Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties
Abstract
:1. Introduction
2. Structure of Exopolysaccharides (EPS)
3. EPS Biosynthesis
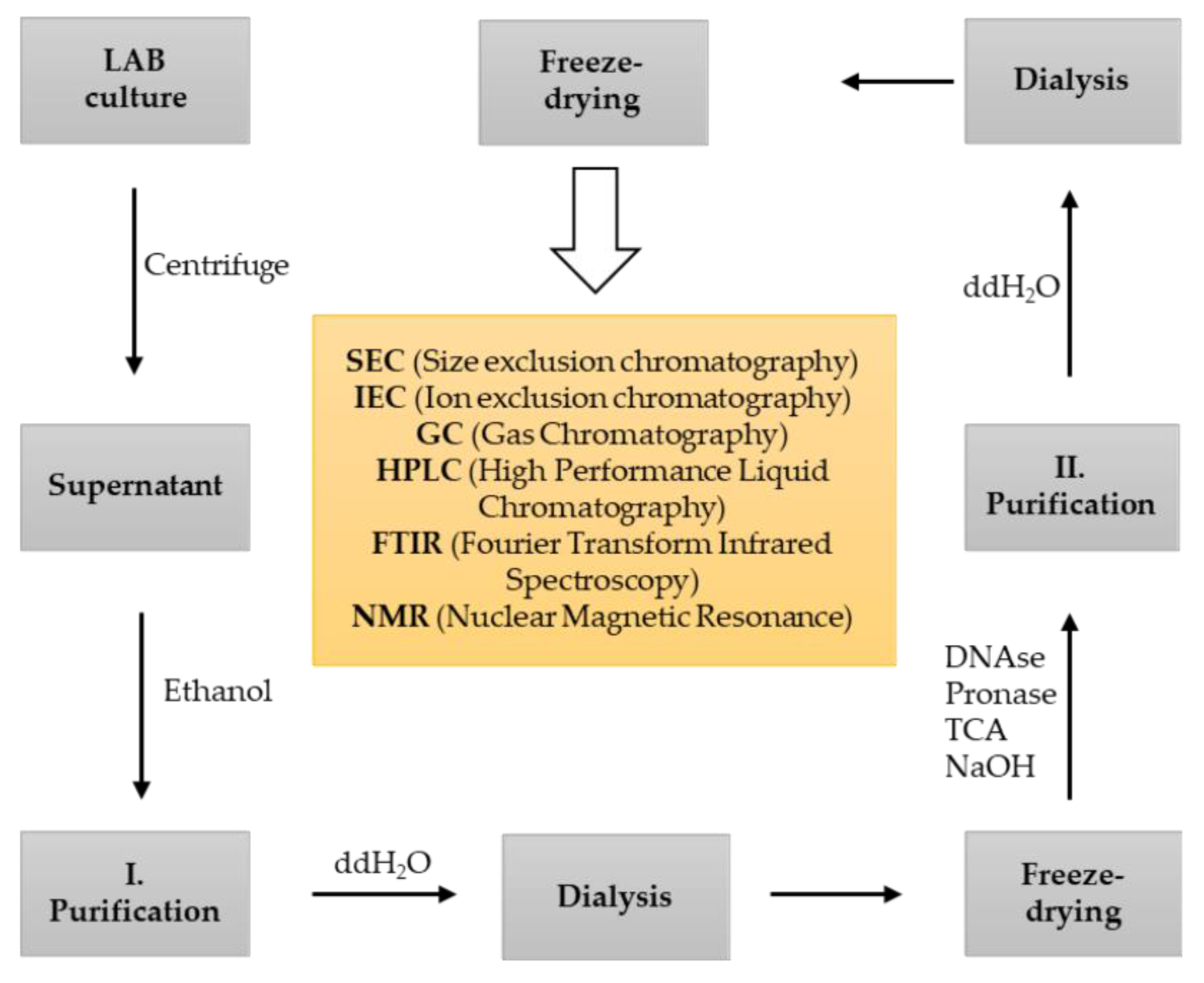
4. Methods of EPS Screening, Isolation and Characterization
5. Application of EPS-Producing LAB in Food Products
5.1. Yoghurt
5.2. Cheese
5.3. Kefir
5.4. Application of EPS-Producing LAB in Plant-Based Beverages
5.5. Application of EPS in Bakery
5.6. Application of EPS in Meat Industry
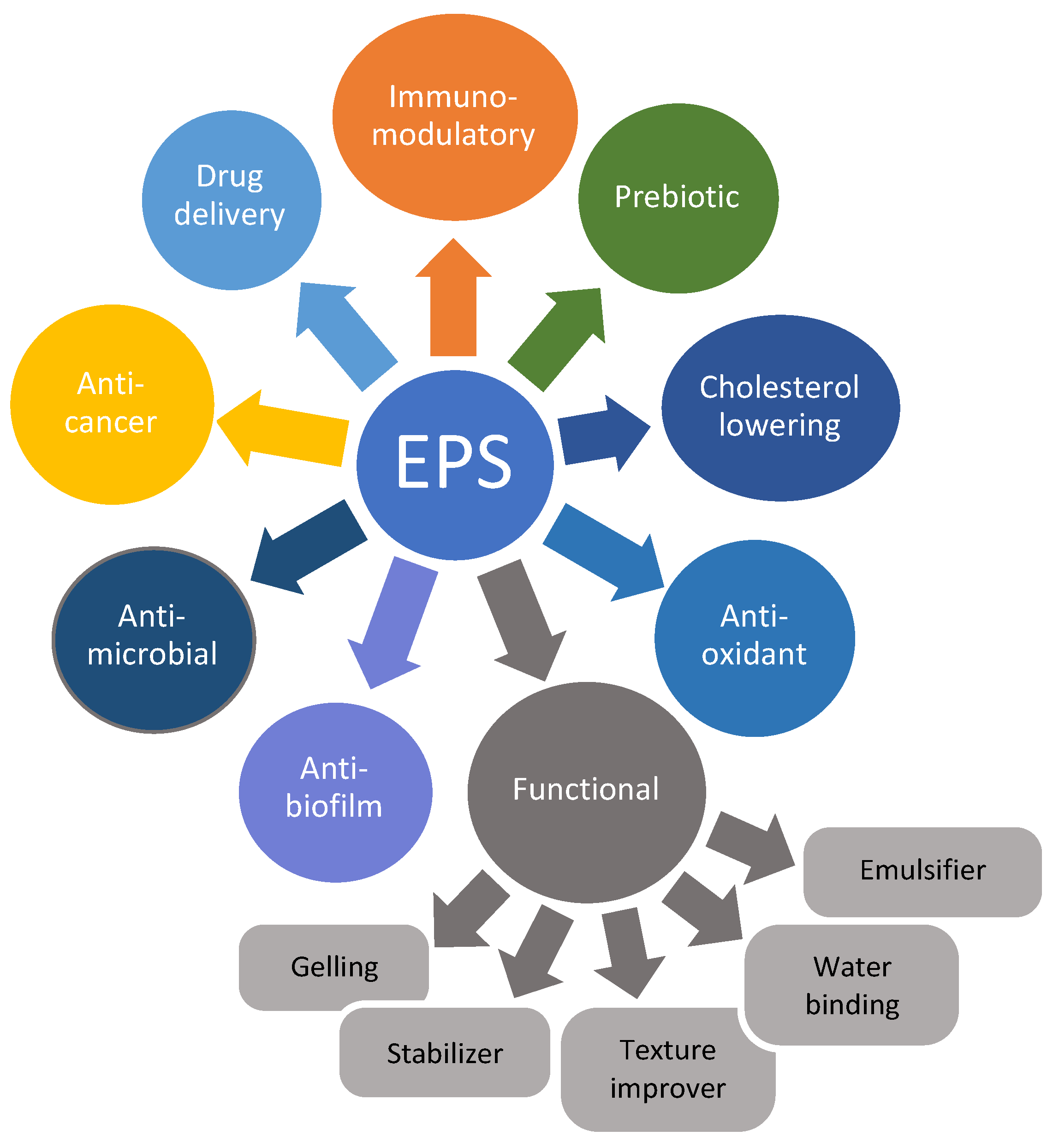
6. Health-Promoting Effects
6.1. Prebiotic Activity
6.2. Immunomodulatory Activity
6.3. Antioxidant Activity
6.4. Cholesterol Lowering Abilities
6.5. Anti-Biofilm Formation
6.6. Antimicrobial Activity
6.7. Anti-Cancer Activity
6.8. Drug Delivery Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manjanna, K.M.; Shivakumar, B.; Pramodkumar, T.M. Natural exopolysaccharides as novel excipients in drug delivery: A Review. Arch. Appl. Sci. Res. 2009, 1, 230–253. [Google Scholar]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Górska, S.; Grycko, P.; Rybka, J.; Gamian, A. Egzopolisacharydy bakterii kwasu mlekowego—Biosynteza i struktura. Exopolysaccharides of Lactic Acid Bacteria: Structure and Biosynthesis. Postepy Hig. Med. Dosw. Online 2007, 61, 805–818. [Google Scholar] [PubMed]
- Harapanahalli, A.K.; Younes, J.A.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Chemical signals and mechanosensing in bacterial responses to their environment. PLoS Pathog. 2015, 11, e1005057. [Google Scholar] [CrossRef]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 450–462. [Google Scholar]
- Oleksy-Sobczak, M.; Klewicka, E.; Piekarska-Radzik, L. Exopolysaccharides production by Lactobacillus rhamnosus strains—Optimization of synthesis and extraction conditions. LWT 2020, 122, 109055. [Google Scholar] [CrossRef]
- Ullrich, M. Bacterial Polysaccharides: Current Innovations and Future Trends; Horizon Scientific Press: Poole, UK, 2009; ISBN 1-904455-45-X. [Google Scholar]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Jha, B. Microbial Exopolysaccharides. In The Prokaryotes: Applied Bacteriology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 179–192. ISBN 978-3-642-31331-8. [Google Scholar]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (eps) producing lactic acid bacteria strains. LWT Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive exopolysaccharides from a S. thermophilus strain: Screening, purification and characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and composites of exopolysaccharides; properties and applications: A review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Microbial polysaccharides from gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Vanhaverbeke, C.; Heyraud, A.; Mazeau, K. Conformational Analysis of the exopolysaccharide from Burkholderia caribensis strain MWAP71: Impact on the interaction with soils. Biopolym. Orig. Res. Biomol. 2003, 69, 480–497. [Google Scholar]
- Torino, M.I.; Font de Valdez, G.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Majumder, A.; Goyal, A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J. Microbiol. 2012, 52, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Gangoiti, J.; Pijning, T.; Dijkhuizen, L. Biotechnological potential of novel glycoside hydrolase family 70 enzymes synthesizing α-glucans from starch and sucrose. Biotechnol. Adv. 2018, 36, 196–207. [Google Scholar] [CrossRef]
- Aburas, H.; İspirli, H.; Taylan, O.; Yilmaz, M.T.; Dertli, E. Structural and physicochemical characterisation and antioxidant activity of an α-D-Glucan produced by sourdough isolate Weissella cibaria MED17. Int. J. Biol. Macromol. 2020, 161, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ni, D.; Zhang, W.; Stressler, T.; Mu, W. Lactic acid bacteria-derived α-Glucans: From enzymatic synthesis to miscellaneous applications. Biotechnol. Adv. 2021, 47, 107708. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, S.S.; Kralj, S.; van Geel-Schutten, I.H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural analysis of the α-D-Glucan (EPS180) produced by the Lactobacillus reuteri strain 180 glucansucrase GTF180 Enzyme. Carbohydr. Res. 2008, 343, 1237–1250. [Google Scholar] [CrossRef] [Green Version]
- Argüello-Morales, M.A.; Remaud-Simeon, M.; Pizzut, S.; Sarçabal, P.; Willemot, R.-M.; Monsan, P. Sequence Analysis of the gene encoding alternansucrase, a sucrose glucosyltransferase from Leuconostoc mesenteroides NRRL B-1355. FEMS Microbiol. Lett. 2000, 182, 81–85. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Majumder, R.; Rather, I.A.; Kim, K. Extraction, isolation and purification of exopolysaccharide from lactic acid bacteria using ethanol precipitation method. Bangladesh J. Pharmacol. 2016, 11, 573–576. [Google Scholar] [CrossRef] [Green Version]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Bounaix, M.-S.; Gabriel, V.; Robert, H.; Morel, S.; Remaud-Siméon, M.; Gabriel, B.; Fontagné-Faucher, C. Characterization of Glucan-producing Leuconostoc strains isolated from sourdough. Int. J. Food Microbiol. 2010, 144, 1–9. [Google Scholar] [CrossRef]
- Chellapandian, M.; Larios, C.; Sanchez-Gonzalez, M.; Lopez-Munguia, A. Production and properties of a dextransucrase from Leuconostoc mesenteroides IBT-PQ isolated from ‘Pulque’, a traditional Aztec alcoholic beverage. J. Ind. Microbiol. Biotechnol. 1998, 21, 51–56. [Google Scholar] [CrossRef]
- Fabre, E.; Bozonnet, S.; Arcache, A.; Willemot, R.-M.; Vignon, M.; Monsan, P.; Remaud-Simeon, M. Role of the Two catalytic domains of DSR-E dextransucrase and their involvement in the formation of highly α-1, 2 branched dextran. J. Bacteriol. 2005, 187, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-K.; Kim, Y.-M.; Kim, D.-M. Functional, genetic, and bioinformatic characterization of dextransucrase (dsrbcb4) gene in Leuconostoc mesenteroides B-1299CB4. J. Microbiol. Biotechnol. 2008, 18, 1050–1058. [Google Scholar]
- Kang, H.-K.; Oh, J.-S.; Kim, D. Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α (1→6) glucan. FEMS Microbiol. Lett. 2009, 292, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralj, S.; van Geel-Schutten, G.H.; Dondorff, M.M.G.; Kirsanovs, S.; van der Maarel, M.J.E.C.; Dijkhuizen, L.Y. Glucan synthesis in the genus Lactobacillus: Isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology 2004, 150, 3681–3690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272. [Google Scholar] [CrossRef] [Green Version]
- Monchois, V.; Remaud-Simeon, M.; Monsan, P.; Willemot, R.-M. Cloning and sequencing of a gene coding for an extracellular dextransucrase (DSRB) from Leuconostoc mesenteroides NRRL B-1299 synthesizing only a α (1–6) glucan. FEMS Microbiol. Lett. 1998, 159, 307–315. [Google Scholar] [CrossRef]
- Mukasa, H.; Shimamura, A.; Tsumori, H. Purification and characterization of basic glucosyltransferase from Streptococcus mutans serotype C. Biochim. Biophys. Acta BBA Gen. Subj. 1982, 719, 81–89. [Google Scholar] [CrossRef]
- Neubauer, H.; Bauche, A.; Mollet, B. Molecular characterization and expression analysis of the dextransucrase DsrD of Leuconostoc mesenteroides Lcc4 in homologous and heterologous Lactococcus lactis cultures. Microbiology 2003, 149, 973–982. [Google Scholar] [CrossRef]
- Simpson, C.L.; Cheetham, N.W.; Jacques, N.A. Four glucosyltransferases, GtfJ, GtfK, GtfL and GtfM, from Streptococcus salivarius ATCC 25975. Microbiology 1995, 141, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Bechtner, J.; Wefers, D.; Schmid, J.; Vogel, R.F.; Jakob, F. Identification and comparison of two closely related dextransucrases released by water kefir borne Lactobacillus hordei TMW 1.1822 and Lactobacillus nagelii TMW 1.1827. Microbiology 2019, 165, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.G.; Collins, M.D.; Lawson, P.A.; Rodriguez, A.V. Lactobacillus nagelii sp. nov., an organism isolated from a partially fermented wine. Int. J. Syst. Evol. Microbiol. 2000, 50, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Okada, S. Lactobacillus satsumensis sp. Nov., isolated from mashes of shochu, a traditional japanese distilled spirit made from fermented rice and other starchy materials. Int. J. Syst. Evol. Microbiol. 2005, 55, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Kaneuchi, C.; Seki, M.; Komagata, K. Taxonomic study of Lactobacillus mali Carr and Davis 1970 and related strains: Validation of Lactobacillus mali Carr and Davis 1970 over Lactobacillus yamanashiensis Nonomura 1983. Int. J. Syst. Evol. Microbiol. 1988, 38, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A Taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. 2020, 2782–2858. [Google Scholar] [CrossRef]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Ikeda, T.; Kuramitsu, H.K. Expression of Streptococcus mutans Gtf Genes in Streptococcus milleri. Infect. Immun. 1992, 60, 2815–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monchois, V.; Arguello-Morales, M.; Russell, R.R. Isolation of an active catalytic core of Streptococcus downei MFe28 GTF-I glucosyltransferase. J. Bacteriol. 1999, 181, 2290–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerning, J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol. Lett. 1990, 87, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Côté, G.L.; Skory, C.D. Cloning, expression, and characterization of an insoluble glucan-producing glucansucrase from Leuconostoc mesenteroides NRRL B-1118. Appl. Microbiol. Biotechnol. 2012, 93, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Lamosa, P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Stripling, E.; Sanders, P.; van Geel-Schutten, G.H.; Dijkhuizen, L. Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl. Environ. Microbiol. 2005, 71, 3942–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Garai-Ibabe, G.; Dueñas, M.T.; Irastorza, A.; Sierra-Filardi, E.; Werning, M.L.; López, P.; Corbí, A.L.; Fernández de Palencia, P. Naturally occurring 2-substituted (1,3)-β-D-glucan producing Lactobacillus suebicus and Pediococcus parvulus strains with potential utility in the production of functional foods. Bioresour. Technol. 2010, 101, 9254–9263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Hou, Y.; Xu, Y.; Mørkeberg Krogh, K.B.R.; Tenkanen, M. Enzymatic analysis of levan produced by lactic acid bacteria in fermented doughs. Carbohydr. Polym. 2019, 208, 285–293. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Ozimek, L.K.; Kralj, S.; Van der Maarel, M.J.; Dijkhuizen, L. The levansucrase and inulosucrase enzymes of Lactobacillus reuteri 121 catalyse processive and non-processive transglycosylation reactions. Microbiology 2006, 152, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Mozzi, F.; Vaningelgem, F.; Hébert, E.M.; Van der Meulen, R.; Foulquié Moreno, M.R.; Font de Valdez, G.; De Vuyst, L. Diversity of heteropolysaccharide-producing lactic acid bacterium strains and their biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435. [Google Scholar] [CrossRef] [Green Version]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.-M.; Remaud-Siméon, M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Robyt, J.F. Mechanisms in the glucansucrase synthesis of polysaccharides and oligosaccharides from sucrose. Adv. Carbohydr. Chem. Biochem. 1995, 51, 133–168. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Jahanbin, K.; Gharibzahedi, S.M.T.; Taheri, S. Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 2012, 133, 383–389. [Google Scholar] [CrossRef]
- West, T.P. Synthesis of the microbial polysaccharide gellan from dairy and plant-based processing coproducts. Polysaccharides 2021, 2, 16. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Muddada, S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed Res. Int. 2017, 2017, 4201809. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Zamfir, M.; Mozzi, F.; Adriany, T.; Marshall, V.; Degeest, B.; Vaningelgem, F. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int. Dairy J. 2003, 13, 707–717. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Q.; Zhang, H.; Wu, Y.; Hang, X.; Ai, L. Exopolysaccharide produced by Streptococcus thermophiles S-3: Molecular, partial structural and rheological properties. Carbohydr. Polym. 2018, 194, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Gentès, M.-C.; St-Gelais, D.; Turgeon, S.L. Gel formation and rheological properties of fermented milk with in situ exopolysaccharide production by lactic acid bacteria. Dairy Sci. Technol. 2011, 91, 645–661. [Google Scholar] [CrossRef] [Green Version]
- Gentès, M.-C.; Turgeon, S.L.; St-Gelais, D. Impact of starch and exopolysaccharide-producing lactic acid bacteria on the properties of set and stirred yoghurts. Int. Dairy J. 2016, 55, 79–86. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, W.; Guo, Q.; Xiong, Z.; Wang, G.; Xia, Y.; Lai, P.; Yin, B.; Ai, L. Characterization of a yogurt-quality improving exopolysaccharide from Streptococcus thermophilus AR333. Food Hydrocoll. 2018, 81, 220–228. [Google Scholar] [CrossRef]
- Bouzar, F.; Cerning, J.; Desmazeaud, M. Exopolysaccharide production and texture-promoting abilities of mixed-strain starter cultures in yogurt production. J. Dairy Sci. 1997, 80, 2310–2317. [Google Scholar] [CrossRef]
- Loeffler, M.; Hilbig, J.; Velasco, L.; Weiss, J. Usage of in situ exopolysaccharide-forming lactic acid bacteria in food production: Meat products—A new field of application? Compr. Rev. Food Sci. Food Saf. 2020, 19, 2932–2954. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, H.; Hu, Z.; Liang, Y.; Guo, S.; Yang, M.; Du, R.; Wang, X. Immunostimulatory activity of exopolysaccharides from probiotic Lactobacillus casei WXD030 strain as a novel adjuvant in vitro and in vivo. Food Agric. Immunol. 2018, 29, 1086–1105. [Google Scholar] [CrossRef] [Green Version]
- Rani, R.P.; Anandharaj, M.; David Ravindran, A. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, Q.; Dong, M. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, X.; Suzuki, S.; Suzuki, K.; Kitamura, S. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2BT. J. Agric. Food Chem. 2004, 52, 5533–5538. [Google Scholar] [CrossRef]
- Górska, S.; Jachymek, W.; Rybka, J.; Strus, M.; Heczko, P.B.; Gamian, A. Structural and immunochemical studies of neutral exopolysaccharide produced by Lactobacillus johnsonii 142. Carbohydr. Res. 2010, 345, 108–114. [Google Scholar] [CrossRef]
- Degeest, B.; Janssens, B.; De Vuyst, L. Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0–1: Production kinetics, enzyme activities and EPS yields. J. Appl. Microbiol. 2001, 91, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and Antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef]
- Hu, G.; Fu, S.; Liu, H.; Lucia, L.A. Adsorption of cationized Eucalyptus heteropolysaccharides onto chemical and mechanical pulp fibers. Carbohydr. Polym. 2015, 123, 324–330. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-Functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-Coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S. Purification, partial structural characterization and health benefits of exopolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process. Biochem. 2020, 99, 79–86. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active enzymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Monnet, V.; Chapot-Chartier, M.-P. Cell walls and exopolysaccharides of lactic acid bacteria. In Proceedings of the The 10th LAB Symposium—Thirty Years of Research on Lactic Acid Bacteria; Media Labs: Rotterdam, The Netherlands, 2011; pp. 37–59. [Google Scholar]
- Korakli, M.; Vogel, R.F. Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl. Microbiol. Biotechnol. 2006, 71, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; Dijkstra, B.W.; Dijkhuizen, L. Glycosidic bond specificity of glucansucrases: On the role of acceptor substrate binding residues. Biocatal. Biotransform. 2012, 30, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Laws, A.; Gu, Y.; Marshall, V. Biosynthesis, characterisation, and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Monchois, V.; Willemot, R.-M.; Monsan, P. Glucansucrases: Mechanism of action and structure–function relationships. FEMS Microbiol. Rev. 1999, 23, 131–151. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; de los Reyes-Gavilán, C.G. Invited review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Growth and exopolysaccharide production during free and immobilized cell chemostat culture of Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2005, 98, 272–284. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Salazar, N.; De los Reyes-Gavilan, C.G. Biosynthesis and chemical composition of exopolysaccharides produced by lactic acid bacteria. Bact. Polysacch. Curr. Innov. Future Trends 2009, 279–310. [Google Scholar]
- Cirrincione, S.; Breuer, Y.; Mangiapane, E.; Mazzoli, R.; Pessione, E. “Ropy” phenotype, exopolysaccharides and metabolism: Study on food isolated potential probiotics LAB. Microbiol. Res. 2018, 214, 137–145. [Google Scholar] [CrossRef]
- Fukuda, K.; Shi, T.; Nagami, K.; Leo, F.; Nakamura, T.; Yasuda, K.; Senda, A.; Motoshima, H.; Urashima, T. Effects of carbohydrate source on physicochemical properties of the exopolysaccharide produced by Lactobacillus fermentum TDS030603 in a chemically defined medium. Carbohydr. Polym. 2010, 79, 1040–1045. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Madhuri, K.V.; Prabhakar, K.V. Microbial exopolysaccharides: Biosynthesis and potential applications. Orient. J. Chem. 2014, 30, 1401. [Google Scholar] [CrossRef] [Green Version]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic acid bacteria exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Mozzi, F.; Rollán, G.; De Giori, G.S.; De Valdez, G.F. Effect of galactose and glucose on the exopolysaccharide production and the activities of biosynthetic enzymes in Lactobacillus casei CRL 87. J. Appl. Microbiol. 2001, 91, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Petry, S.; Furlan, S.; Crepeau, M.-J.; Cerning, J.; Desmazeaud, M. Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl. Environ. Microbiol. 2000, 66, 3427–3431. [Google Scholar] [CrossRef] [Green Version]
- Robitaille, G.; Moineau, S.; St-Gelais, D.; Vadeboncoeur, C.; Britten, M. Detection and quantification of capsular exopolysaccharides from Streptococcus thermophilus using lectin probes. J. Dairy Sci. 2006, 89, 4156–4162. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [Green Version]
- Vuyst, D.; de Ven, V. Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J. Appl. Microbiol. 1998, 84, 1059–1068. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using uv spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Sun, L.; Sadiq, F.A.; Yang, Y.; Gao, J.; Sang, Y. Preparation screening, production optimization and characterization of exopolysaccharides produced by Lactobacillus sanfranciscensis Ls-1001 isolated from chinese traditional sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.M.; Pluta, A.S.; Garbowska, M.; Stasiak-Różańska, L. Exopolysaccharide-producing lactic acid bacteria—Health-promoting properties and application in the dairy industry. Adv. Microbiol. 2019, 58, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Zarour, K.; Vieco, N.; Pérez-Ramos, A.; Nácher-Vázquez, M.; Mohedano, M.L.; López, P. Chapter 4—Food ingredients synthesized by lactic acid bacteria. In Microbial Production of Food Ingredients and Additives; Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 89–124. ISBN 978-0-12-811520-6. [Google Scholar]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady, P.S. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the texture of yogurt by use of exopolysaccharide producing lactic acid bacteria. BioMed Res. Int. 2016, 2016, e7945675. [Google Scholar] [CrossRef]
- Patel, A.; Prajapati, J. Food and Health Applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- London, L.E.E.; Chaurin, V.; Auty, M.A.E.; Fenelon, M.A.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Use of Lactobacillus mucosae DPC 6426, an exopolysaccharide-producing strain, positively influences the techno-functional properties of yoghurt. Int. Dairy J. 2015, 40, 33–38. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-Starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Broadbent, J.R.; McMahon, D.J.; Oberg, C.J.; Welker, D.L. Use of exopolysaccharide-producing cultures to improve the functionality of low fat cheese. Int. Dairy J. 2001, 11, 433–439. [Google Scholar] [CrossRef]
- Costa, N.E.; Hannon, J.A.; Guinee, T.P.; Auty, M.A.E.; McSweeney, P.L.H.; Beresford, T.P. Effect of exopolysaccharide produced by isogenic strains of Lactococcus lactis on half-fat Cheddar cheese. J. Dairy Sci. 2010, 93, 3469–3486. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Xiao, H.; Zhou, Z.; Han, Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process. Biochem. 2019, 87, 187–195. [Google Scholar] [CrossRef]
- Şanli, T.; Gursel, A.; Şanli, E.; Acar, E.; Benli, M. The effect of using an exopolysaccharide-producing culture on the physicochemical properties of low-fat and reduced-fat Kasar cheeses. Int. J. Dairy Technol. 2013, 66, 535–542. [Google Scholar] [CrossRef]
- Surber, G.; Schäper, C.; Wefers, D.; Rohm, H.; Jaros, D. Exopolysaccharides from Lactococcus lactis affect manufacture, texture and sensory properties of concentrated acid milk gel suspensions (fresh cheese). Int. Dairy J. 2021, 112, 104854. [Google Scholar] [CrossRef]
- Coelho Nepomuceno, R.S.C.; Gonçalves Costa, L.C., Jr.; Bueno Costa, R.G. Exopolysaccharide-producing culture in the manufacture of prato cheese. LWT Food Sci. Technol. 2016, 72, 383–389. [Google Scholar] [CrossRef]
- Rehman, R.; Wang, Y.; Wang, J.; Geng, W. Physicochemical analysis of Mozzarella cheese produced and developed by the novel EPS-producing strain Lactobacillus kefiranofaciens ZW3. Int. J. Dairy Technol. 2018, 71, 90–98. [Google Scholar] [CrossRef]
- Carrero-Puentes, S.; Fuenmayor, C.; Jiménez-Pérez, C.; Guzmán-Rodríguez, F.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Alatorre-Santamaría, S.; García-Garibay, M.; Cruz-Guerrero, A. Development and characterization of an exopolysaccharide-functionalized acid whey cheese (Requesón) using Lactobacillus delbrueckii ssp. bulgaricus. J. Food Process. Preserv. 2021, e16095. [Google Scholar] [CrossRef]
- Hamet, M.F.; Piermaria, J.A.; Abraham, A.G. Selection of EPS-producing Lactobacillus strains isolated from kefir grains and rheological characterization of the fermented milks. LWT Food Sci. Technol. 2015, 63, 129–135. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Song, X.; Guo, M. Effects of kefir grains from different origins on proteolysis and volatile profile of goat milk kefir. Food Chem. 2021, 339, 128099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q. Isolation, purification, characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef]
- Botelho, P.S.; Maciel, M.I.S.; Bueno, L.A.; Marques, M.d.F.F.; Marques, D.N.; Sarmento Silva, T.M. Characterisation of a new exopolysaccharide obtained from of fermented kefir grains in soymilk. Carbohydr. Polym. 2014, 107, 1–6. [Google Scholar] [CrossRef]
- Egea, M.B.; dos Santos, D.C.; de Oliveira Filho, J.G.; da Costa Ores, J.; Takeuchi, K.P.; Lemes, A.C. A review of nondairy kefir products: Their characteristics and potential human health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 1–17. [Google Scholar] [CrossRef]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of selected lactic acid bacteria and quinoa flour for manufacturing novel yogurt-like beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Păcularu-Burada, B.; Georgescu, L.A.; Bahrim, G.-E. Current approaches in sourdough production with valuable characteristics for technological and functional applications. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2020, 44, 132–148. [Google Scholar] [CrossRef]
- Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Sugar reduction in bakery products: Current strategies and sourdough technology as a potential novel approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef]
- Tinzl-Malang, S.K.; Grattepanche, F.; Rast, P.; Fischer, P.; Sych, J.; Lacroix, C. Purified exopolysaccharides from Weissella confusa 11GU-1 and Propionibacterium freudenreichii JS15 act synergistically on bread structure to prevent staling. LWT 2020, 127, 109375. [Google Scholar] [CrossRef]
- Tang, X.; Liu, R.; Huang, W.; Zhang, B.; Wu, Y.; Zhuang, J.; Omedi, J.O.; Wang, F.; Zheng, J. Impact of in situ formed exopolysaccharides on dough performance and quality of Chinese steamed bread. LWT 2018, 96, 519–525. [Google Scholar] [CrossRef]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of amaranth and quinoa flours on exopolysaccharide production and protein profile of liquid sourdough fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef]
- Zhang, B.; Omedi, J.O.; Zheng, J.; Huang, W.; Jia, C.; Zhou, L.; Zou, Q.; Li, N.; Gao, T. Exopolysaccharides in sourdough fermented by Weissella confusa QS813 protected protein matrix and quality of frozen gluten-red bean dough during freeze-thaw cycles. Food Biosci. 2021, 43, 101180. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Singh, P.; Kumar, M.; Al Khodor, S. Role of the gut microbiota in the pathogenesis of coeliac disease and potential therapeutic implications. Eur. J. Nutr. 2020, 59, 3369–3390. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the functionality of exopolysaccharides produced by sourdough lactic acid bacteria in bread and steamed bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef]
- Kumar, Y. Development of low-fat/reduced-fat processed meat products using fat replacers and analogues. Food Rev. Int. 2021, 37, 296–312. [Google Scholar] [CrossRef]
- Andrès, S.; Zaritzky, N.; Califano, A. The effect of whey protein concentrates and hydrocolloids on the texture and colour characteristics of chicken sausages. Int. J. Food Sci. Technol. 2006, 41, 954–961. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Schuh, V.; Weiss, J. Effects of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) as fat replacers on the microstructure and sensory characteristics of fried beef patties. Food Hydrocoll. 2015, 45, 236–246. [Google Scholar] [CrossRef]
- Verbeken, D.; Neirinck, N.; Van Der Meeren, P.; Dewettinck, K. Influence of κ-Carrageenan on the thermal gelation of salt-soluble meat proteins. Meat Sci. 2005, 70, 161–166. [Google Scholar] [CrossRef]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. The influence of exopolysaccharide-producing lactic acid bacteria on reconstructed ham. Int. J. Food Sci. Technol. 2019, 54, 2763–2769. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (Sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef]
- Harutoshi, T. Exopolysaccharides of lactic acid bacteria for food and colon health applications. In Lactic Acid Bacteria-R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013. [Google Scholar]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Singh, P.; Saini, P. Food and health potentials of exopolysaccharides derived from Lactobacilli. Microbiol. Res. J. Int. 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Trapet, L.; de Vries, E.; Abee, T.; Hugenholtz, J. Physiological function of exopolysaccharides produced by Lactococcus lactis. Int. J. Food Microbiol. 2001, 64, 71–80. [Google Scholar] [CrossRef]
- Sharma, S.; Padhi, S.; Kumari, M.; Rai, A.K.; Sahoo, D. Bioactive Compounds in Fermented Foods. Health Aspects; CRC Press: Boca Raton, FL, USA, 2021; p. 48. [Google Scholar]
- Balzaretti, S.; Taverniti, V.; Guglielmetti, S.; Fiore, W.; Minuzzo, M.; Ngo, H.N.; Ngere, J.B.; Sadiq, S.; Humphreys, P.N.; Laws, A.P. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 2017, 83, e02702–e02716. [Google Scholar] [CrossRef] [Green Version]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef]
- Hooshdar, P.; Kermanshahi, R.K.; Ghadam, P.; Khosravi-Darani, K. A review on production of exopolysaccharide and biofilm in probiotics like Lactobacilli and methods of analysis. Biointerface Res. Appl. Chem. 2020, 10, 6058–6075. [Google Scholar]
- Oerlemans, M.M.P.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Patten, D.A.; Laws, A.P. Lactobacillus-produced exopolysaccharides and their potential health benefits: A review. Benef. Microbes 2015, 6, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; dos Santos, K.M.O.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, High production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-Mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Ripari, V. Techno-functional role of exopolysaccharides in cereal-based, yogurt-like beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [Green Version]
- Ruas-Madiedo, P. Biosynthesis and bioactivity of exopolysaccharides produced by probiotic bacteria. Food Oligosacch. Prod. Anal. Bioact. 2014, 118–133. [Google Scholar]
- Darilmaz, D.O.; Beyatli, Y. Investigating hydrophobicity and the effect of exopolysaccharide on aggregation properties of dairy propionibacteria isolated from Turkish homemade cheeses. J. Food Prot. 2012, 75, 359–365. [Google Scholar] [CrossRef]
- Abedfar, A.; Hossininezhad, M. Overview of the most important characterization of exopolysaccharides produced by probiotics bacteria and their biological function. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 47–55. [Google Scholar]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Farrag, A.R.H.; Esawy, M.A. In Vivo Assessment of possible probiotic properties of Bacillus subtilis and prebiotic properties of levan. Biocatal. Agric. Biotechnol. 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In vitro prebiotic activities of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Foods 2020, 67, 103853. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; Clara, G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Wang, Y.; Xu, C.; Lu, Z.; Huang, M.; Wang, Y. Preparation and biological activities of an exopolysaccharide produced by Enterobacter cloacae Z0206. Carbohydr. Polym. 2010, 81, 607–611. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kano, H.; Sashihara, T.; Sugano, H.; Horiuchi, H.; Saito, T.; Oda, M. Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2006, 89, 2873–2881. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Cantabrana, C.; Nikolic, M.; López, P.; Suárez, A.; Miljkovic, M.; Kojic, M.; Margolles, A.; Golic, N.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strains and their polymers elicit different responses on immune cells from blood and gut associated lymphoid tissue. Anaerobe 2014, 26, 24–30. [Google Scholar] [CrossRef]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.-K.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nampoothiri, K.M. Exposition of antitumour activity of a chemically characterized exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510. Biologia 2013, 68, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, H.; Harata, T.; Uemura, J.; Saito, T.; Kaneko, T.; Itoh, T. Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int. J. Food Microbiol. 1998, 40, 169–175. [Google Scholar] [CrossRef]
- Vitlic, A.; Sadiq, S.; Ahmed, H.I.; Ale, E.C.; Binetti, A.G.; Collett, A.; Humpreys, P.N.; Laws, A.P. Evidence for the modulation of the immune response in peripheral blood mononuclear cells after stimulation with a high molecular weight β-glucan isolated from Lactobacillus fermentum Lf2. bioRxiv 2018, 400267. [Google Scholar] [CrossRef] [Green Version]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Jones, S.E.; Paynich, M.L.; Knight, K.L. Exopolysaccharides: Sweet success with probiotic therapeutics. Inflamm. Cell Signal. 2014, 1, e334. [Google Scholar] [CrossRef]
- Liu, C.-T.; Chu, F.-J.; Chou, C.-C.; Yu, R.-C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 721, 157–162. [Google Scholar] [CrossRef]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Nagy, A.; Stanton, C.; Ross, P.R.; Gelencsér, E.; Silva, C.C.G. Immunomodulatory activity of exopolysaccharide producing Leuconostoc citreum strain isolated from Pico cheese. J. Funct. Foods 2017, 33, 235–243. [Google Scholar] [CrossRef]
- Schiavi, E.; Plattner, S.; Rodriguez-Perez, N.; Barcik, W.; Frei, R.; Ferstl, R.; Kurnik-Lucka, M.; Groeger, D.; Grant, R.; Roper, J.; et al. Exopolysaccharide from Bifidobacterium longum subsp. longum 35624TM modulates murine allergic airway responses. Benef. Microbes 2018, 9, 761–773. [Google Scholar] [CrossRef]
- Nowak, B.; Śróttek, M.; Ciszek-Lenda, M.; Skałkowska, A.; Gamian, A.; Górska, S.; Marcinkiewicz, J. Exopolysaccharide from Lactobacillus rhamnosus KL37 inhibits T cell-dependent immune response in mice. Arch. Immunol. Ther. Exp. 2020, 68, 17. [Google Scholar] [CrossRef]
- Matsuzaki, C.; Hayakawa, A.; Matsumoto, K.; Katoh, T.; Yamamoto, K.; Hisa, K. Exopolysaccharides produced by Leuconostoc mesenteroides strain NTM048 as an immunostimulant to enhance the mucosal barrier and influence the systemic immune response. J. Agric. Food Chem. 2015, 63, 7009–7015. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Zhang, J.; Li, Z.; Wang, C.; Chen, M.; Wang, Y.; Sun, Y.; Wang, L.; Luo, C. A Compatibility assay of ursolic acid and foodborne microbial exopolysaccharides by antioxidant power and anti-proliferative properties in hepatocarcinoma cells. J. Food Agric. Environ. 2012, 10, 111–114. [Google Scholar]
- Nguyen, N.D.; Le, A.Q.; Nguyen, Q.H. Electron beam/γ-ray irradiation synthesis of gold nanoparticles and investigation of antioxidant activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 045002. [Google Scholar] [CrossRef] [Green Version]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT 2018, 87, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco-2 cell model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.-S.; Kim, C.-S.; Lee, S.-P. A new in vitro assay of cholesterol adsorption by food and microbial polysaccharides. J. Med. Food 2003, 6, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Suzuki, Y.; Hirota, T. Cholesterol lowering activity of ropy fermented milk. J. Food Sci. 1992, 57, 1327–1329. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, H.; Guo, B.; Chen, W.; Wu, Z.; Wu, Y. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W. Carbohydr. Polym. 2008, 74, 353–357. [Google Scholar] [CrossRef]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Amor, A.B.; Mastouri, M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from Kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of antioxidant, antibacterial and cytotoxicity activities of exopolysaccharide from Enterococcus strains isolated from traditional Iranian kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ktari, N.; Ben Slima, S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben Salah, R. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus Sp.Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Popoola, A.O. Biogenic synthesis and antimicrobial activity of silver nanoparticle using exopolysaccharides from lactic acid bacteria. Int. J. Nano Dimens. 2017, 8, 61–69. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, S.; Ikegami, S.; Itoh, H.; Yamada, H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Abd El Ghany, K.; Hamouda, R.; Abd Elhafez, E.; Mahrous, H.; Salem-Bekhit, M.; Hamza, H.A. A potential role of Lactobacillus acidophilus LA1 and its exopolysaccharides on cancer cells in male albino mice. Biotechnol. Biotechnol. Equip. 2015, 29, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Deepak, V.; Ram Kumar Pandian, S.; Sivasubramaniam, S.D.; Nellaiah, H.; Sundar, K. Optimization of anticancer exopolysaccharide production from probiotic Lactobacillus acidophilus by response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 288–297. [Google Scholar] [CrossRef]
- Gao, F.; Li, L.; Zhang, H.; Yang, W.; Chen, H.; Zhou, J.; Zhou, Z.; Wang, Y.; Cai, Y.; Li, X. Deoxycholic acid modified-carboxymethyl curdlan conjugate as a novel carrier of epirubicin: In vitro and in vivo studies. Int. J. Pharm. 2010, 392, 254–260. [Google Scholar] [CrossRef]
- Liang, T.-W.; Wu, C.-C.; Cheng, W.-T.; Chen, Y.-C.; Wang, C.-L.; Wang, I.-L.; Wang, S.-L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, Y.; Lin, Q.; Liu, H.; Shen, C.; Nan, K.; Chen, H. In vitro and in vivo evaluation of xanthan gum–succinic anhydride hydrogels for the ionic strength-sensitive release of antibacterial agents. J. Mater. Chem. B 2016, 4, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
| HoPS | LAB | Mw/Structure | Reference | |
|---|---|---|---|---|
| α-D-glucans | Dextran | Leuconostoc mesenteroides Leuconostoc citreum Leuconostoc pseudomesenteroides Lentilactobacillus parabuchneri (formerly Lactobacillus parabuchneri) Limosilactobacillus fermentum (formerly Lactobacillus fermentum) Limosilactobacillus reuteri (formerly Lactobacillus reuteri) Latilactobacillus sakei (formerly Lactobacillus sakei) Latilactobacillus curvatus (formerly Lactobacillus curvatus) Lactobacillus hordei Lactobacillus nagelli Lactobacillus mali Lactobacillus satsumensis Weissella confusa Weissella cibaria Streptococcus mutans Streptococcus salivarius | Mw: 103–107 Da α-D-Glc(1,6) | [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| Mutan | Limosilactobacillus reuteri Streptococcus downei Streptococcus mutans Streptococcus salivarius | Mw: >106 Da α-D-Glc(1,3) | [35,36,40,48,49,50] | |
| Alternan | Leuconostoc mesenteroides Streptococcus salivarius Leuconostoc citreum | Mw: >106 Da (α-D-Glc(1,6)/α-D-Glc(1,3) | [51,52,53] | |
| Reuteran | Limosilactobacillus reuteri | Mw: 107 Da α-D-Glc(1,4)/α-D-Glc(1,6) | [40,54] | |
| β-Glucans | Lactobacillus suebicus Pediococcus parvulus | Mw: 105–106 Da [β-D-Glc(1,3) with side chain linked (1,2)] | [55,56] | |
| Fructans | Levans | Leuconostoc mesenteroides Limosilactobacillus reuteri Streptococcus mutans Bacillus subtilis | Mw: 104–108 Da β-D-Fru(2,6) | [47,57] |
| Inulin-type | Streptococcus mutans Limosilactobacillus reuteri Leuconostoc citreum Lactobacillus johnsonii | Mw: 103–107 Da β-D-Fru(2,1) | [20,55,58,59] | |
| Polygalactan | Lactococcus lactis Lactobacillus delbruecki | pentameric repeating unit of galactose | [47] |
| LAB | HePS Composition | Molecular Weight | Reference |
|---|---|---|---|
| Streptococcus thermophilus CC30 | Glucose, galactose | 58 to 180 kDa | [67] |
| Streptococcus thermophilus CH101 | Glucose, galactose | 8.5 × 105 Da | [68] |
| Streptococcus thermophilus LY03 | Glucose, galactose, N-acetylgalactosamine | 1.8 × 106 Da | [68] |
| Streptococcus thermophilus S-3 | N-acetylgalactosamine, galactose, glucose | 5.7 × 105 Da | [69] |
| Streptococcus thermophilus NIZO2104 | Galactose, ribose, N-acetylgalactosamine, glucose | 0.9 × 106 Da | [70,71] |
| Streptococcus thermophilus AR333 | Galactose, glucose, galactosamine | 3.1 × 105 Da | [72] |
| Lactobacillus delbruecki subsp. bulgaricus CNRZ 1187 | Rhamnose, arabinose, mannose, galactose, glucose | 104–106 Da | [73,74] |
| Lactobacillus delbruecki subsp. bulgaricus DGCC291 | Glucose, galactose | 1.4 × 106 Da | [70,71] |
| Lactobacillus delbruecki subsp. bulgaricus NCIMB702074 | Glucose, galactose | 1.8 × 106 Da | [70,71] |
| Lacticaseibacillus casei (formerly Lactobacillus casei) WXD030 | Glucose, glucosamine, mannose | 37.37 kDa | [75] |
| Lactobacillus gasseri FR4 | Glucose, mannose, galactose, rhamnose, fucose | 1.9 × 105 Da | [76] |
| Lactobacillus helveticus MB2-1 | Glucose, mannose, galactose, rhamnose, arabinose | 1.83 × 105 Da | [77] |
| Lactobacillus kefiranofaciens WT-2B | Kefiran: glucose, galactose | 7.6 × 105 Da | [78] |
| Lactobacillus johnsonii 142 | D-glucose and D-dalactose | 1.0 × 105 Da | [79] |
| Latilactobacillus sakei O-1 (formerly Lactobacillus sakei) | Glucose, rhamnose | 6 × 106 Da | [80] |
| Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) JLK0142 | Glucose, galactose | 1.34 × 105 Da | [81] |
| Lactiplantibacillus plantarum WLPL04 | Xylose, glucose, galactose | 6.61 × 104 Da | [82] |
| Lactiplantibacillus plantarum YW11 | Glucose, galactose | 1.1 × 105 Da | [83] |
| Lactiplantibacillus plantarum JLAU103 | Arabinose, rhamnose, fucose, xylose, mannose, fructose, galactose, glucose | 12.4 kDa | [84] |
| Lactiplantibacillus plantarum EP56 | Glucose, galactose, rhamnose | 8.5×105 Da | [85] |
| Lactiplantibacillus plantarum C88 | Glucose, galactose | 1.2 × 106 Da | [86] |
| Lactiplantibacillus plantarum C70 | Arabinose, mannose, glucose, galactose | 3.8 × 105 Da | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. https://doi.org/10.3390/foods11020156
Jurášková D, Ribeiro SC, Silva CCG. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods. 2022; 11(2):156. https://doi.org/10.3390/foods11020156
Chicago/Turabian StyleJurášková, Dominika, Susana C. Ribeiro, and Celia C. G. Silva. 2022. "Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties" Foods 11, no. 2: 156. https://doi.org/10.3390/foods11020156
APA StyleJurášková, D., Ribeiro, S. C., & Silva, C. C. G. (2022). Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods, 11(2), 156. https://doi.org/10.3390/foods11020156







