Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Housing and Feeding
2.2. Slaughter Procedure and Collection of Sheep Primals
2.3. pH, Cutup, Packaging and Retail Display
2.4. Instrumental Colour Measurement
2.5. Purge and Cooking Loss
2.6. Warner-Bratzler Shear Force
2.7. Texture Profile Analysis (TPA)
2.8. Lipid Oxidation
2.9. Total Carbonyl Content
2.10. Free Thiol Content
2.11. Statistical Analysis
3. Results
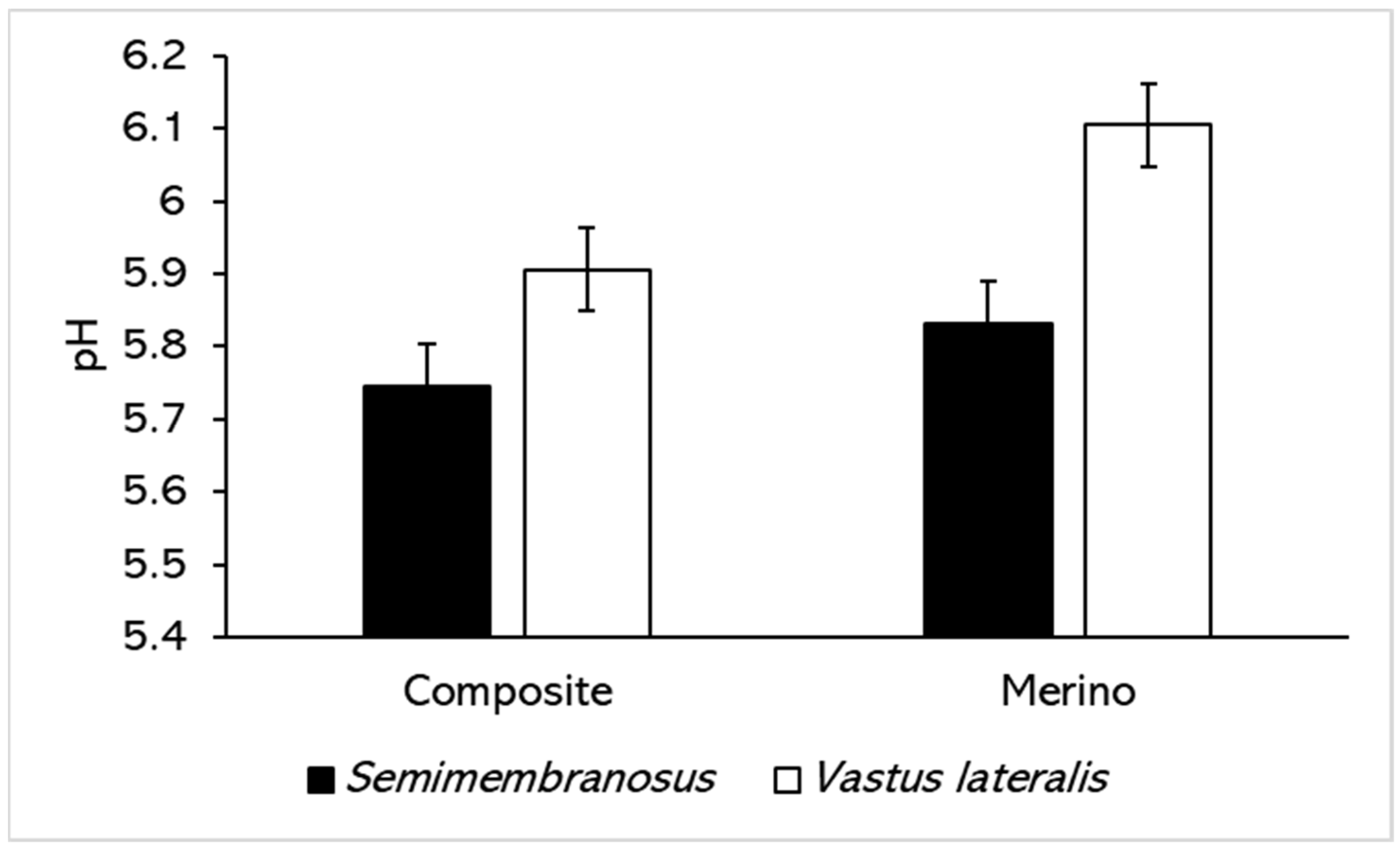
3.1. pH and Colour
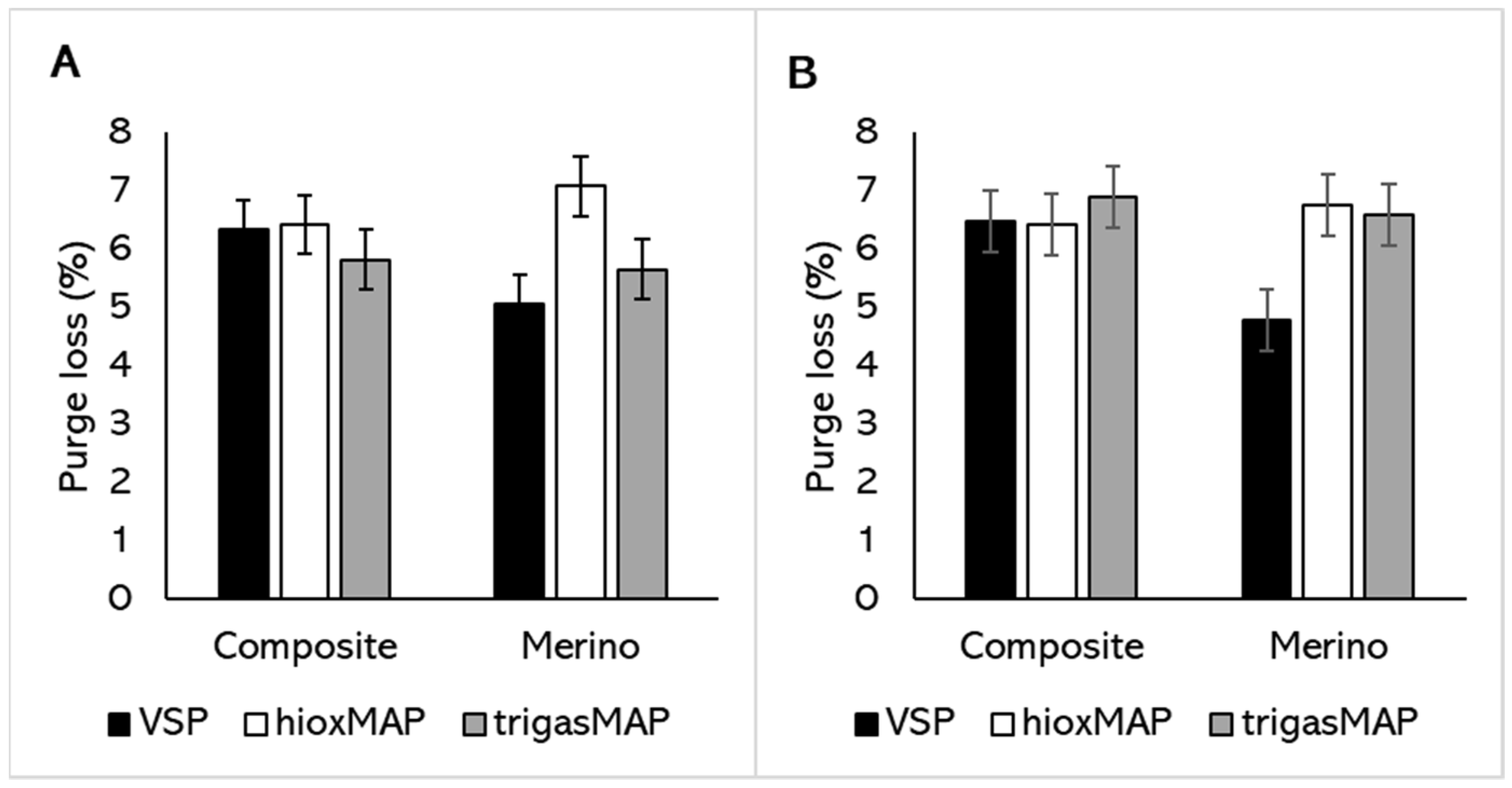
3.2. Water Holding Capacity
3.3. WBSF and Texture Profile Analysis
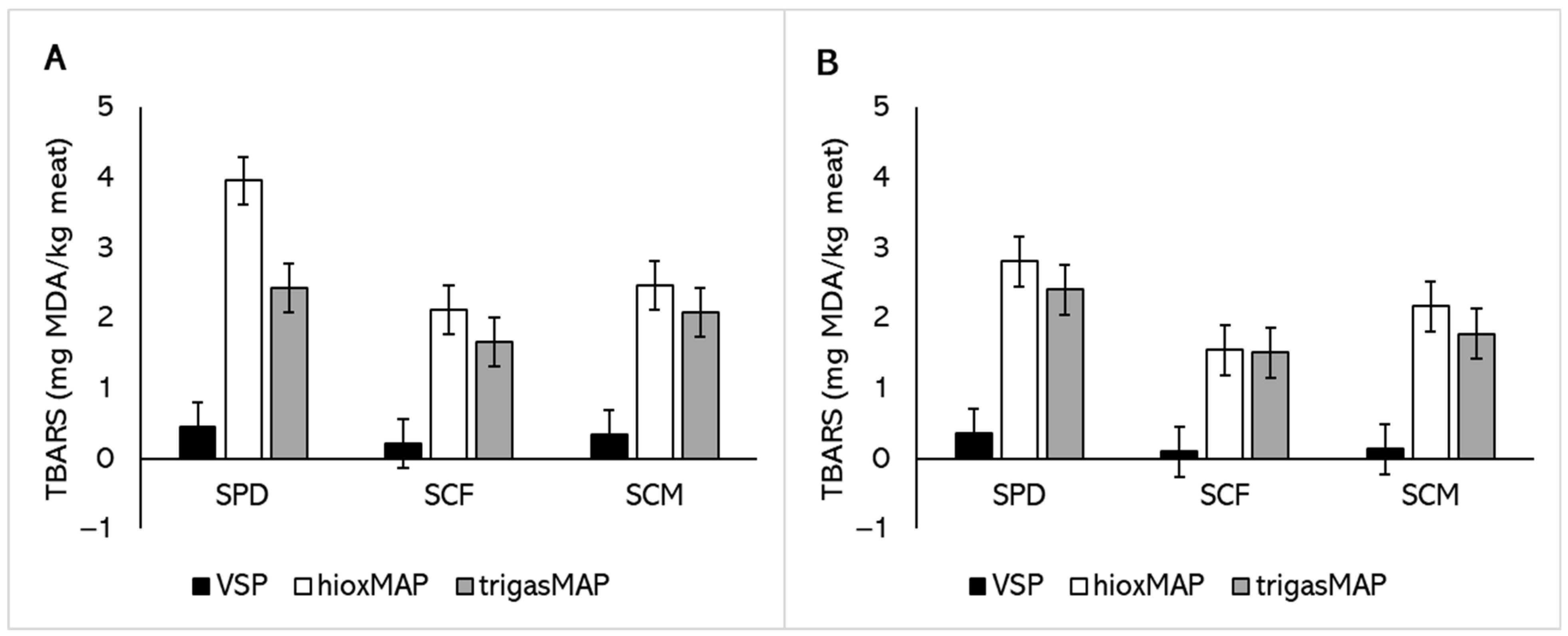
3.4. Lipid Oxidation
3.5. Protein Oxidation
4. Discussion
4.1. Colour and pH
4.2. Water Holding Capacity and Texture
4.3. Lipid Oxidation
4.4. Protein Oxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, D.C.; Geesink, G.; Alyarenga, T.I.R.C.; Polkinghorne, R.; Stark, J.; Lee, M.; Warner, R. Impact of high oxygen and vacuum retail ready packaging formats on lamb loin and topside eating quality. Meat Sci. 2017, 123, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Geesink, G.; Robertson, J.; Ball, A. The effect of retail packaging method on objective and consumer assessment of beef quality traits. Meat Sci. 2015, 104, 85–89. [Google Scholar] [CrossRef]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Torngren, M.A.; Darre, M.; Gunvig, A.; Bardenshtein, A. Case studies of packaging and processing solutions to improve meat quality and safety. Meat Sci. 2018, 144, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Pennisi, P.; Bella, M.; Priolo, A. Lipid and colour stability of meat from lambs fed fresh herbage or concentrate. Meat Sci. 2009, 82, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Dunshea, F.R.; Warner, R.D. Use of lucerne hay in ruminant feeds to improve animal productivity, meat nutritional value and meat preservation under a more variable climate. Meat Sci. 2020, 170, 108235. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; Muir, S.K.; Plozza, T.E.; Kerr, M.G.; Brown, W.G.; Jacobs, J.L.; Knight, M.I. Lipid oxidation and colour stability of lamb and yearling meat (muscle longissimus lumborum) from sheep supplemented with camelina-based diets after short-, medium-, and long-term storage. Antioxidants 2021, 10, 166. [Google Scholar] [CrossRef]
- Quezada, N.; Cherian, G. Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid. Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Cloete, J.; Hoffman, L.; Cloete, S. A comparison between slaughter traits and meat quality of various sheep breeds: Wool, dual-purpose and mutton. Meat Sci. 2012, 91, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.I.; van der Werf, J.H.; Jacob, R.H.; Hopkins, D.L.; Pannier, L.; Pearce, K.L.; Gardner, G.E.; Warner, R.D.; Geesink, G.H.; Edwards, J.E.; et al. Genetic parameters for meat quality traits of Australian lamb meat. Meat Sci. 2014, 96, 1016–1024. [Google Scholar] [CrossRef]
- Peng, Y.L.; Adhiputra, K.; Padayachee, A.; Channon, H.; Ha, M.; Warner, R.D. High oxygen modified atmosphere packaging negatively influences consumer acceptability traits of pork. Foods 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, G.; Jorgensen, S.S. A critical examination of some experimental variables in the 2-thiobarbituric acid (TBA) test for lipid oxidation in meat products. Z. Lebensm. Unters. Forsch. 1996, 202, 205–210. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Jacob, R.H.; D’Antuono, M.F.; Gilmour, A.R.; Warner, R.D. Phenotypic characterisation of colour stability of lamb meat. Meat Sci. 2014, 96, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.; Morton, J.D.; Bhat, Z.F.; Kong, L. Meat color: Factors affecting color stability. In Encyclopedia of Food Chemistry; Varelis, P., Melton, L., Shahidi, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2, pp. 202–210. [Google Scholar]
- Resconi, V.C.; Escudero, A.; Beltran, J.A.; Olleta, J.L.; Sanudo, C.; Campo, M.D. Color, lipid oxidation, sensory quality, and aroma compounds of beef steaks displayed under different levels of oxygen in a modified atmosphere package. J. Food Sci. 2012, 77, S10–S18. [Google Scholar] [CrossRef]
- Taylor, A.; Down, N.; Shaw, B. A comparison of modified atmosphere and vacuum skin packing for the storage of red meats. Int. J. Food Sci. Technol. 1990, 25, 98–109. [Google Scholar] [CrossRef]
- Renerre, M. Oxidative processes and myoglobin. In Antioxidants in Muscle Foods; Decker, E., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2000; Volume 2000, pp. 113–133. [Google Scholar]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef]
- Sorheim, O.; Nissen, H.; Nesbakken, T. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Sci. 1999, 52, 157–164. [Google Scholar] [CrossRef]
- Khliji, S.; van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef]
- Cayuela, J.M.; Gil, M.D.; Banon, S.; Garrido, M.D. Effect of vacuum and modified atmosphere packaging on the quality of pork loin. Eur. Food Res. Technol. 2004, 219, 316–320. [Google Scholar] [CrossRef]
- Bağdatli, A.; Kayaardi, S. Influence of storage period and packaging methods on quality attributes of fresh beef steaks. CyTA-J. Food 2015, 13, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Lagerstedt, A.; Lundstrom, K.; Lindahl, G. Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. longissimus dorsi steaks after different ageing times. Meat Sci. 2011, 87, 101–106. [Google Scholar] [CrossRef]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Clausen, I.; Jakobsen, M.; Ertbjerg, P.; Madsen, N.T. Modified atmosphere packaging affects lipid oxidation, myofibrillar fragmentation index and eating quality of beef. Packag. Technol. Sci. 2009, 22, 85–96. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, ageing and temperature influence the changes in texture, cooking loss and shrinkage of cooked beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef]
- Vaskoska, R.; Venien, A.; Ha, M.; White, J.D.; Unnithan, R.R.; Astruc, T.; Warner, R.D. Thermal denaturation of proteins in the muscle fibre and connective tissue from bovine muscles composed of type I (masseter) or type II (cutaneous trunci) fibres: DSC and FTIR microspectroscopy study. Food Chem. 2021, 343, 128544. [Google Scholar] [CrossRef] [PubMed]
- Stolowski, G.D.; Baird, B.E.; Miller, R.K.; Savell, J.W.; Sams, A.R.; Taylor, J.F.; Sanders, J.O.; Smith, S.B. Factors influencing the variation in tenderness of seven major beef muscles from three Angus and Brahman breed crosses. Meat Sci. 2006, 73, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.Q.; Liu, R.; Zhang, W.G.; Li, Y.P.; Wang, J.; Zhou, G.H. Effects of different packaging systems on beef tenderness through protein modifications. Food Bioprocess Technol. 2015, 8, 580–588. [Google Scholar] [CrossRef]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; O’Neill, E.E.; Kerry, J.P. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chem. 2012, 131, 527–532. [Google Scholar] [CrossRef]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; Walsh, H.; Allen, P.; Kerry, J.P. Sensory comparison of commercial low and high oxygen modified atmosphere packed sirloin beef steaks. Meat Sci. 2011, 88, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.L.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, G.H.; Zhang, W.G. Effects of high oxygen packaging on tenderness and water holding capacity of pork through protein oxidation. Food Bioprocess Technol. 2015, 8, 2287–2297. [Google Scholar] [CrossRef]
- Jongberg, S.; Wen, J.; Tørngren, M.A.; Lund, M.N. Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packag. Shelf Life 2014, 1, 38–48. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Galvin, K.; Moloney, A.; Troy, D.; O’Sullivan, K.; Kerry, J. Effect of pre-slaughter rations of forage and/or concentrates on the composition and quality of retail packaged beef. Meat Sci. 2003, 63, 279–286. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Rossetti, L.; Grigioni, G.; Irurueta, M.; Sancho, A.M.; Carrete, J.; Pensel, N.A. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 2007, 75, 299–307. [Google Scholar] [CrossRef]
- Cieslak, A.; Stanisz, M.; Wojtowski, J.; Pers-Kamczyc, E.; Szczechowiak, J.; El-Sherbiny, M.; Szumacher-Strabel, M. Camelina sativa affects the fatty acid contents in M-longissimus muscle of lambs. Eur. J. Lipid Sci. Technol. 2013, 115, 1258–1265. [Google Scholar] [CrossRef]
- Spanos, D.; Torngren, M.A.; Christensen, M.; Baron, C.P. Effect of oxygen level on the oxidative stability of two different retail pork products stored using modified atmosphere packaging (MAP). Meat Sci. 2016, 113, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Prache, S.; Schreurs, N.; Guillier, L. Review: Factors affecting sheep carcass and meat quality attributes. Animal 2021, 100330. [Google Scholar] [CrossRef] [PubMed]

| Effect | Treatment | L* (Lightness) | a* (Redness) | b* (Yellowness) | h* (Hue Angle) | C* (Chroma) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | ||
| Semimembranosus Constant 1 | 37.5 ± 1.37 | 20.41 ± 1.02 | 19.63 ± 0.98 | 43.67 ± 2.33 | 28.55 ± 1.16 | ||||||
| Breed | Merino | −2.4 ± 2.56 | <0.001 | 1.87 ± 1.96 | 0.383 | −3.6 ± 1.93 | 0.278 | −8.35 ± 4.63 | 0.402 | −1.14 ± 2.28 | 0.927 |
| Feed | SCF 2 | 2.7 ± 2.44 | 0.194 | −1.89 ± 1.82 | 0.418 | −1.70 ± 1.78 | 0.712 | 1.86 ± 4.27 | 0.671 | −2.93 ± 2.12 | 0.29 |
| SCM 3 | −2.3 ± 2.44 | 0.194 | 0.46 ± 1.82 | 0.418 | 1.01 ± 1.78 | 0.712 | 2.14 ± 4.27 | 0.671 | 0.68 ± 2.12 | 0.29 | |
| Packaging | HioxMAP 4 | 9.91 ± 1.57 | <0.001 | −11.71 ± 1.48 | <0.001 | −6.46 ± 1.24 | <0.001 | 13.3 ± 3.24 | <0.001 | −12.64 ± 1.63 | <0.001 |
| TrigasMAP 5 | 9.72 ± 1.57 | <0.001 | −11.39 ± 1.48 | <0.001 | −7.66 ± 1.24 | <0.001 | 9.6 ± 3.24 | <0.001 | −13.44 ± 1.63 | <0.001 | |
| Vastus lateralis Constant 6 | 38.99 ± 1.52 | 19.33 ± 1.05 | 18.37 ± 0.84 | 42.96 ± 2.5 | 26.74 ± 1.07 | ||||||
| Breed | Merino | 0.62 ± 2.48 | <0.001 | −2.94 ± 1.74 | 0.005 | −5.18 ± 1.37 | 0.043 | −2.75 ± 4.11 | 0.003 | −5.33 ± 1.75 | 0.08 |
| Feed | SCF 2 | 1.38 ± 2.49 | 0.63 | −0.89 ± 1.72 | 0.104 | −0.22 ± 1.39 | 0.174 | 1.52 ± 4.05 | 0.452 | −0.78 ± 1.76 | 0.053 |
| SCM 3 | 1.58 ± 2.49 | 0.63 | −0.35 ± 1.72 | 0.104 | 2.07 ± 1.39 | 0.174 | 4.19 ± 4.05 | 0.452 | 1.21 ± 1.76 | 0.053 | |
| Packaging | HioxMAP 4 | 9.74 ± 1.48 | <0.001 | −6.88 ± 0.98 | <0.001 | −5.99 ± 0.90 | <0.001 | 2.9 ± 2.14 | <0.001 | −9.1 ± 1.09 | <0.001 |
| TrigasMAP 5 | 10.45 ± 1.48 | <0.001 | −9.25 ± 0.98 | <0.001 | −6.59 ± 0.90 | <0.001 | 6.72 ± 2.14 | <0.001 | −11.19 ± 1.09 | <0.001 | |
| Effect | Treatment | Purge Loss (%) | Cooking Loss (%) | WBSF (N) | Hardness (N) | Cohesiveness | Chewiness (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | ||
| Semimembranosus Constant 1 | 6.3 ± 0.5 | 35.3 ± 0.9 | 25.3 ± 2.7 | 33.6 ± 1.5 | 0.16 ± 0.01 | 1.78 ± 0.32 | |||||||
| Breed | Merino | −0.6 ± 1 | 0.506 | −2.3 ± 1.7 | 0.038 | −0.4 ± 4.9 | 0.703 | 2.8 ± 4.3 | 0.001 | −0.01 ± 0.03 | <0.001 | 0.16 ± 0.8 | <0.001 |
| Feed | SCF 2 | −0.3 ± 0.8 | 0.984 | 0.5 ± 1.3 | 0.566 | 3.2 ± 4.1 | 0.18 | 1.7 ± 2.3 | 0.952 | −0.004 ± 0.02 | 0.334 | 0.21 ± 0.49 | 0.508 |
| SCM 3 | 0.5 ± 0.8 | 0.984 | 0.9 ± 1.3 | 0.566 | 4.6 ± 4.1 | 0.18 | 0.8 ± 2.3 | 0.952 | 0.02 ± 0.02 | 0.334 | 0.37 ± 0.49 | 0.508 | |
| Packaging | HioxMAP 4 | 0.4 ± 0.6 | 0.014 | −3 ± 1.1 | <0.001 | 3.8 ± 3.1 | 0.001 | 3.0 ± 3.3 | 0.07 | 0.05 ± 0.02 | <0.001 | 1.14 ± 0.63 | <0.001 |
| TrigasMAP 5 | −0.5 ± 0.6 | 0.014 | −3.3 ± 1.1 | <0.001 | 5.1 ± 3.1 | 0.001 | 6.6 ± 3.3 | 0.07 | 0.03 ± 0.02 | <0.001 | 1.07 ± 0.63 | <0.001 | |
| Vastus lateralis Constant 6 | 6.0 ± 0.5 | 35.3 ± 0.9 | 20.9 ± 1.2 | 32.9 ± 2 | 0.18 ± 0.02 | 2.04 ± 0.56 | |||||||
| Breed | Merino | −0.8 ± 1 | 0.101 | −1.4 ± 1.6 | 0.348 | 0.3 ± 2.5 | 0.688 | −4.8 ± 4.5 | 0.756 | −0.01 ± 0.05 | 0.005 | −0.08 ± 1.22 | 0.01 |
| Feed | SCF 2 | 0.6 ± 0.8 | 0.936 | 1.3 ± 1.3 | 0.742 | 2.9 ± 1.9 | 0.117 | −0.9 ± 2.9 | 0.034 | −0.02 ± 0.03 | 0.926 | −0.31 ± 0.85 | 0.551 |
| SCM 3 | 0.2 ± 0.8 | 0.936 | 1.1 ± 1.3 | 0.742 | 1 ± 1.9 | 0.117 | −1.2 ± 2.9 | 0.034 | −0.02 ± 0.03 | 0.926 | −0.23 ± 0.85 | 0.551 | |
| Packaging | HioxMAP 4 | −0.1 ± 0.7 | 0.031 | −2.7 ± 0.9 | <0.001 | 2.1 ± 1.7 | 0.763 | 0.3 ± 3.2 | 0.695 | 0.05 ± 0.03 | <0.001 | 1.36 ± 0.82 | <0.001 |
| TrigasMAP 5 | 0.3 ± 0.7 | 0.031 | −4.9 ± 0.9 | <0.001 | 1.4 ± 1.7 | 0.763 | 0.7 ± 3.2 | 0.695 | 0.04 ± 0.03 | <0.001 | 1.11 ± 0.82 | <0.001 | |
| Effect | Treatment | TBARS (mg MDA·kg−1 Meat) | Total Carbonyl (nmol·mg−1 Protein) | Free Thiol Content (nmol·mg−1 Protein) | |||
|---|---|---|---|---|---|---|---|
| Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | ||
| Semimembranosus Constant 1 | 0.51 ± 0.33 | 1.52 ± 0.58 | 53.48 ± 3.14 | ||||
| Breed | Merino | −0.13 ± 0.46 | 0.323 | −0.11 ± 0.77 | 0.781 | −14.87 ± 6.85 | 0.661 |
| Feed | SCF 2 | −0.4 ± 0.47 | 0.034 | −0.66 ± 0.81 | 0.395 | 0.85 ± 5.02 | 0.37 |
| SCM 3 | −0.28 ± 0.47 | 0.034 | −0.50 ± 0.81 | 0.395 | 0.02 ± 5.02 | 0.37 | |
| Packaging | HioxMAP 4 | 4.05 ± 0.45 | <0.001 | 1.75 ± 0.58 | <0.001 | −10.62 ± 2.52 | <0.001 |
| TrigasMAP 5 | 1.80 ± 0.45 | <0.001 | 1.74 ± 0.58 | <0.001 | −8.92 ± 2.52 | <0.001 | |
| Vastus lateralis Constant 6 | 0.29 ± 0.36 | 1.2 ± 0.29 | 54.62 ± 3.15 | ||||
| Breed | Merino | 0.14 ± 0.50 | 0.716 | 0.28 ± 0.41 | 0.404 | −13.21 ± 6.51 | 0.424 |
| Feed | SCF 2 | −0.23 ± 0.54 | 0.06 | −0.36 ± 0.42 | 0.162 | 0.36 ± 5 | 0.382 |
| SCM 3 | −0.11 ± 0.54 | 0.06 | −0.23 ± 0.41 | 0.162 | −1.62 ± 5 | 0.382 | |
| Packaging | HioxMAP 4 | 2.59 ± 0.41 | <0.001 | 2.06 ± 0.39 | <0.001 | −10.29 ± 2.43 | <0.001 |
| TrigasMAP 5 | 2.67 ± 0.41 | <0.001 | 1.96 ± 0.39 | <0.001 | −8.22 ± 2.43 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, M.; Warner, R.D.; King, C.; Wu, S.; Ponnampalam, E.N. Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed. Foods 2022, 11, 144. https://doi.org/10.3390/foods11020144
Ha M, Warner RD, King C, Wu S, Ponnampalam EN. Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed. Foods. 2022; 11(2):144. https://doi.org/10.3390/foods11020144
Chicago/Turabian StyleHa, Minh, Robyn Dorothy Warner, Caitlin King, Sida Wu, and Eric N. Ponnampalam. 2022. "Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed" Foods 11, no. 2: 144. https://doi.org/10.3390/foods11020144
APA StyleHa, M., Warner, R. D., King, C., Wu, S., & Ponnampalam, E. N. (2022). Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed. Foods, 11(2), 144. https://doi.org/10.3390/foods11020144







