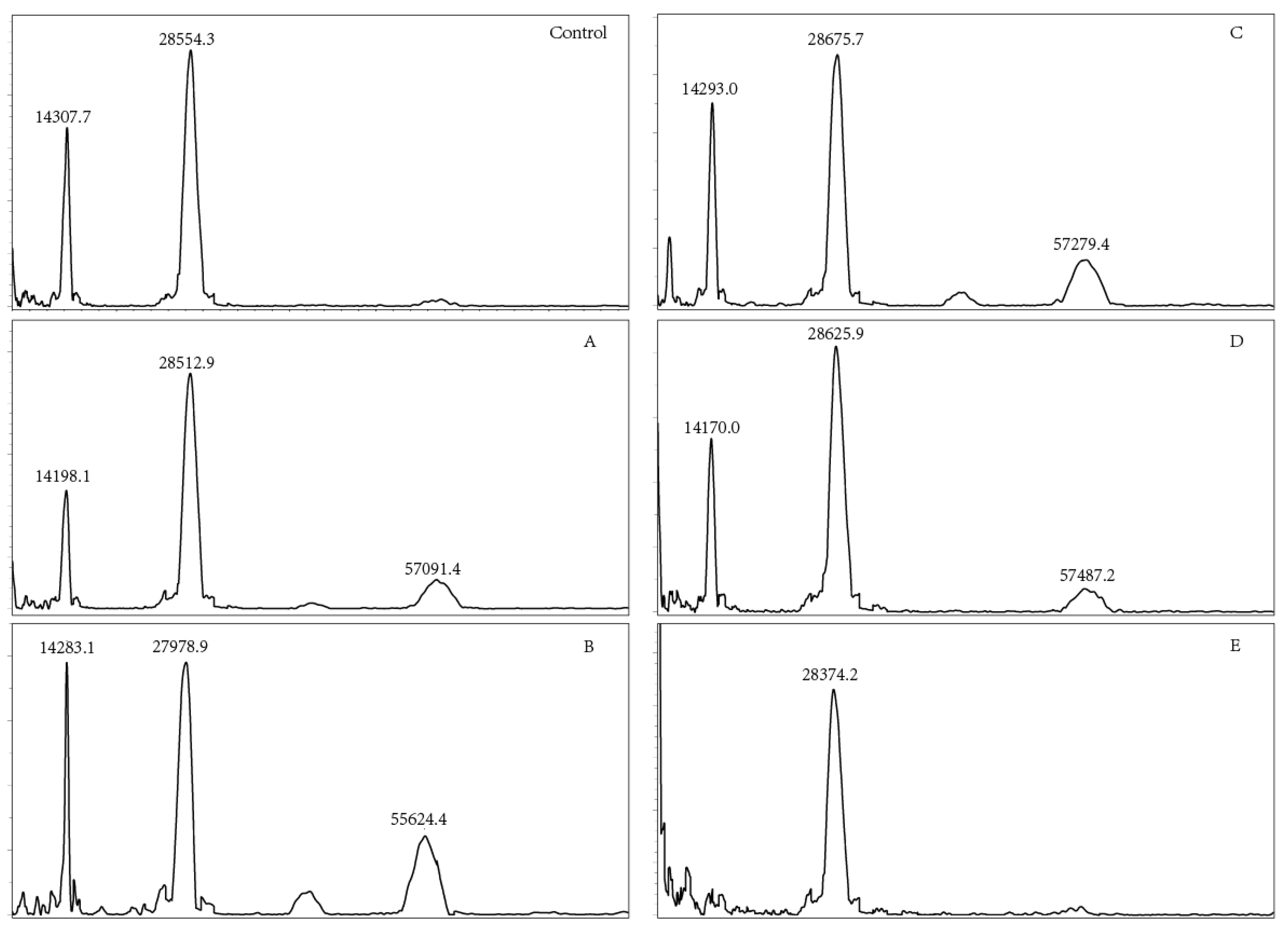
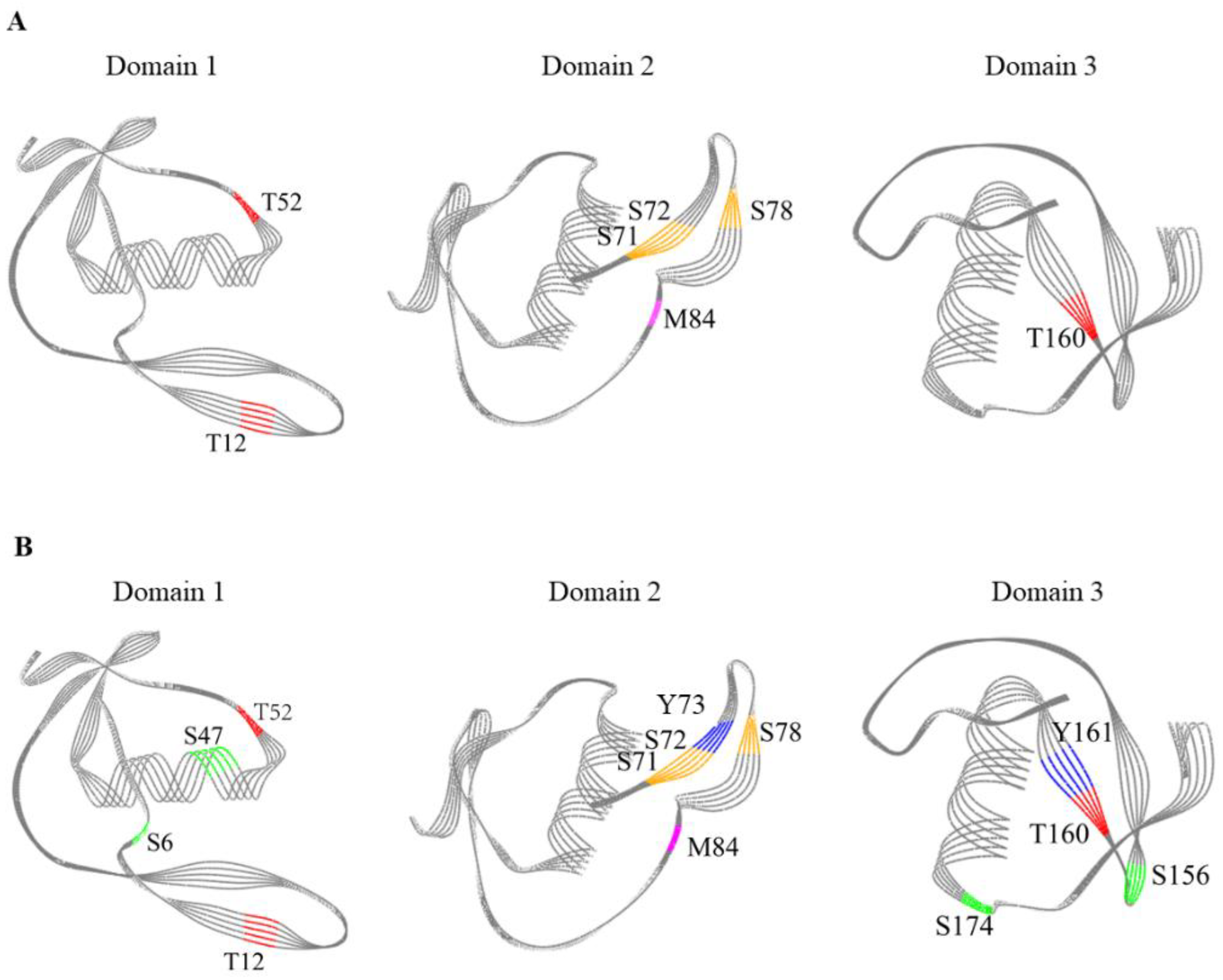
| Control | 120 °C for 10 min | 140 °C for 10 min | 200 °C for 10 min | ||
|---|---|---|---|---|---|
| Coverage | 86.67% | 99% | 99% | 100% | |
| Signal sequence 1–24 | |||||
| Modification | Oxidation | M3 | M3 | ||
| Nitro | M3 | M3 | |||
| Phosphor | S11 | S11 | S11 | S11 | |
| Carboxymethyl | C15 | C15 | C15 | C15 | |
| Protein signal sequence 1–186 | |||||
| Modification | Oxidation | M84 | M84 | M84 | |
| Nitro | Y102, Y141 | Y102, Y141 | Y102, Y141 | Y73, Y102, Y141, Y161 | |
| Phosphor | S6, T38 | S6, T12, T52, T160 | S6, T12, T52, T160 | S6, T12, T52, T160 | |
| Carboxymethyl | C5, C22, C30, C41, C44, C87, C95, C106, C109, C138, C146, C154, C165, C168 | C5, C22, C30, C41, C44, C70, C87, C95, C106, C109, C138, C146, C154, C165, C168 | C5, C22, C30, C41, C44, C70, C87, C95, C106, C109, C138, C146, C154, C165, C168 | C5, C22, C30, C41, C44, C70, C87, C95, C106, C109, C138, C146, C154, C165, C168 | |
| Glygly | K14, K56, T32, S55, K112, K121 | K14, K56, T32, S55, K112, K121, S71, S72, S78 | K14, K56, T32, S55, K112, K121, S71, S72, S78 | K14, K56, T32, S55, K112, K121, S71, S72, S78 | |
| Sulfo | T36 | T36 | T36 | T36, S6, S47, S156, S174 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, P.-W.; Tu, Z.-C.; Hu, Y.-M.; Wang, H. Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid. Foods 2022, 11, 238. https://doi.org/10.3390/foods11020238
Wen P-W, Tu Z-C, Hu Y-M, Wang H. Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid. Foods. 2022; 11(2):238. https://doi.org/10.3390/foods11020238
Chicago/Turabian StyleWen, Ping-Wei, Zong-Cai Tu, Yue-Ming Hu, and Hui Wang. 2022. "Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid" Foods 11, no. 2: 238. https://doi.org/10.3390/foods11020238
APA StyleWen, P.-W., Tu, Z.-C., Hu, Y.-M., & Wang, H. (2022). Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid. Foods, 11(2), 238. https://doi.org/10.3390/foods11020238





