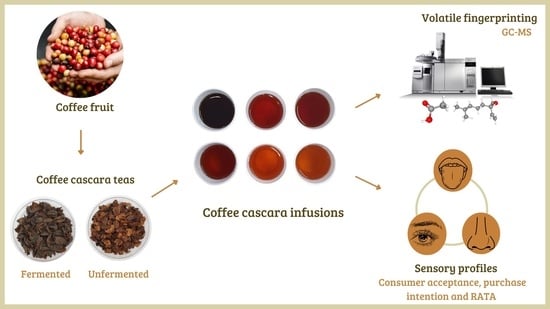
Volatile Fingerprinting and Sensory Profiles of Coffee Cascara Teas Produced in Latin American Countries
Abstract
1. Introduction
2. Materials and Methods
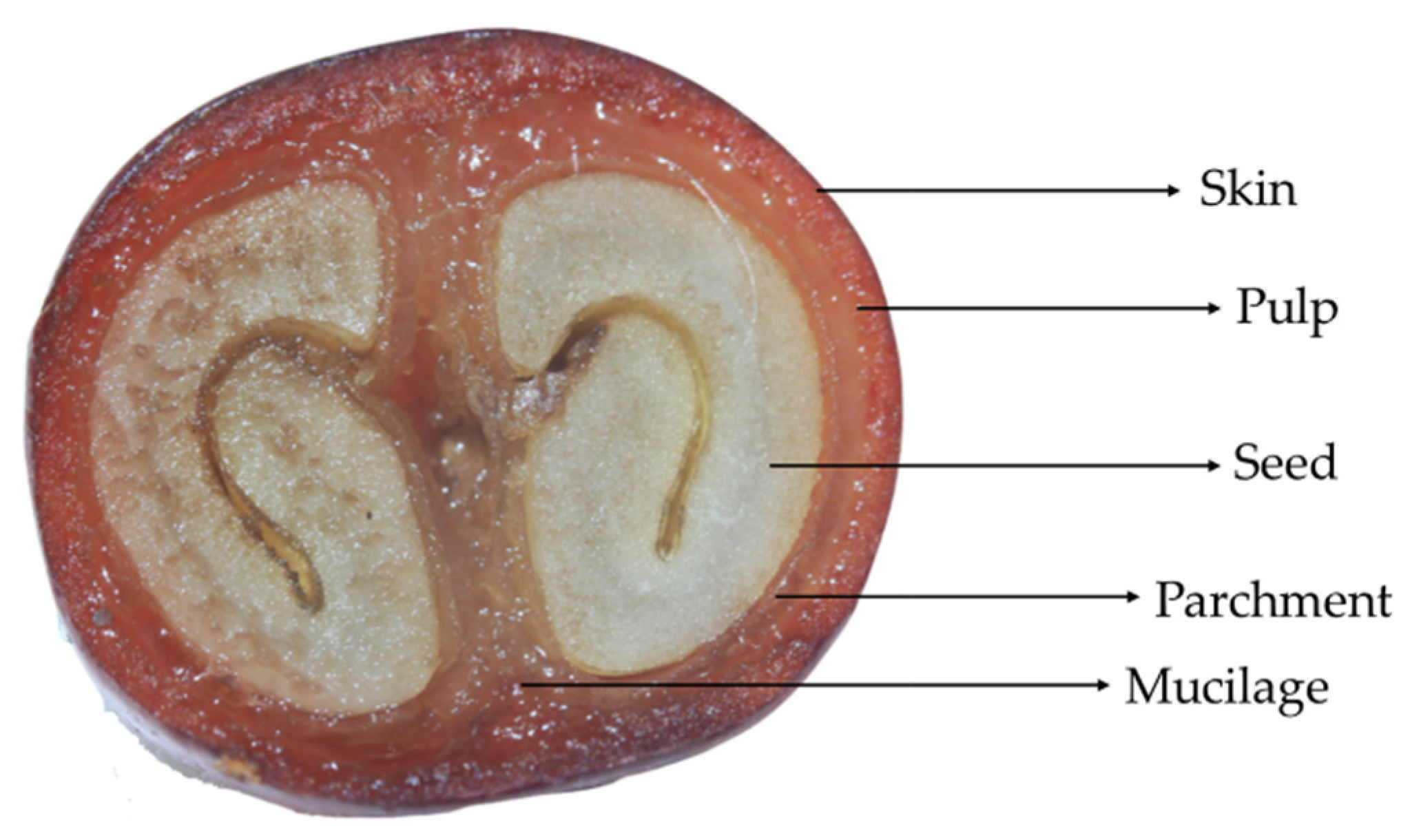
2.1. Samples and Study Design
2.2. Infusions Preparation
2.3. Physical-Chemical Analyses
2.4. Analyses of Volatile Organic Compounds
2.5. Microbiological Analyses for Food Safety
2.6. Sensory Analysis
2.6.1. Consumer Acceptance and Purchase Intention
2.6.2. Rate All that Apply (RATA)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
3.2. Physical-Chemical Analyses
3.3. Volatile Organic Compounds
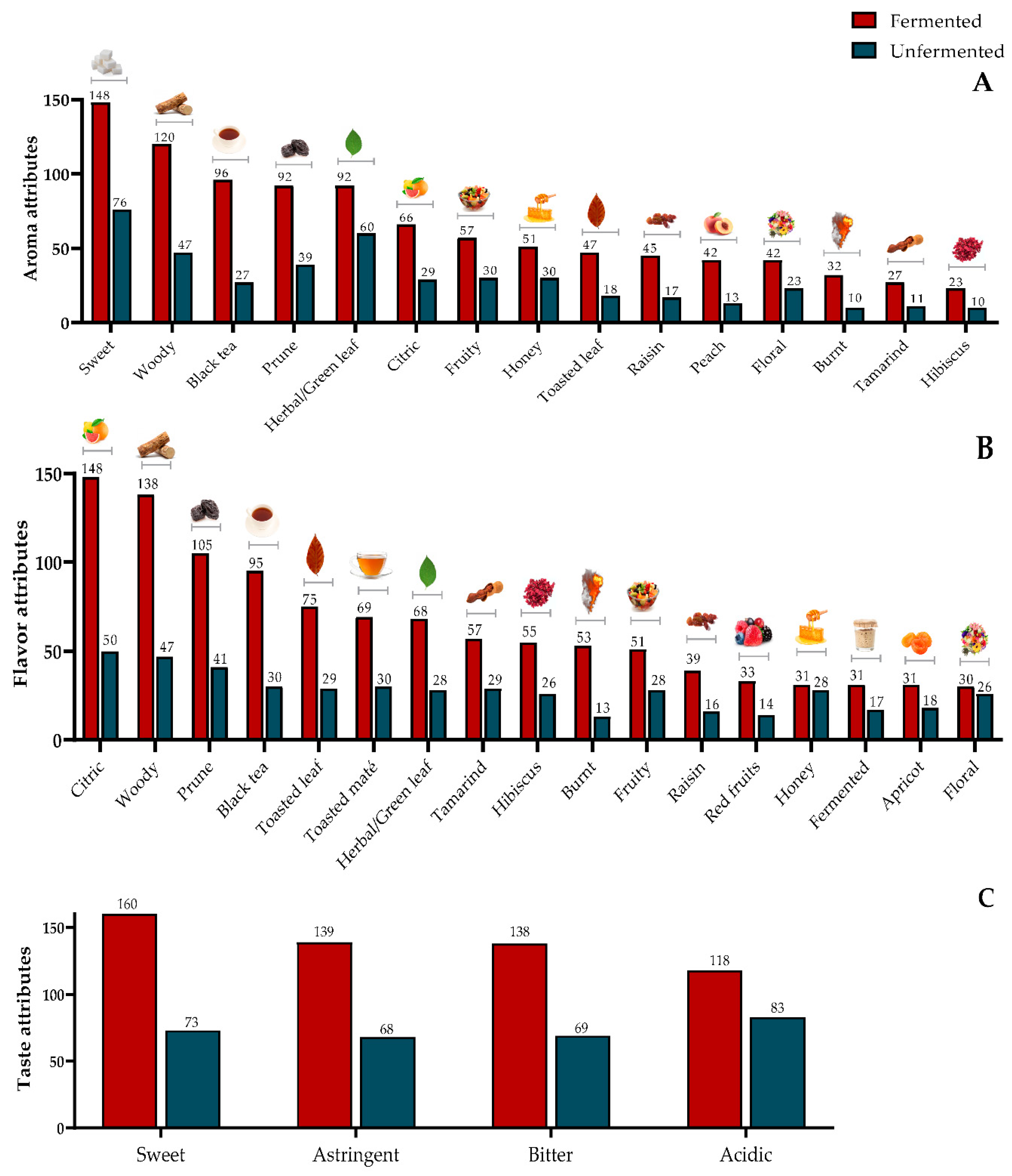
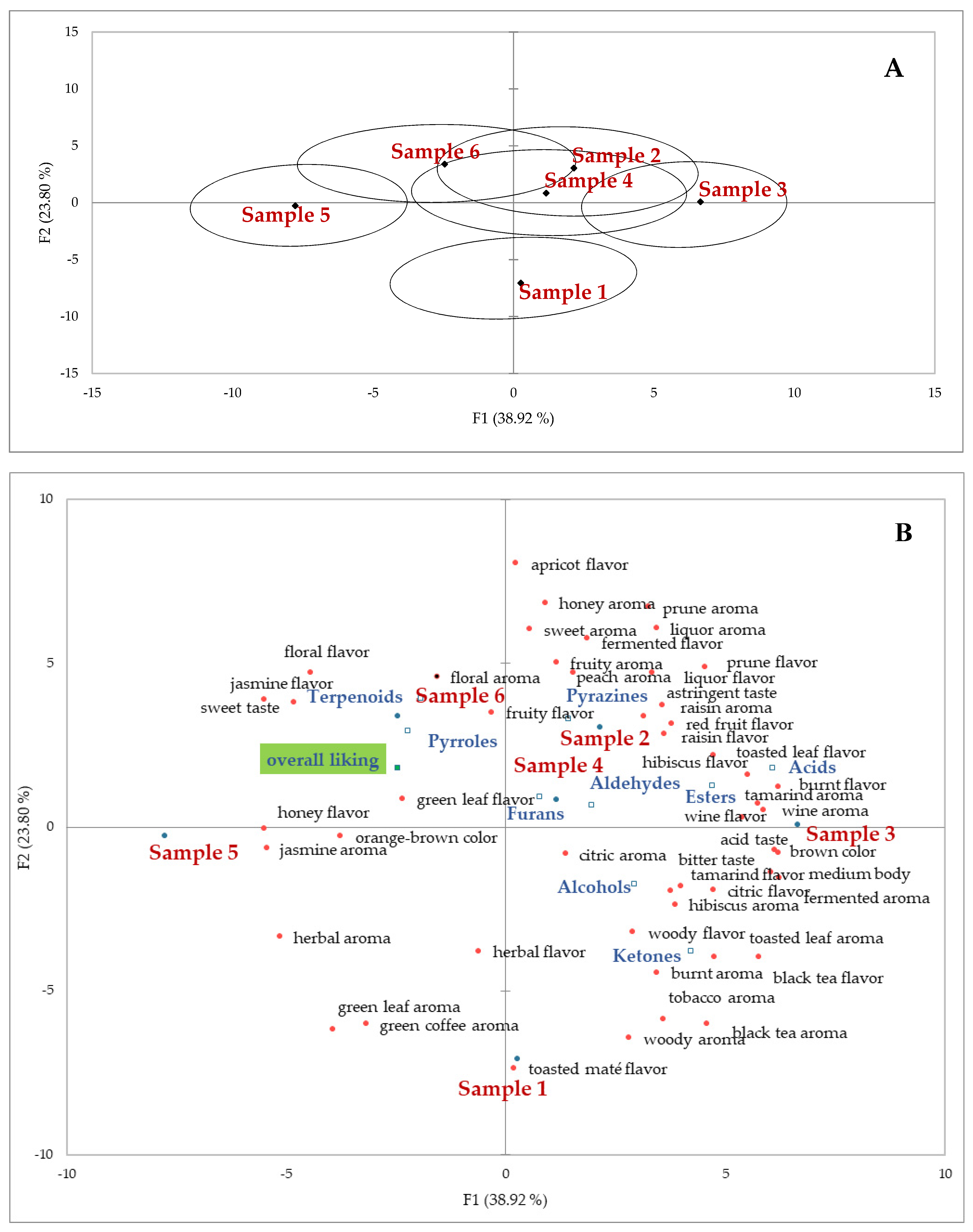
3.4. Sensory Tests
3.4.1. Assessors’ Characteristics
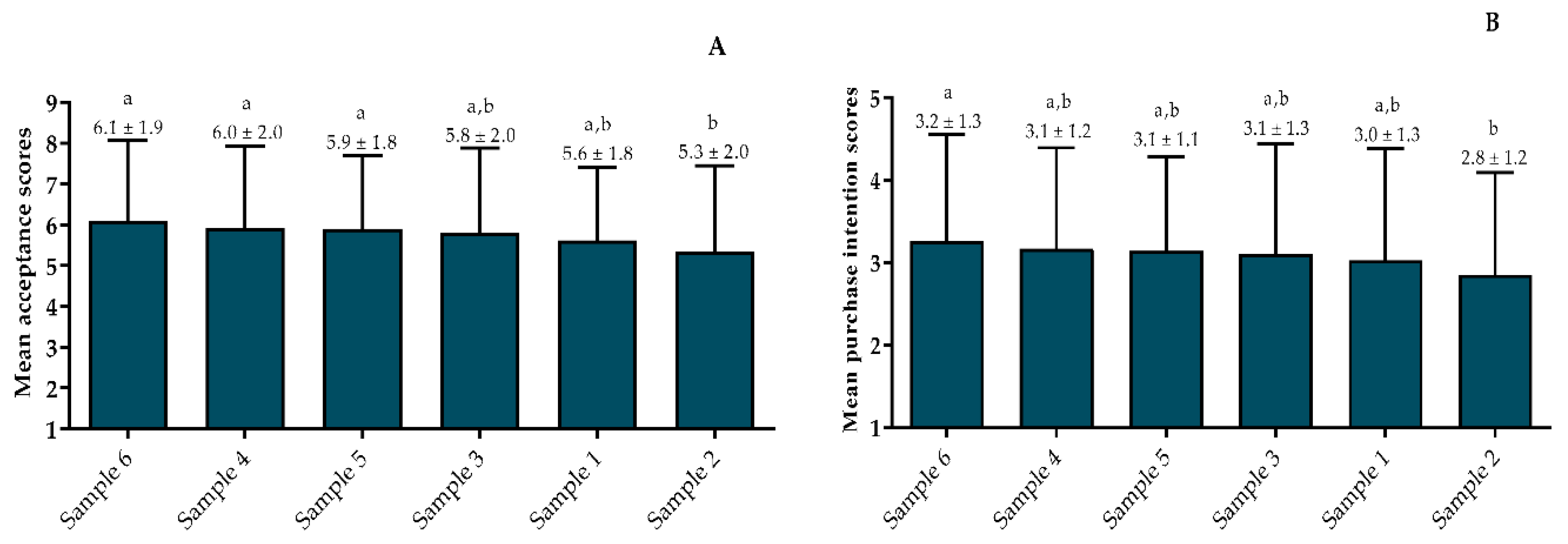
3.4.2. Consumer Acceptance and Purchase Intention Test Scores
3.4.3. Rate All that Apply (RATA) Test
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization. World Coffee Production. Available online: https://ico.org/prices/po-production.pdf (accessed on 19 July 2022).
- Farah, A. Flavor development during roasting. In Drying and Roasting of Cocoa and Coffee, 1st ed.; Hii, C.L., Borém, F.M., Eds.; CRC Press: New York, NY, USA, 2019; pp. 267–309. ISBN 9781315113104. [Google Scholar]
- Heuzé, V.; Tran, G. Coffee Hulls, Fruit Pulp and by-Products. Feediédir, a Programme by INRAE, CIRAD. AFZ and FAO. 2015. Available online: https://www.feedipedia.org/node/549 (accessed on 15 August 2022).
- Veenstra, S. Recovery of biogas from landfill sites. IHE Delft. In Curso Taller Internacional Sobre Tratamiento Anaerobio de Aguas Residuales; SFC: Santiago de Cali, Colombia, 1995. [Google Scholar]
- Beyene, A.; Kassahun, Y.; Addis, T.; Assefa, F.; Amsalu, A.; Legesse, W.; Kloos, H.; Triest, L. The impact of traditional coffee processing on river water quality in Ethiopia and the urgency of adopting sound environmental practices. Environ. Monit. Assess. 2012, 184, 7053–7063. [Google Scholar] [CrossRef]
- Awoke, A.; Beyene, A.; Kloos, H.; Goethals, P.L.; Triest, L. River water pollution status and water policy scenario in Ethiopia: Raising awareness for better implementation in developing countries. Environ. Manag. 2016, 58, 694–706. [Google Scholar] [CrossRef] [PubMed]
- DePaula, J.; Farah, A. Phenolic compounds in coffee and by-products. In Coffee Science: Biotechnological Advances, Economics, and Health Benefits, 1st ed.; Ramakrishna, A., Parvatam, G., Jeszka-Skowron, M., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 119–134. ISBN 9781003043133. [Google Scholar]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–26. ISBN 9780128112908. [Google Scholar]
- Dorsey, B.M.; Jones, M.A. Healthy components of coffee processing by-products. In Handbook of Coffee Processing By-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 27–62. ISBN 9780128112908. [Google Scholar]
- del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Mesa, M.D. Coffee by-products. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 309–334. [Google Scholar] [CrossRef]
- Prata, E.R.; Oliveira, L.S. Fresh coffee husks as potential sources of anthocyanins. LWT Food Sci. Technol. 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; Velázquez Escobar, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- EFSA. Panel on Dietetic Products Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on the safety of dried coffee husk (cascara) from Coffea arabica L. as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens). EFSA J. 2022, 20, 7085. [Google Scholar] [CrossRef]
- Brasil-ANVISA: Resolution of the Collegiate Board of Directors (RDC) 216/2004. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2004/res0216_15_09_2004.html (accessed on 20 August 2022).
- Food and Agriculture Organization of the United Nations. Codex Alimentarius Commission. 1997. Available online: https://www.fao.org/3/W6419E/W6419E00.htm#Contents (accessed on 20 August 2022).
- American Society for Testing and Materials. Proceedings. 1973, Volume 73. Available online: https://books.google.com.br/books/about/Proceedings_American_Society_for_Testing.html?id=GEFIAQAAIAAJ&redir_esc=y (accessed on 20 August 2022).
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- Adhikari, J.; Chambers, E.; Koppel, K. Impact of consumption temperature on sensory properties of hot brewed coffee. Food Res. Int. 2019, 115, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Bary, S.; Zhou, W. Changes in the color, chemical stability and antioxidant capacity of thermally treated anthocyanin aqueous solution over storage. Food Chem. 2016, 192, 516–524. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos; ANVISA: São Paulo, Brazil, 2008. [Google Scholar]
- Wang, Z.; Han, B.; Jing, W.; Yi, Z.; Zhang, Y.; Ren, D.; Yi, L. Effects of Different Steeping Temperatures on the Leaching of Aroma Components in Black Tea by SPME–GC–MS Coupled with Chemometric Method. J. AOAC Int. 2019, 102, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- NIST V2.2 (National Institute of Standards and Technology, USA) Library Database. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 15 August 2022).
- Galvan-Lima, A.; Cunha, S.C.; Martins, Z.E.; Soares, A.G.; Ferreira, I.M.; Farah, A. Headspace volatolome of peel flours from citrus fruits grown in Brazil. Food Res. Int. 2021, 150, 110801. [Google Scholar] [CrossRef]
- Brasil-ANVISA: Resolution of the Collegiate Board of Directors (RDC) 724/2022 and Normative Instruction (IN) 161/2022. Available online: https://antigo.anvisa.gov.br/documents/10181/2718376/RDC_724_2022_.pdf/33c61081-4f32-43c2-9105-c318fa6069ce (accessed on 20 August 2022).
- Meilgaard, M.; Civille, G.V.; Carr, B.T. techniques. In Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 1, pp. 173–186. [Google Scholar]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a rating-based variant of check-all-that-apply questions: Rate-all-that-apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Meyners, M.; Jaeger, S.R.; Ares, G. On the analysis of Rate-All-That-Apply (RATA) data. Food Qual. Prefer. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Vital, P.G.; Caballes, M.B.D.; Rivera, W.L. Antimicrobial resistance in Escherichia coli and Salmonella spp. isolates from fresh produce and the impact to food safety. J. Environ. Sci. Health B 2017, 52, 683–689. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Hu, L.; Melka, D.; Wang, H.; Laasri, A.; Brown, E.W.; Strain, E.; Allard, M.; Bunning, V.K.; et al. Survey of Foodborne Pathogens, Aerobic Plate Counts, Total Coliform Counts, and Escherichia coli Counts in Leafy Greens, Sprouts, and Melons Marketed in the United States. J. Food Prot. 2018, 81, 400–411. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Microbiological hazards in fresh leafy vegetables and herbs. In Microbiological Risk Assessment Series; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Beuchat, L.R.; Mann, D.A. Comparison of new and traditional culture-dependent media for enumerating foodborne yeasts and molds. J. Food Prot. 2016, 79, 95–111. [Google Scholar] [CrossRef]
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls. Appendix 4: Bacterial Pathogen Growth and Inactivation. 2022. Available online: https://www.fda.gov/media/159301/download (accessed on 20 August 2022).
- Gamboa-Gaitán, M.Á. Presencia de aspergillus y otros simbiontes fúngicos em granos de café procedentes de Colombia. Acta Biolo. Colomb. 2012, 17, 39–49. [Google Scholar]
- European Commission. Commission Regulation (EC) No 123/2005 of 26 January 2005 amending Regulation (EC) No 466/2001 as regards ochratoxin. A. Off. J. Eur. Union 2005, 2005, 2004–2006. [Google Scholar]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Murlida, E.; Noviasari, S.; Nilda, C.; Rohaya, S.; Rahmi, F.; Muzaifa, M. Chemical characteristics of cascara tea from several varieties of coffee in Aceh Province. IOP Conf. Series: Earth Environ. Sci. 2021, 667, 012078. [Google Scholar] [CrossRef]
- Lima, J.D.P.; Farah, A. Methylxanthines in stimulant foods and beverages commonly consumed in Brazil. J. Food Compos. Anal. 2019, 78, 75–85. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zou, C.; Gao, Y.; Chen, J.-X.; Wang, F.; Chen, G.-S.; Yin, J.-F. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chem. 2017, 236, 142–151. [Google Scholar] [CrossRef]
- Maharani, S.; Mustikawati, I.; Nailufhar, L.; Istiqomah, S. The effect of brewing time on pH values, polyphenols content, and antioxidant activities of coffee husk tea (cascara tea). J. Physics: Conf. Ser. 2021, 1869, 012050. [Google Scholar] [CrossRef]
- Rahardjo, Y.P.; Syamsu, K.; Rahardja, S.; Samsudin; Mangunwijaya, D. Impact of controlled fermentation on the volatile aroma of roasted cocoa. Braz. J. Food Technol. 2022, 25. [Google Scholar] [CrossRef]
- Pua, A.; Choo, W.X.D.; Goh, R.M.V.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A systematic study of key odourants, non-volatile compounds, and antioxidant capacity of cascara (dried Coffea arabica pulp). LWT 2021, 138, 110630. [Google Scholar] [CrossRef]
- Mathieu, F.; Malosse, C.; Frérot, B. Identification of the volatile components released by fresh coffee berries at different stages of ripeness. J. Agric. Food Chem. 1998, 46, 1106–1110. [Google Scholar] [CrossRef]
- Buck, N.; Wohlt, D.; Winter, A.; Ortner, E. Aroma-Active compounds in robusta coffee pulp puree—Evaluation of physicochemical and sensory properties. Molecules 2021, 26, 3925. [Google Scholar] [CrossRef] [PubMed]
- Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 15 August 2022).
- The Good Scents Company Information System. Available online: https://www.thegoodscentscompany.com/ (accessed on 15 August 2022).
- Xiao, Z.; Ma, S.; Niu, Y.; Chen, F.; Yu, D. Characterization of odour-active compounds of sweet orange essential oils of different regions by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. Flavour Fragr. J. 2015, 31, 41–50. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Márquez, V.; Martínez, N.; Guerra, M.; Fariña, L.; Boido, E.; Dellacassa, E. Characterization of aroma-impact compounds in yerba mate (Ilex paraguariensis) using GC–olfactometry and GC–MS. Food Res. Int. 2013, 53, 808–815. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT 2022, 163, 113492. [Google Scholar] [CrossRef]
- Dongmo, S.N.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key volatile aroma compounds of lactic acid fermented malt based beverages–impact of lactic acid bacteria strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef]
- Schieberle, P.; Schuh, C. Aroma compounds in black tea powders of different origins-changes induced by preparation of the infusion. In Developments in Food Science; Bredie, W.L.P., Petersen, M.A., Eds.; Elsevier: London, UK, 2006; Volume 43, pp. 151–156. [Google Scholar]
- Wang, Y.; Wang, S.; Fabroni, S.; Feng, S.; Rapisarda, P.; Rouseff, R. The genus citrus. In Chemistry of Citrus Flavor; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Elsevier: London, UK, 2020; pp. 447–470. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Rouseff, R.; Perez-Cacho, P.R. Citrus flavor. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 117–134. [Google Scholar] [CrossRef]
- Selli, S.; Kelebek, H. Aromatic profile and odour-activity value of blood orange juices obtained from Moro and Sanguinello (Citrus sinensis L. Osbeck). Ind. Crop. Prod. 2011, 33, 727–733. [Google Scholar] [CrossRef]
- Mathieu, F.; Malosse, C.; Cain, A.-H.; Frérot, B. Comparative headspace analysis of fresh red coffee berries from different cultivated varieties of coffee trees. J. High Resolut. Chromatogr. 1996, 19, 298–300. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Y.; Zhou, J.; Lian, M.; Li, C.; Xu, Y.; Liu, S.; Wang, C.; Meng, Q. The Study of Fingerprint Characteristics of Dayi Pu-Erh Tea Using a Fully Automatic HS-SPME/GC–MS and Combined Chemometrics Method. PLoS ONE 2014, 9, e116428. [Google Scholar] [CrossRef]
- Rodrigues, J.; Almeida, P. E-2-nonenal and β-Damascenone in beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 395–402. ISBN 9780123738912. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Eric, F.; Darriet, P.; Dubourdieu, D. Identification of Volatile Compounds Responsible for Prune Aroma in Prematurely Aged Red Wines. J. Agric. Food Chem. 2008, 56, 5285–5290. [Google Scholar] [CrossRef]
- Greger, V.; Schieberle, P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef]
- Wang, D.; Duan, C.-Q.; Shi, Y.; Zhu, B.-Q.; Javed, H.U.; Wang, J. Free and glycosidically bound volatile compounds in sun-dried raisins made from different fragrance intensities grape varieties using a validated HS-SPME with GC–MS method. Food Chem. 2017, 228, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Institute of Geography and Statistics IBGE. Pesquisa de Orçamentos Familiares 2017–2018. 2020. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101742.pdf (accessed on 15 August 2022).
- Suplements 101, Tea Statistics. 2021. Available online: https://supplements101.net/tea-statistics/ (accessed on 15 August 2022).
- Market Research. Gourmet Food Market Research Reports & Industry Analysis. Available online: https://www.marketresearch.com/Food-Beverage-c84/Food-c167/Gourmet-Food-c487/ (accessed on 15 August 2022).
- Wang, D.; Cai, J.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS–SPME with GC–MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography–olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]
- An, H.; Ou, X.; Zhang, Y.; Li, S.; Xiong, Y.; Li, Q.; Huang, J.; Liu, Z. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef]
- Toci, A.; Farah, A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Neto, D.P.D.C.; Pereira, G.V.D.M.; Tanobe, V.O.A.; Soccol, V.T.; Da Silva, B.J.G.; Rodrigues, C.; Soccol, C.R.; Da Silva, B.G. Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef] [PubMed]
| Fermented | ||
|---|---|---|
| Sample | Origin/Cultivar | |
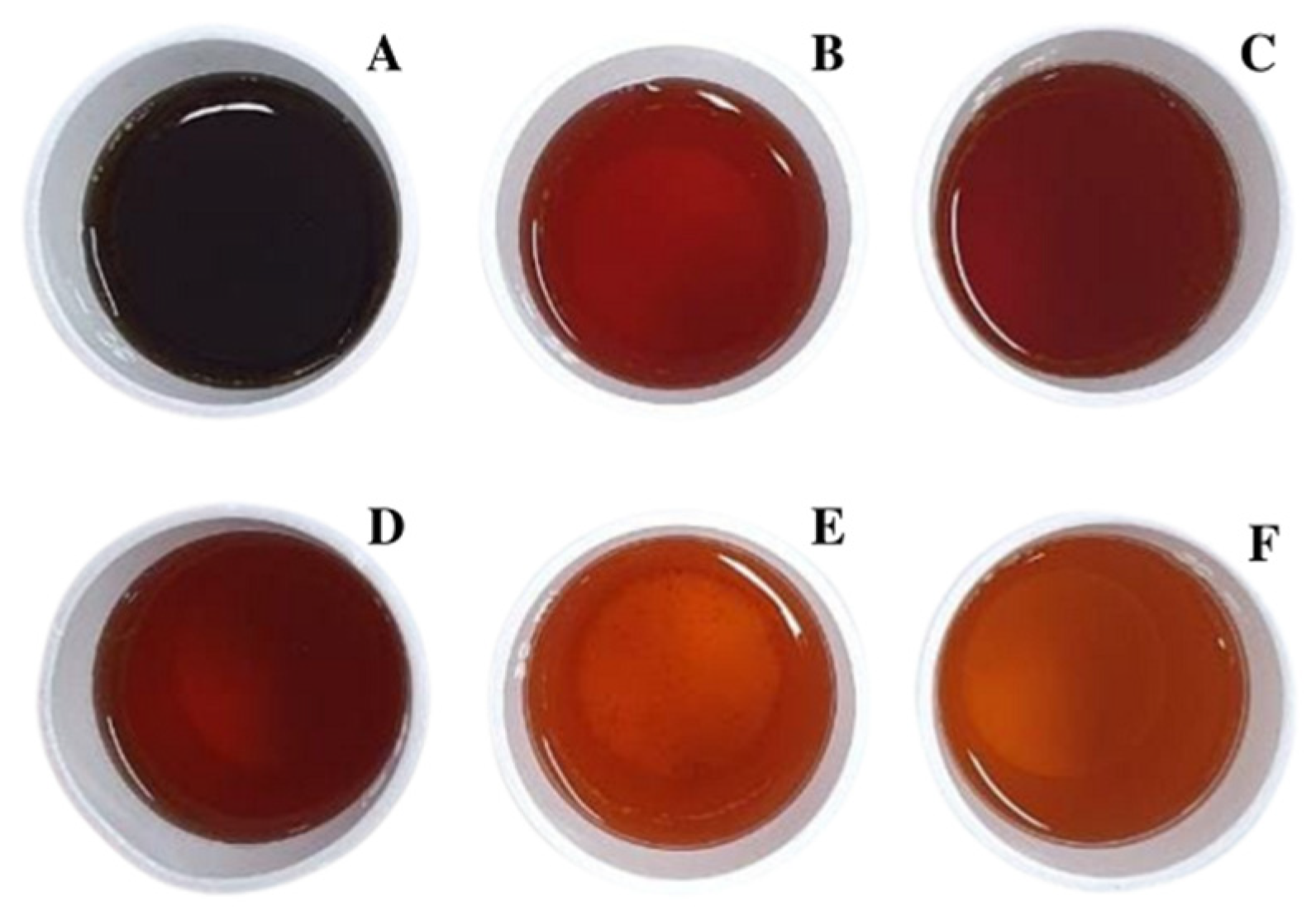
 Typical appearance of fermented arabica coffee cascara teas. | 1 | Brazil/Mix of six cultivars: Iapar 59, Bourbon Amarelo, Catucaí Açu, Catuaí 44, Caturra, IcatuPrecoce/Sold in Brazil |
| 2 | Bolivia/Nr/Sold in Canada | |
| 3 | El Salvador/Nr/Sold in the USA | |
| 4 | Nicaragua/Caturra and Bourbon/Sold in the USA | |
| Unfermented | ||
| Sample | Origin/Cultivar | |
 Typical appearance of unfermented arabica coffee cascara teas. | 5 | Brazil/Mix of six cultivars: Iapar 59, Bourbon Amarelo, Catucaí Açu, Catuaí 44, Caturra/Sold in Brazil |
| 6 | Nicaragua/Caturra/Sold in the USA | |
| 7 | Brazil/IcatuPrecoce/Sold in Brazil | |
| Samples | Instrumental Color | SS (°Brix) | pH | TA (mEq NaOH/L) | ||
|---|---|---|---|---|---|---|
| L* | C* | H° | ||||
| 1 | 70.97 ± 0.86 d | 52.11 ± 1.39 b | 88.47 ± 0.88 d | 1.17 ± 0.06 ab | 4.21 ± 0.01 a | 6.0 ± 0.3 c |
| 2 | 79.69 ± 0.50 a | 32.54 ± 1.50 d | 97.58 ± 0.70 a | 1.13 ± 0.06 c | 4.22 ± 0.01 a | 6.5 ± 0.0 b |
| 3 | 56.69 ± 1.50 f | 51.42 ± 0.23 b | 76.71 ± 1.61 f | 1.30 ± 0.00 a | 4.20 ± 0.00 a | 6.5 ± 0.1 b |
| 4 | 68.98 ± 1.19 e | 58.01 ± 1.46 a | 85.56 ± 1.19 e | 1.27 ± 0.06 a | 4.19 ± 0.01 ab | 7.2 ± 0.3 a |
| 5 | 73.68 ± 0.45 c | 45.75 ± 0.92 c | 90.96 ± 0.49 c | 1.20 ± 0.00 ab | 4.18 ± 0.01 b | 6.2 ± 0.3 bc |
| 6 | 77.89 ± 0.69 b | 34.27 ± 1.40 d | 96.03 ± 0.38 b | 1.10 ± 0.00 c | 4.22 ± 0.02 a | 7.0 ± 0.4 ab |
| Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fermented | Unfermented | |||||||||
| Volatile Compound | Odor Description | aCAS# | bELRI | cLRI | 1 | 2 | 3 | 4 | 5 | 6 |
| Aldehydes | ||||||||||
| Pentanal | Almond, pungent, coffee,chocolate [47,48] | 110-62-3 | 749 | 873 | □ | ■d,e | □ | □ | □ | □ |
| Hexanal * | Green, vegetable, fruity, tallow, fat [47,48] | 66-25-1 | 759 | 874 | ■e | ■d,e | ■d,e | ■d,e | □ | ■e |
| Hexenal | Apple, green [47] | 505-57-7 | 892 | 920 | □ | ■e | □ | □ | □ | □ |
| Heptanal * | Fresh, herbal, fatty, citrus, wine-lees [47,48] | 111-71-7 | 785 | 843 | ■e | ■e | ■d,e | □ | □ | ■e |
| Octanal * | Citrus, soap, lemon, herbal, honey [47,48] | 124-13-0 | 726 | 805 | ■e | ■e | □ | ■e | □ | ■e |
| Nonanal * | Fat, citrus, fresh, orange, green [47,48] | 124-19-6 | 798 | 880 | ■e | ■d,e | ■d,e | ■e | ■d,e | ■d,e |
| Decanal * | Sweet, citrus, floral, soap, orange [47,48] | 112-31-2 | 797 | 838 | ■e | ■d,e | ■e | □ | □ | ■e |
| Undecanal | Floral, citrus, green, fresh [47] | 112-44-7 | 847 | 901 | ■e | □ | □ | □ | □ | □ |
| Dodecanal * | Citrus, green, floral [47] | 112-54-9 | 839 | 922 | ■e | ■e | □ | □ | ■e | ■e |
| Benzaldehyde * | Almond, burnt sugar, tropical fruit [47,48] | 100-52-7 | 689 | 875 | ■e | ■e | ■d,e | ■e | ■e | ■e |
| α-methylbutanal | Cocoa, almond, malt, fermented [47,48] | 96-17-3 | 683 | 836 | □ | ■d,e | □ | □ | □ | ■d,e |
| β-methylbutanal | Chocolate, peach [48] | 590-86-3 | 742 | 861 | □ | □ | □ | □ | □ | ■d,e |
| Phenylethanal | Honey, sweet, floral, fermented [47,48] | 122-78-1 | 741 | 880 | ■e | ■e | ■e | □ | □ | ■e |
| Safranal | Herb, sweet, fresh, spicy [47,48] | 116-26-7 | 762 | 882 | □ | ■d,e | ■e | □ | □ | □ |
| (E)-cinnamaldehyde | Sweet, cinnamon, balsamic, honey [47,48] | 14371-10-9 | 767 | 868 | □ | ■e | □ | □ | □ | □ |
| Vanillin * | Sweet, vanilla, creamy, chocolate [47,48] | 121-33-5 | 652 | 721 | ■e | □ | □ | □ | ■e | □ |
| Acids | ||||||||||
| Acetic acid * | Acidic, sour, pungent, vinegar [47,48] | 64-19-7 | 589 | 600 | ■e | ■e | ■d,e | ■d,e | □ | ■d,e |
| Hexanoic acid | Sour, fatty, sweat, cheesy [47,48] | 142-62-1 | 817 | 848 | □ | ■e | ■d,e | □ | □ | □ |
| Heptanoic acid | Rancid, sour, cheesy, sweat [48] | 111-14-8 | 785 | 750 | □ | ■e | □ | □ | □ | □ |
| Octanoic acid | Acid, sweat, cheese, fruity [47,48] | 124-07-2 | 789 | 837 | ■e | ■d,e | ■e | ■e | □ | ■e |
| Nonanoic acid | Green, cheese, fatty [47,48] | 112-05-0 | 800 | 830 | ■e | ■d,e | ■d,e | ■d,e | □ | ■e |
| Decanoic acid | Rancid, fatty, sour, citrus [47,48] | 334-48-5 | 773 | 814 | ■d,e | ■e | ■d,e | ■d,e | ■d,e | ■d,e |
| Isovaleric acid | Sweet, acid, fermented, berry [47,48] | 503-74-2 | 792 | 859 | □ | ■d,e | ■d,e | □ | □ | ■d,e |
| Isobutyric acid | Acidic, sour, cheese, rancid [47,48] | 79-31-2 | 754 | 870 | ■d,e | ■d,e | ■d,e | ■d,e | □ | □ |
| Methylbutyric acid | Fruity, cheese, sweat [47,48] | 759-05-7 | 787 | 839 | □ | □ | ■d,e | □ | □ | □ |
| Alcohols | ||||||||||
| 1,2-epoxylinalool | Floral, alcohol [47,48] | 14049-11-7 | 755 | 767 | ■e | ■e | ■e | ■e | □ | □ |
| Phenylethylalcohol * | Honey, spice, rose, lilac, floral, fresh [47,48] | 60-12-8 | 740 | 849 | ■e | ■e | ■e | ■e | ■e | ■e |
| 3-methylpentanol | Pungent, green, fruity [47,48] | 589-35-5 | 790 | 919 | ■e | ■e | □ | □ | □ | □ |
| Ethylhexanol | Citrus, fresh, floral, rose, green [47,48] | 104-76-7 | 819 | 906 | ■e | ■e | ■d,e | ■e | ■e | ■e |
| Benzyl alcohol | Floral, rose, balsamic, sweet [47,48] | 100-51-6 | 830 | 855 | ■e | ■e | □ | □ | ■e | ■e |
| Lauryl alcohol | Fatty, waxy, honey, coconut [47,48] | 112-53-8 | 797 | 929 | □ | ■e | ■e | □ | □ | □ |
| Esters | ||||||||||
| Methyl salicylate | Peppermint [47], wintergreen [48] | 119-36-8 | 690 | 865 | ■d,e | ■d,e | □ | ■d,e | ■e | □ |
| Ethyl salicylate | Wintergreen, mint, floral, spicy [47,48] | 118-61-6 | 792 | 955 | ■e | ■e | ■d,e | □ | ■e | ■d,e |
| Methyl octanoate | Orange, vegetable, herbal [47,48] | 111-11-5 | 632 | 742 | □ | □ | ■e | □ | □ | □ |
| Ethyl octanoate | Fruity, banana, pear [47,48] | 106-32-1 | 690 | 854 | □ | ■e | ■e | □ | □ | □ |
| Esters | ||||||||||
| Methyl palmitate | Waxy, fatty, candle [48] | 112-39-0 | 851 | 888 | □ | □ | □ | □ | □ | ■e |
| Ethyl palmitate | Fruity, milky, balsamic [48] | 628-97-7 | 573 | 635 | ■e | □ | ■e | ■e | □ | □ |
| Benzyl acetate | Fresh, boiled vegetable, fruity, floral [47,48] | 140-11-4 | 622 | 722 | □ | ■e | □ | □ | □ | □ |
| Isopropyl myristate | Faint, oily, fatty [48] | 110-27-0 | 714 | 782 | □ | □ | □ | □ | □ | ■d,e |
| Terpenoid | ||||||||||
| Linalool * | Citrus, floral, lavender, sweet, green [47,48] | 78-70-6 | 788 | 838 | ■e | ■e | □ | ■d,e | ■e | ■e |
| α-terpineol | Oil, anise, mint, lemon, citrus [47,48] | 98-55-5 | 730 | 838 | □ | □ | □ | ■e | □ | □ |
| Menthol | Fresh, peppermint [47,48] | 2216-51-5 | 761 | 801 | □ | □ | □ | □ | □ | ■e |
| β-ionone * | Sweet, violet, floral, raspberry [47] | 14901-07-6 | 708 | 807 | □ | ■e | □ | □ | □ | □ |
| Ketones | ||||||||||
| β-damascenone * | Apple, rose, honey, sweet, tobacco [47,48] | 23726-93-4 | 823 | 927 | ■e | ■d,e | ■e | □ | □ | □ |
| γ-nonalactone | Coconut, peach, sweet [47,48] | 104-61-0 | 827 | 899 | ■d,e | ■d,e | ■e | ■d,e | ■e | □ |
| Geranyl acetone | Magnolia, fruity, rose, pear, guava [47,48] | 3796-70-1 | 758 | 873 | ■e | ■e | ■e | □ | □ | □ |
| Furans | ||||||||||
| Furfural | Bread, almond, sweet, woody [47,48] | 98-01-1 | 867 | 902 | ■d,e | ■d,e | ■d,e | ■d,e | ■d,e | ■d,e |
| 5-methylfurfural | Almond, caramel, burnt sugar [47] | 620-02-0 | 696 | 863 | □ | □ | □ | ■d,e | □ | □ |
| 2-acetylfuran | Balsamic, almond, nutty, toasted [47,48] | 1192-62-7 | 670 | 848 | □ | □ | □ | ■e | □ | □ |
| Pyrroles | ||||||||||
| 2-acetylpyrrole | Nutty, walnut, bread [47] | 1072-83-9 | 680 | 835 | □ | □ | □ | ■e | ■d,e | □ |
| Formyl pyrrole | Chocolate [48] | 1003-29-8 | 657 | 819 | □ | ■e | □ | □ | □ | □ |
| Pyrazines | ||||||||||
| 2,3,5-trimethyl-pyrazine | Roast, potato, musty, nutty, cocoa [47,48] | 14667-55-1 | 689 | 865 | □ | ■e | □ | □ | □ | □ |
| 2,6-dimethyl-pyrazine | Nutty, butter, cocoa, caramel [47,48] | 5910-89-4 | 695 | 845 | □ | ■e | □ | □ | □ | □ |
| Gender | Age | ||||||
|---|---|---|---|---|---|---|---|
| Male | Female | 18–24 | 25–34 | 34–44 | 45–59 | ||
| 26% | 74% | 39% | 47% | 8% | 6% | ||
| Level of education | |||||||
| Basic education | Complete high school | Incomplete graduation | Complete graduation | Master’s or Doctoral degree | |||
| 6% | 10% | 31% | 7% | 46% | |||
| Family income (MW: minimum wages) | |||||||
| 1 MW | 2–3 MW | 4–5 MW | >5 MW | ||||
| 1% | 25% | 25% | 46% | ||||
| Frequency of tea/herbal tea consumption | Portionsize | ||||||
| Daily | Twicedaily | Weekly | 50 mL | Regular cup (150 mL) | Largecup (240 mL) | ||
| 30% | 16% | 54% | 12% | 33% | 55% | ||
| Types of teas commonly consumed | |||||||
| Black tea | Green tea | White Tea | Toasted maté | Chamomile | Lemonbalm | Fruity | Other teas * |
| 35% | 27% | 6% | 57% | 65% | 50% | 44% | 28% |
| Bulk orsachet? | Brands | ||||||
| Only sachets | Only bulk teas ** | Sachetand bulk teas | Only traditional | Only imported | Traditionaland imported | ||
| 48% | 7% | 45% | 82% | 6% | 12% | ||
| Drinking temperature | Consumption of ready-to-drink infusions *** | Habit of sweetening tea | |||||
| Only hot | Only cold | Hot and cold | Yes | No | Yes **** | No | |
| 51% | 4% | 45% | 53% | 47% | 45% | 55% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DePaula, J.; Cunha, S.C.; Cruz, A.; Sales, A.L.; Revi, I.; Fernandes, J.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; Farah, A. Volatile Fingerprinting and Sensory Profiles of Coffee Cascara Teas Produced in Latin American Countries. Foods 2022, 11, 3144. https://doi.org/10.3390/foods11193144
DePaula J, Cunha SC, Cruz A, Sales AL, Revi I, Fernandes J, Ferreira IMPLVO, Miguel MAL, Farah A. Volatile Fingerprinting and Sensory Profiles of Coffee Cascara Teas Produced in Latin American Countries. Foods. 2022; 11(19):3144. https://doi.org/10.3390/foods11193144
Chicago/Turabian StyleDePaula, Juliana, Sara C. Cunha, Adriano Cruz, Amanda L. Sales, Ildi Revi, José Fernandes, Isabel M. P. L. V. O. Ferreira, Marco A. L. Miguel, and Adriana Farah. 2022. "Volatile Fingerprinting and Sensory Profiles of Coffee Cascara Teas Produced in Latin American Countries" Foods 11, no. 19: 3144. https://doi.org/10.3390/foods11193144
APA StyleDePaula, J., Cunha, S. C., Cruz, A., Sales, A. L., Revi, I., Fernandes, J., Ferreira, I. M. P. L. V. O., Miguel, M. A. L., & Farah, A. (2022). Volatile Fingerprinting and Sensory Profiles of Coffee Cascara Teas Produced in Latin American Countries. Foods, 11(19), 3144. https://doi.org/10.3390/foods11193144