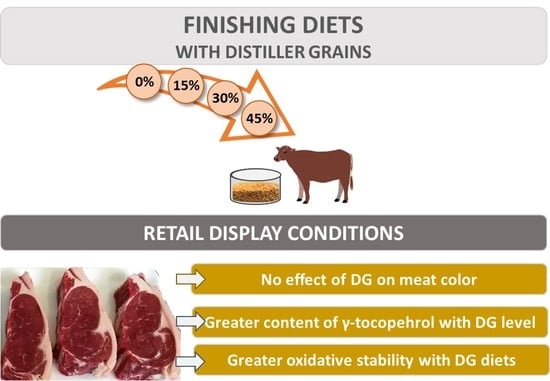
Effect of Aging and Retail Display Conditions on the Color and Oxidant/Antioxidant Status of Beef from Steers Finished with DG-Supplemented Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Sample Preparation
- R1: the steaks were individually placed on Styrofoam trays, overwrapped with oxygen-permeable polyvinylchloride film, and placed under refrigeration at 4 ± 2 °C for four days in darkness and then for three days with 7 h of illumination (D65, 700 lux) per day.
- R2: the steaks were placed under vacuum-packed conditions at 1 ± 1 °C and darkness for 25 days, plus a retail display period under aerobic exposure. For this last stage, the steaks were placed on a Styrofoam tray overwrapped with oxygen-permeable polyvinylchloride film for three days under refrigeration at 4 ± 2 °C and with 7 h of illumination (D65, 700 lux) per day.
2.3. α- and γ-Tocopherol, β-Carotene, Retinol and Lutein Content
2.4. Muscle Ferric Reducing Antioxidant Power (FRAP)
2.5. Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Meat Color
2.7. Statistical Analysis
3. Results and Discussion
3.1. Content and Animal Intake of Antioxidant Compounds in Feedstuffs
3.2. Antioxidant Compounds in Fresh LTL Muscle
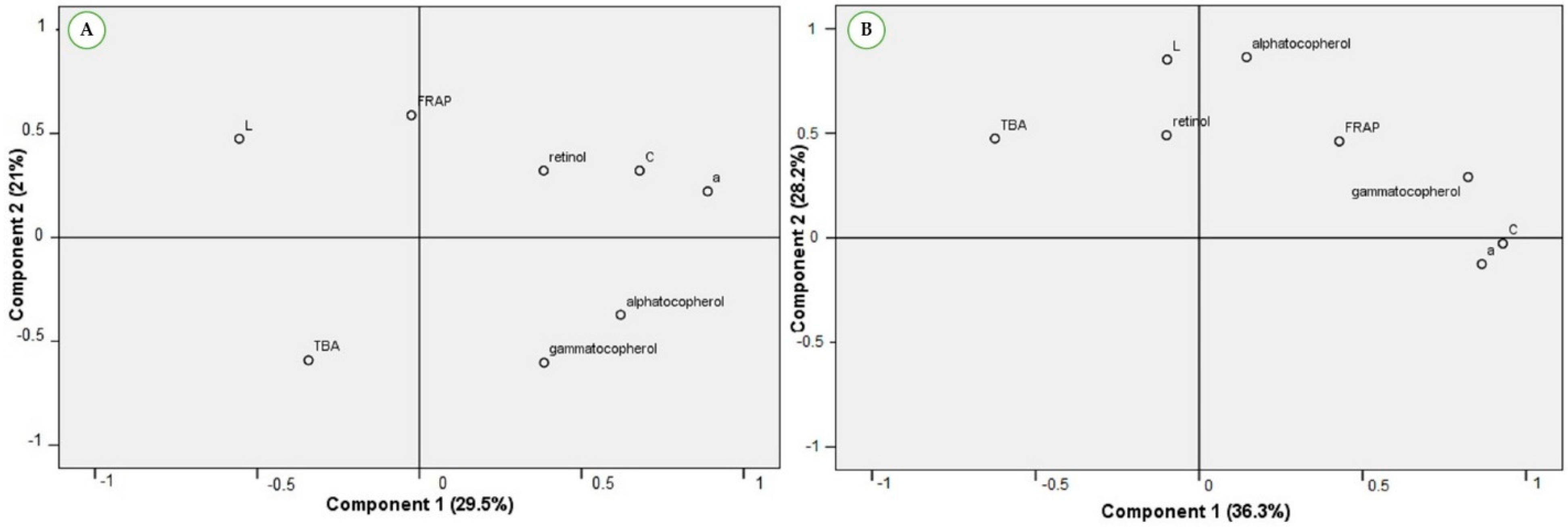
3.3. Antioxidant Compounds, Oxidation and Color Stability of LTL under Storage Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, W.A. Ethanol Industry: Surpassing Uncertainties and Looking Forward. In Global Bioethanol; Academic Press: Cambridge, MA, USA, 2016; pp. 1–33. ISBN 978-0-12-803141-4. [Google Scholar]
- Renewable Fuels Association Annual Ethanol Production. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 3 January 2022).
- Merayo, M.; Pighin, D.; Grigioni, G. Meat quality traits and feeding distillers grains to cattle: A review. Anim. Prod. Sci. 2020, 60, 1123–1135. [Google Scholar] [CrossRef]
- Klopfenstein, T.J.; Erickson, G.E.; Bremer, V.R. Board-Invited Review: Use of distillers by-products in the beef cattle feeding industry. J. Anim. Sci. 2008, 86, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scollan, N.D.; Dannenberger, D.; Nuernberg, K.; Richardson, I.; MacKintosh, S.; Hocquette, J.-F.; Moloney, A.P. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Faustman, C.; Suman, S.P. The Eating Quality of Meat: I-Color. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Lawrie´s Meat Science; Toldrá, F., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 329–356. ISBN 9780081006979. [Google Scholar]
- Testa, M.L.; Grigioni, G.; Panea, B.; Pavan, E. Color and Marbling as Predictors of Meat Quality Perception of Argentinian Consumers. Foods 2021, 10, 21. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef]
- Gobert, M.; Gruffat, D.; Habeanu, M.; Parafita, E.; Bauchart, D.; Durand, D. Plant extracts combined with vitamin E in PUFA-rich diets of cull cows protect processed beef against lipid oxidation. Meat Sci. 2010, 85, 676–683. [Google Scholar] [CrossRef]
- Natalello, A.; Priolo, A.; Valenti, B.; Codini, M.; Mattioli, S.; Pauselli, M.; Puccio, M.; Lanza, M.; Stergiadis, S.; Luciano, G. Dietary pomegranate by-product improves oxidative stability of lamb meat. Meat Sci. 2020, 162, 108037. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.S.; Jenschke, B.E.; Senaratne, L.S.; Carr, T.P.; Erickson, G.E.; Calkins, C.R. Effects of feeding modified distillers grains plus solubles on marbling attributes, proximate composition, and fatty acid profile of beef. J. Anim. Sci. 2012, 90, 4634–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mello, A.S.; Jenschke, B.E.; Senaratne, L.S.; Carr, T.P.; Erickson, G.E.; Calkins, C.R. Effects of finishing diets containing wet distillers grains plus solubles on beef quality attributes and fatty acid profile. Meat Sci. 2018, 136, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; VanOverbeke, D.L.; Depenbusch, B.; Drouillard, J.S.; DiCostanzo, A. Impact of beef cattle diets containing corn or sorghum distillers grains on beef color, fatty acid profiles, and sensory attributes. J. Anim. Sci. 2008, 86, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Depenbusch, B.E.; Coleman, C.M.; Higgins, J.J.; Drouillard, J.S. Effects of increasing levels of dried corn distillers grains with solubles on growth performance, carcass characteristics, and meat quality of yearling heifers. J. Anim. Sci. 2009, 87, 2653–2663. [Google Scholar] [CrossRef]
- Latimori, N.; Carduza, F.; Merayo, M.; Soteras, T.; Grigioni, G.; Garis, M. Efecto de la incorporación de burlanda de maíz en la dieta de bovinos para carne. In Producción Bovinos para Carne (2013–2017) Programa Nacional de Producción Animal Alimentación de Bovinos Para Carne; Pasinato, A., Grigioni, G., Alende, M., Eds.; INTA, Anguil: La Pampa, Argentina, 2018; ISBN 978-1-873671-00-9. [Google Scholar]
- Merayo, M.; Pighin, D.; Latimori, N.; Kloster, A.; Cunzolo, S.; Grigioni, G. Utilización de subproductos de la industria de biocombustibles en la alimentación de bovinos para carne en argentina. In Tecnologías Aplicadas en la Producción, Calidad y Competitividad de la Carne de Especies Pecuarias; Dominguez Vara, I.A., Morales Almaraz, E., Ramirez Bribiesca, E., Eds.; Ediciones y Gráficos Eon: Toluca, Mexico, 2018; pp. 305–323. ISBN 9786078559671. [Google Scholar]
- Buttriss, J.; Diplock, A. High-Performance Liquid Cromatography Methods for Vitamin E in Tissues. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 131–138. ISBN 0-12-182005-X. [Google Scholar]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Rossetti, L.; Grigioni, G.; Irurueta, M.; Sancho, A.M.; Carrete, J.; Pensel, N.A. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 2007, 75, 299–307. [Google Scholar] [CrossRef]
- Pensel, N.A. Influence of Experimental Conditions on Porcine Muscle and Its Effect on Oxidation; The Ohio State University: Columbus, OH, USA, 1990. [Google Scholar]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo, C. InfoStat Versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina, 2020. Available online: http://infostat.com.ar (accessed on 3 January 2022).
- Li, M.; Tian, L.; Zhao, G.; Zhang, Q.; Gao, X.; Huang, X.; Sun, L. Formation of biogenic amines and growth of spoilage-related microorganisms in pork stored under different packaging conditions applying PCA. Meat Sci. 2014, 96, 843–848. [Google Scholar] [CrossRef]
- Shin, E.-C.; Shurson, G.C.; Gallaher, D.D. Antioxidant capacity and phytochemical content of 16 sources of corn distillers dried grains with solubles (DDGS). Anim. Nutr. 2018, 4, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Loy, D.D.; Lundy, E.L. Nutritional Properties and Feeding Value of Corn and Its Coproducts. In Corn; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 633–659. ISBN 978-0-12-811971-6. [Google Scholar]
- Koger, T.J.; Wulf, D.M.; Weaver, A.D.; Wright, C.L.; Tjardes, K.E.; Mateo, K.S.; Engle, T.E.; Maddock, R.J.; Smart, A.J. Influence of feeding various quantities of wet and dry distillers grains to finishing steers on carcass characteristics, meat quality, retail-case life of ground beef, and fatty acid profile of longissimus muscle. J. Anim. Sci. 2010, 88, 3399–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.; Ripoll, G.; Casasús, I.; Bertolín, J.R.; Joy, M. Carotenoids and tocopherol in plasma and subcutaneous fat colour to trace forage-feeding in growing steers. Livest. Sci. 2019, 219, 104–110. [Google Scholar] [CrossRef]
- Pouzo, L.B.; Descalzo, A.M.; Zaritzky, N.E.; Rossetti, L.; Pavan, E. Antioxidant status, lipid and color stability of aged beef from grazing steers supplemented with corn grain and increasing levels of flaxseed. Meat Sci. 2016, 111, 1–8. [Google Scholar] [CrossRef]
- Dunne, P.G.; Monahan, F.J.; O’Mara, F.P.; Moloney, A.P. Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Sci. 2009, 81, 28–45. [Google Scholar] [CrossRef]
- da Silva Hampel, V.; Poli, C.H.E.C.; Devincenzi, T.; Pötter, L. Feeding systems and tocopherol level in the diet and their effects on the quality of lamb meat: A meta-analysis. Rev. Bras. Zootec. 2019, 48, 48. [Google Scholar] [CrossRef] [Green Version]
- Salami, S.A.; O’Grady, M.N.; Luciano, G.; Priolo, A.; McGee, M.; Moloney, A.P.; Kerry, J.P. Fatty acid composition, shelf-life and eating quality of beef from steers fed corn or wheat dried distillers’ grains with solubles in a concentrate supplement to grass silage. Meat Sci. 2021, 173, 108381. [Google Scholar] [CrossRef]
- Chao, M.D.; Domenech-Perez, K.I.; Senaratne-Lenagala, L.S.; Calkins, C.R. Feeding wet distillers grains plus solubles contributes to sarcoplasmic reticulum membrane instability. Anim. Prod. Sci. 2018, 58, 2215–2223. [Google Scholar] [CrossRef] [Green Version]
- Saunders, C.; Wolf, F.; Sauber, T.; Owens, F. Method of Improving Animal Tissue Quality. US Patent No. 6,977,269 B1, 20 December 2005. [Google Scholar]
- Merayo, M. Granos de Destilería en Alimentación Animal: Su Incidencia en Calidad de Carne Bovina, Universidad de Buenos Aires, 2020. Available online: http://hdl.handle.net/20.500.12123/10199 (accessed on 4 February 2022).
- Chao, M.D.; Domenech-Pérez, K.I.; Voegele, H.R.; Kunze, E.K.; Calkins, C.R. Effects of dietary antioxidant supplementation of steers finished with 30% wet distillers grains plus solubles on fatty acid profiles and display life of strip loins. Anim. Prod. Sci. 2018, 58, 1949–1957. [Google Scholar] [CrossRef]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef]
- Prandl, O.; Fischer, A.; Schmidhofer, T.; Sinell, H. Tecnología e Higiene de la Carne; Ciencia y tecnología de la carne; Acribia: Zaragoza, Spain, 1994; ISBN 9788420007656. [Google Scholar]
- Insani, E.M.; Eyherabide, A.; Grigioni, G.; Sancho, A.M.; Pensel, N.A.; Descalzo, A.M. Oxidative stability and its relationship with natural antioxidants during refrigerated retail display of beef produced in Argentina. Meat Sci. 2008, 79, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, C.; McGilchrist, P. Ageing as a method to increase bloom depth and improve retail colour in beef graded AUS-MEAT colour 4. Meat Sci. 2022, 183, 108665. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef]
- Holman, B.W.B.; van de Ven, R.J.; Mao, Y.; Coombs, C.E.O.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef]
| Item | Dietary Treatment 1 | |||
|---|---|---|---|---|
| 0DG | 15DG | 30DG | 45DG | |
| Ingredient (% DM) | ||||
| Cracked corn grain | 84 | 74 | 64 | 48 |
| Distiller grains (DG) | 0 | 15 | 30 | 45 |
| Soybean meal | 10 | 5 | 0 | 0 |
| Alfalfa hay | 6 | 6 | 6 | 6 |
| Nucleus 2 | 0.3 | 0.3 | 0.3 | 0.3 |
| Chemical composition (% DM) | ||||
| Crude protein | 11.72 | 12.82 | 14.02 | 16.80 |
| Fat | 4.16 | 5.37 | 6.44 | 7.56 |
| Ash | 2.87 | 3.14 | 3.27 | 3.73 |
| Item | 0DG 1 | 15DG | 30DG | 45DG | SEM 2 | p-Value | L 3 | Q |
|---|---|---|---|---|---|---|---|---|
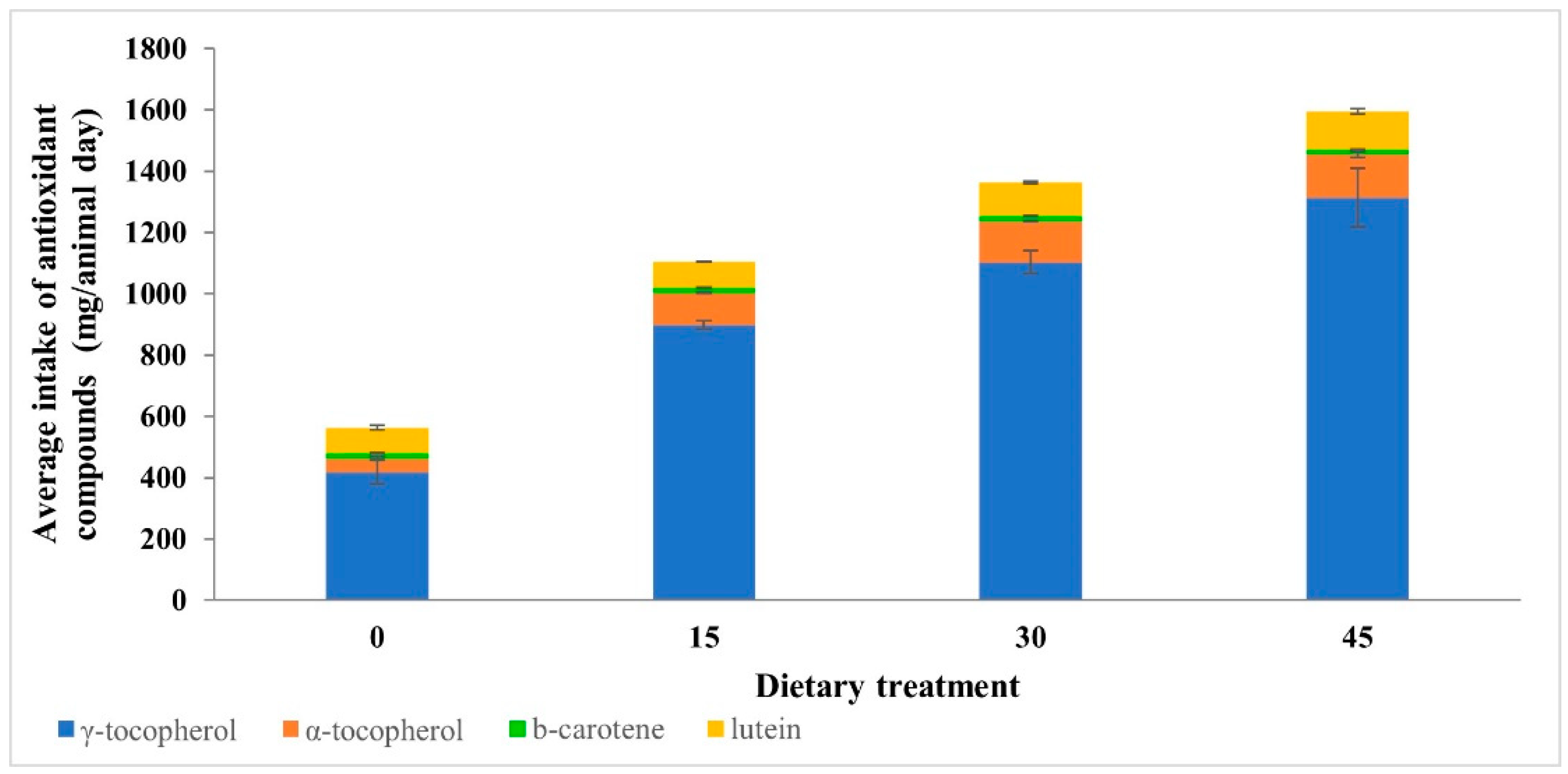
| γ-tocopherol | 48.37 c | 102.43 b | 132.80 a,b | 160.68 a | 9.84 | 0.0002 | <0.0001 | NS |
| α-tocopherol | 5.25 b | 12.00 a | 16.15 a | 17.27 a | 1.45 | 0.002 | 0.0003 | NS |
| β-carotene | 1.91 c | 1.95 b,c | 2.04 a,b | 2.11 a | 0.02 | 0.0002 | <0.0001 | NS |
| α-carotene | n.d. 4 | n.d. | n.d. | n.d. | ||||
| Lutein | 9.62 b | 9.53 b | 13.18 a | 15.01 a | 0.75 | 0.002 | 0.0004 | NS |
| Item | 0DG 1 | 15DG | 30DG | 45DG | SEM 2 | p-Value | L 3 | Q |
|---|---|---|---|---|---|---|---|---|
| Antioxidant compounds (µg/g meat) | ||||||||
| γ-tocopherol | 0.83 b | 1.02 a,b | 1.05 a,b | 1.44 a | 0.14 | 0.04 | 0.007 | 0.39 |
| α-tocopherol | 0.78 | 0.98 | 0.98 | 1.41 | 0.22 | NS | 0.06 | 0.52 |
| Retinol | 0.017 | 0.025 | 0.030 | 0.024 | 0.003 | 0.099 | 0.12 | 0.93 |
| Oxidation stability | ||||||||
| FRAP (eq Fe+2/µM) | 204 | 229 | 226 | 216 | 17 | NS | 0.73 | 0.33 |
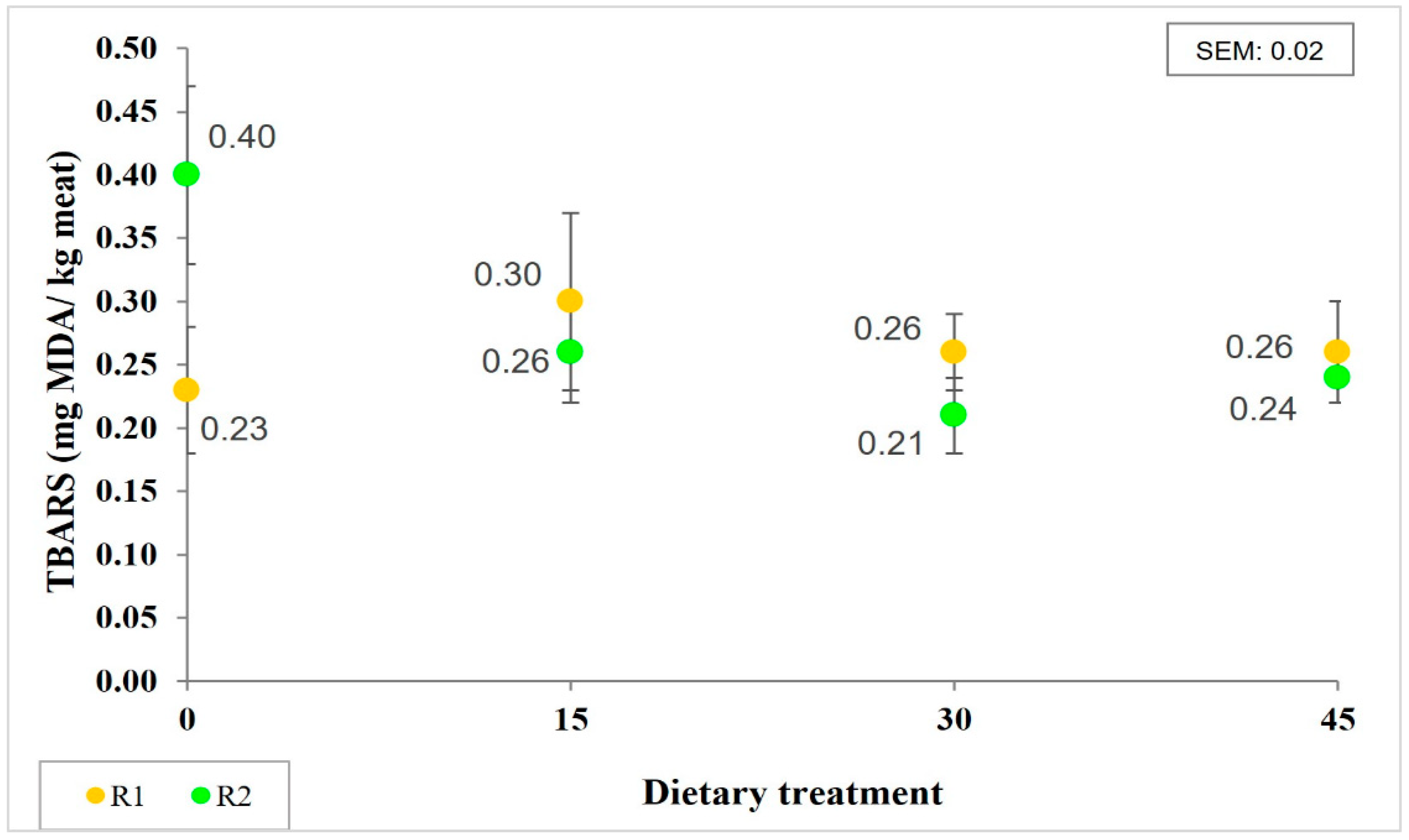
| TBARS (mg MDA/kg meat) | 0.39 | 0.29 | 0.25 | 0.22 | 0.06 | NS | 0.09 | 0.27 |
| Item | Dietary Treatment 1 | Retail Treatment | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0DG | 15DG | 30DG | 45DG | SEM 2 | R1 | R2 | SEM | D*R 3 | D | R | |
| Antioxidant compounds (µg/g meat) | |||||||||||
| γ-tocopherol | 0.62 b | 0.88 a | 0.81 a,b | 0.75 a,b | 0.05 | 0.78 | 0.75 | 0.04 | NS | 0.04 | NS |
| α-tocopherol | 0.52 | 0.65 | 0.63 | 0.51 | 0.09 | 0.57 | 0.58 | 0.04 | NS | NS | NS |
| Retinol | 0.023 b | 0.026 a,b | 0.030 a | 0.024 a,b | 0.001 | 0.027 | 0.025 | 0.002 | NS | 0.03 | NS |
| Antioxidant capacity | |||||||||||
| FRAP (eq Fe+2/µM) | 261 | 250 | 261 | 251 | 9.20 | 270 a | 241 b | 6.82 | NS | NS | 0.004 |
| Item | Dietary Treatment 1 | Retail Treatment | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0DG | 15DG | 30DG | 45DG | SEM 2 | R1 | R2a | R2b | SEM | D*R 3 | D | R | |
| L* | 43.24 | 42.50 | 41.85 | 41.37 | 0.57 | 40.93 b | 42.84 a | 42.94 a | 0.37 | NS | NS | 0.0002 |
| a* | 17.10 | 18.32 | 18.02 | 18.08 | 0.47 | 15.72 c | 20.09 a | 17.83 b | 0.26 | NS | NS | <0.0001 |
| C* | 19.96 | 21.45 | 20.98 | 21.01 | 0.45 | 19.03 c | 22.71 a | 20.82 b | 0.31 | NS | NS | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merayo, M.; Rizzo, S.A.; Rossetti, L.; Pighin, D.; Grigioni, G. Effect of Aging and Retail Display Conditions on the Color and Oxidant/Antioxidant Status of Beef from Steers Finished with DG-Supplemented Diets. Foods 2022, 11, 884. https://doi.org/10.3390/foods11060884
Merayo M, Rizzo SA, Rossetti L, Pighin D, Grigioni G. Effect of Aging and Retail Display Conditions on the Color and Oxidant/Antioxidant Status of Beef from Steers Finished with DG-Supplemented Diets. Foods. 2022; 11(6):884. https://doi.org/10.3390/foods11060884
Chicago/Turabian StyleMerayo, Manuela, Sergio Aníbal Rizzo, Luciana Rossetti, Dario Pighin, and Gabriela Grigioni. 2022. "Effect of Aging and Retail Display Conditions on the Color and Oxidant/Antioxidant Status of Beef from Steers Finished with DG-Supplemented Diets" Foods 11, no. 6: 884. https://doi.org/10.3390/foods11060884
APA StyleMerayo, M., Rizzo, S. A., Rossetti, L., Pighin, D., & Grigioni, G. (2022). Effect of Aging and Retail Display Conditions on the Color and Oxidant/Antioxidant Status of Beef from Steers Finished with DG-Supplemented Diets. Foods, 11(6), 884. https://doi.org/10.3390/foods11060884