Gene Expression Analyses Reveal Mechanisms of Inhibited Yellowing by Applying Selenium-Chitosan on Fresh-Cut Broccoli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Chromatic Aberration
2.3. Ethylene Release Rate and Respiratory Intensity
2.4. Determination of Chlorophyll Content
2.5. Determination of Carotenoids and Their Components
2.6. Quantitative Real-Time PCR (RT-qPCR) Assay
2.7. Statistical Analysis
3. Results
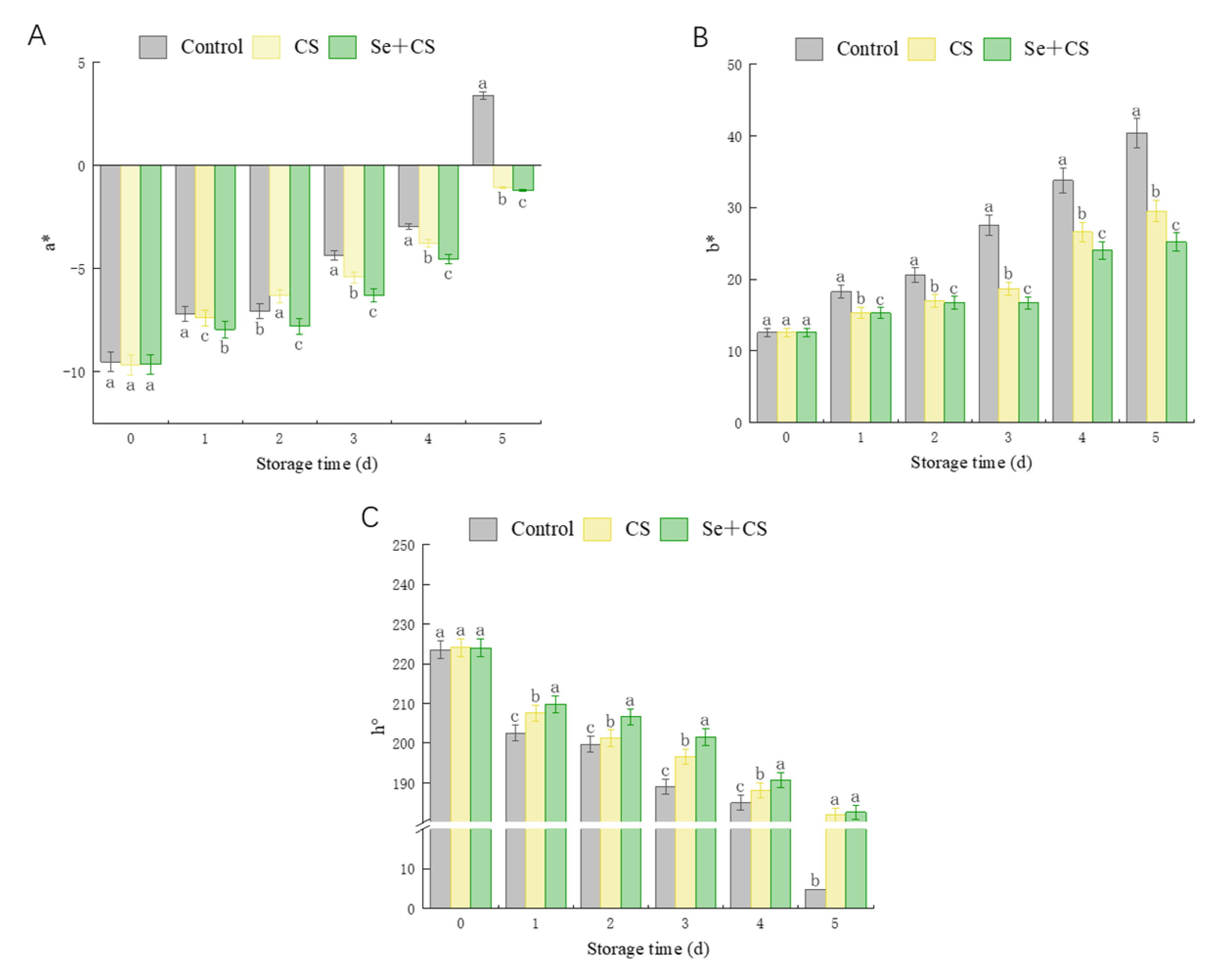
3.1. Chromatic Aberration Comparison between Treatments
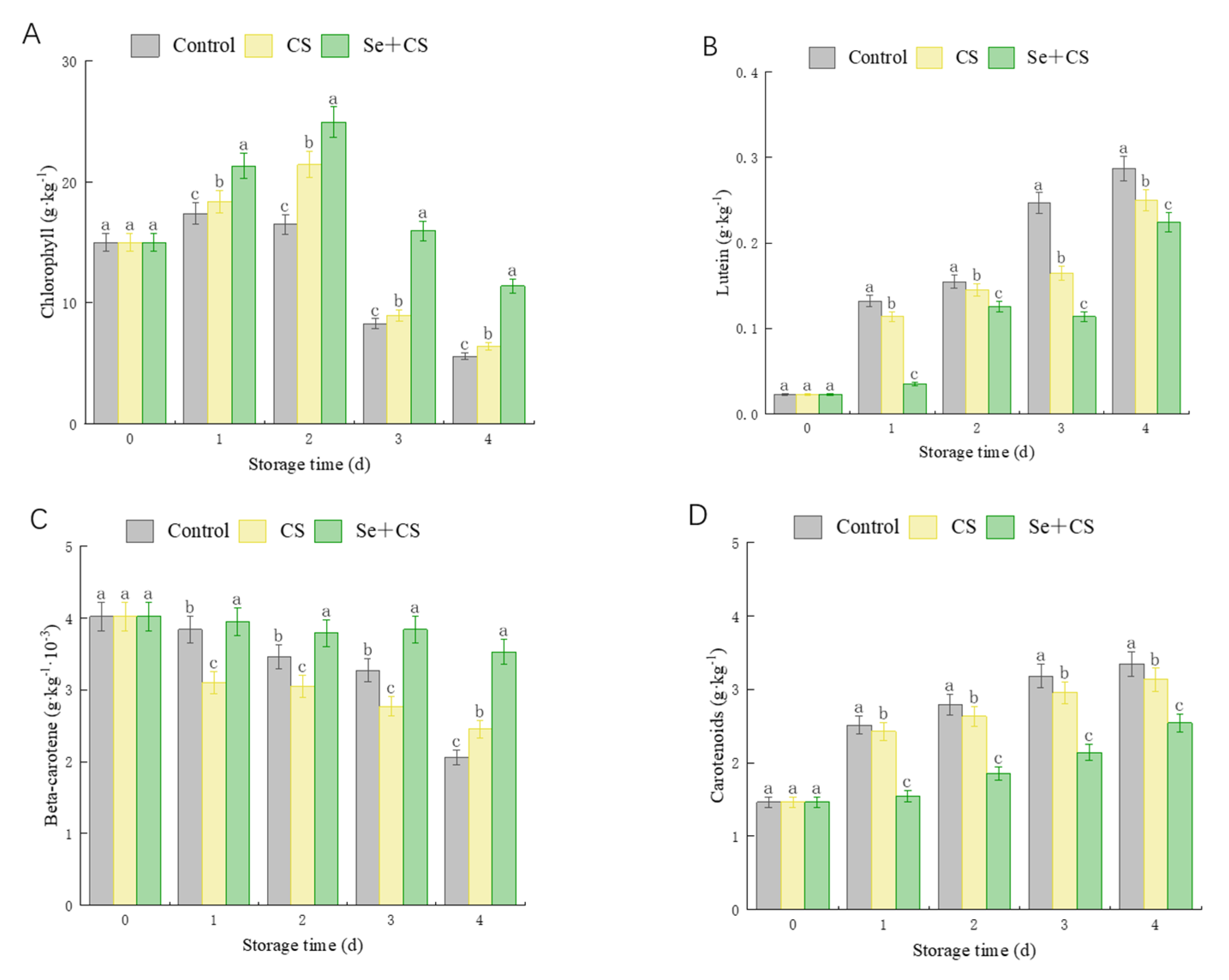
3.2. Analysis of Pigment Content
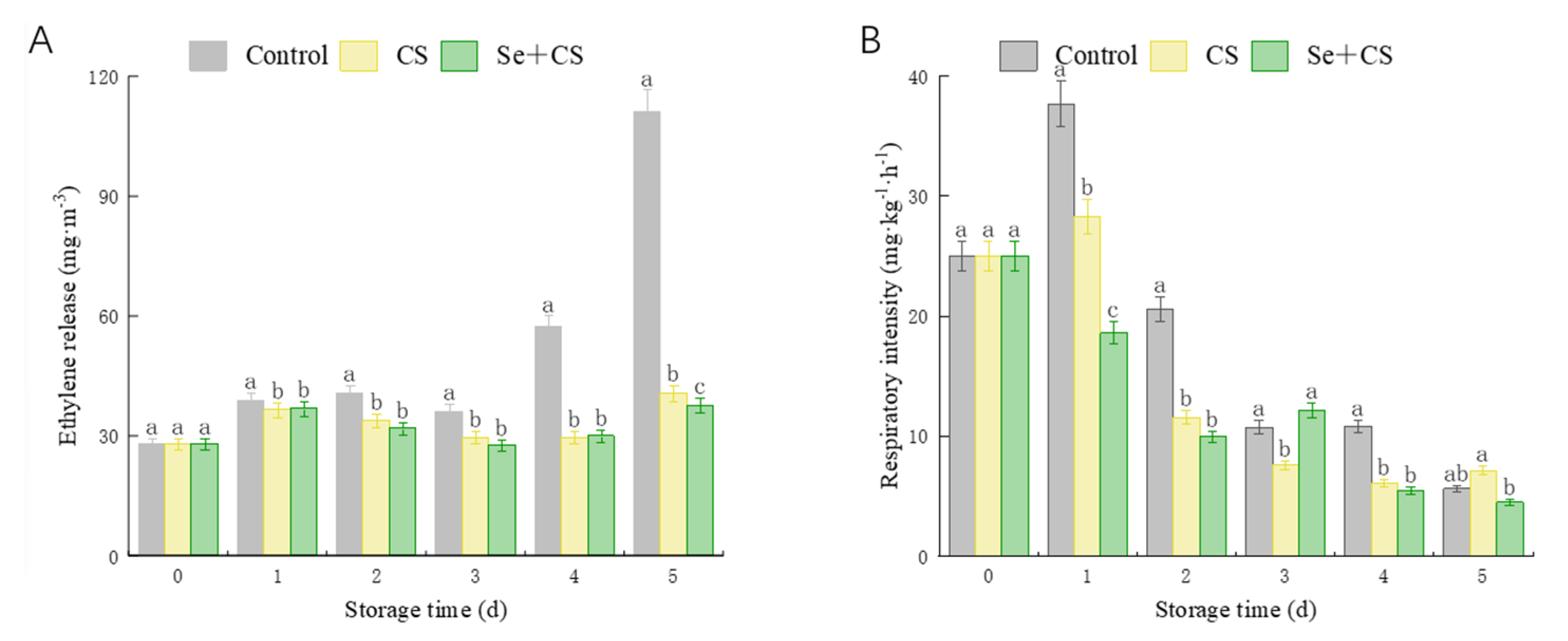
3.3. Analysis of Ethylene Release Rate and Respiration Intensity
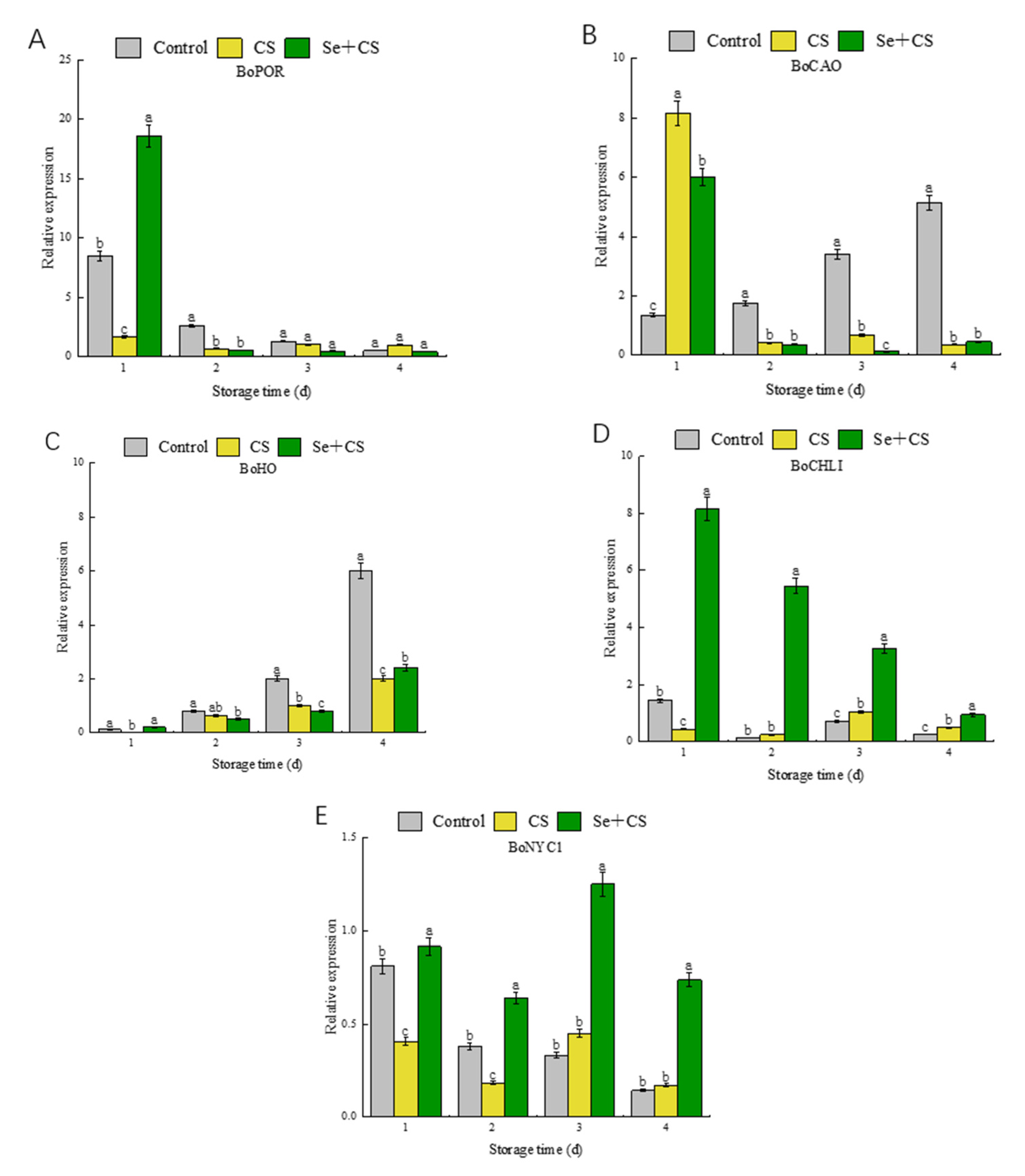
3.4. Analysis of Differentially Expressed Genes in Chlorophyll Metabolism
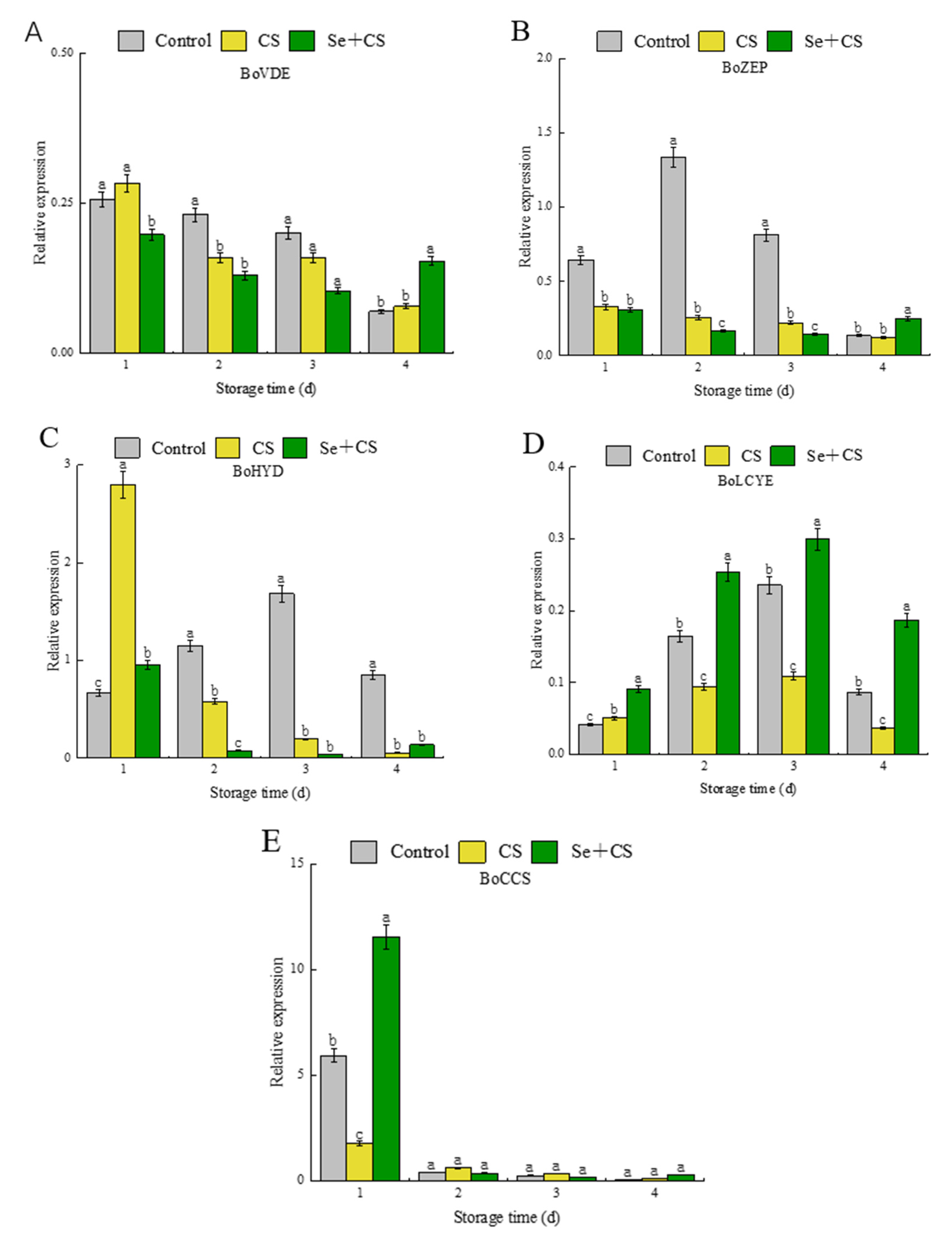
3.5. Analysis of Differentially Expressed Genes in Carotenoid Synthesis
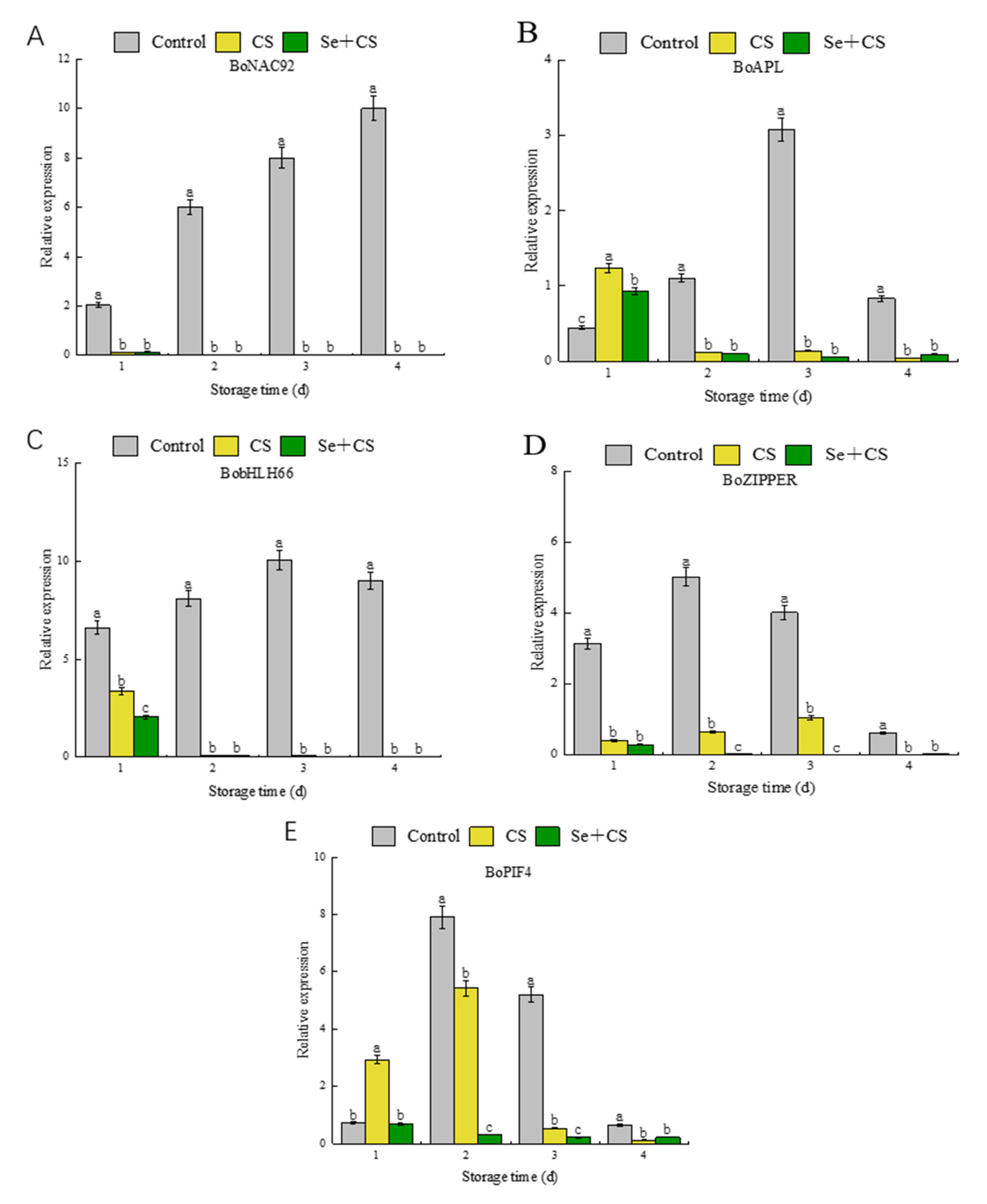
3.6. Analysis of Transcription Factors in Pigment Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Jobson, H.E.; Li, J.; Stephenson, K.K.; Wade, K.; Fahey, J.W. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol. Cancer Ther. 2006, 5, 935–944. [Google Scholar]
- Rex, M.; Paulette, M.-F.; Munday, C.; Paonessa, J.D.; Tang, L.; Munday, J.; Lister, C.; Wilson, P.; Warren, D.; Zhang, Y. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008, 68, 1593–1600. [Google Scholar]
- Latté, K.P.; Appel, K.-E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhou, Q.; Cheng, S.; Zhou, X.; Wei, B.; Zhao, Y.; Ji, S. 24-epibrassinolide alleviates postharvest yellowing of broccoli via improving its antioxidant capacity. Food Chem. 2021, 365, 130529. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Lai, Y.; Lo, H.; Chen, L.O. Proteomics and transcriptomics of broccoli subjected to exogenously supplied and transgenic senescence-induced cytokinin for amelioration of postharvest yellowing. J. Proteom. 2013, 93, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Liu, C.; Wang, J.; Younas, S.; Zheng, H.; Zheng, L. Melatonin immersion affects the quality of fresh-cut broccoli (Brassica oleracea L.) during cold storage: Focus on the antioxidant system. J. Food Process. Preserv. 2020, 44, e14691. [Google Scholar] [CrossRef]
- Soleimani, A.M.; Borja, F.F. Employing phytosulfokine α (PSKα) for delaying broccoli florets yellowing during cold storage. Food Chem. 2021, 355, 129626. [Google Scholar]
- Martínez-Hernández, G.B.; Huertas, J.-P.; Navarro-Rico, J.; Gómez, P.A.; Artés, F.; Palop, A.; Artés-Hernández, F. Inactivation kinetics of foodborne pathogens by UV-C radiation and its subsequent growth in fresh-cut kailan-hybrid broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariantonia, L.; Davide, M.; Rossella, D.R.; Mauro, A.; Daniele, M.; Massimo, S.; Stefano, F. In vivo antiaging effects of alkaline water supplementation. J. Enzym. Inhib. Med. Chem. 2020, 35, 657–664. [Google Scholar]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Chung, M.Y.; Vrebalov, J.; Alba, R.; Lee, J.; McQuinn, R.; Chung, J.D.; Klein, P.; Giovannoni, J. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 2010, 64, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Tavadyan, L.; Manukyan, Z.; Harutyunyan, L.; Musayelyan, M.; Sahakyan, A.; Tonikyan, H. Antioxidant Properties of Selenophene, Thiophene and Their Aminocarbonitrile Derivatives. Antioxidants 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Maryam, N.; Alireza, I.; Rahim, A.; Oraghi, A.Z.; Mostafa, E. Correction: Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2021, 16, e0250192. [Google Scholar]
- Kyriaki, R.P.; Marinos, X.; Maroula, G.K.; Violetta, C.K. Cruciferous vegetables as functional foods: Effects of selenium biofortification. Int. J. Veg. Sci. 2022, 28, 191–210. [Google Scholar]
- Lv, J.; Wu, J.; Zuo, J.; Fan, L.; Shi, J.; Gao, L.; Li, M.; Wang, Q. Effect of Se treatment on the volatile compounds in broccoli. Food Chem. 2017, 216, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, D.; Chen, Y.; Cheng, J.; Zhao, G.; Fahima, T.; Yan, J. Selenium mitigates salt-induced oxidative stress in durum wheat (Triticum durum Desf.) seedlings by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. 3 Biotech 2020, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Dar, Z.M.; Malik, M.A.; Aziz, M.A.; Masood, A.; Dar, A.R.; Hussan, S.; Dar, Z.A. Role of Selenium in Regulation of Plant Antioxidants, Chlorophyll Retention and Osmotic Adjustment under Drought Conditions: A Review. Int. J. Plant Soil Sci. 2021, 33, 52–60. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Mudassir, M.; Ramzan, M.; Hussain, I.; Siddiqui, M.H.; Ali, H.M.; Shabbir, Z.; Ali, A.; Ahmed, S.; et al. Iron oxide nanoparticles and selenium supplementation improve growth and photosynthesis by modulating antioxidant system and gene expression of chlorophyll synthase (CHLG) and protochlorophyllide oxidoreductase (POR) in arsenic-stressed Cucumis melo. Environ. Pollut. 2022, 307, 119413. [Google Scholar] [CrossRef] [PubMed]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; Smedt, S.C.D. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Van, H.L.; Lindong, Z.; Chan, K.H.; Panicker, P.S.; Hoa, P.D.; Jaehwan, K. Chitosan Nanofiber and Cellulose Nanofiber Blended Composite Applicable for Active Food Packaging. Nanomaterials 2020, 10, 1752. [Google Scholar]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; DiVenere, D.; Salerno, M. Effects of Pre- and Postharvest Chitosan Treatments to Control Storage Grey Mold of Table Grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Patre, D.D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 1–152. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Chen, J.; Gan, Z.; Wan, C. Mitigating effects of chitosan coating on postharvest senescence and energy depletion of harvested pummelo fruit response to granulation stress. Food Chem. 2021, 348, 129113–129116. [Google Scholar] [CrossRef]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 1089. [Google Scholar] [CrossRef]
- Huang, C.; Tian, Y.; Zhang, B.; Jawad, H.M.; Li, Z.; Zhu, Y. Chitosan (CTS) Alleviates Heat-Induced Leaf Senescence in Creeping Bentgrass by Regulating Chlorophyll Metabolism, Antioxidant Defense, and the Heat Shock Pathway. Molecules 2021, 26, 5337. [Google Scholar] [CrossRef]
- Qin, S.; Huang, B.; Ma, J.; Wang, X.; Zhang, J.; Li, L.; Chen, F. Effects of selenium-chitosan on blood selenium concentration, antioxidation status, and cellular and humoral immunity in mice. Biol. Trace Elem. Res. 2015, 165, 145–152. [Google Scholar] [CrossRef]
- Khajeh, B.M.; Afsharmanesh, M.; Salarmoini, M.; Ebrahimnejad, H. Effects of selenium-chitosan on growth performance, carcass traits, meat quality, and blood indices of broiler chickens. Livest. Sci. 2021, 250, 104562. [Google Scholar]
- Maiyo, F.; Singh, M. Folate-Targeted mRNA Delivery Using Chitosan-Functionalized Selenium Nanoparticles: Potential in Cancer Immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Luo, F.; Jia, H.C.; Zhang, X.; Tao, D.B.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Effects of methyl jasmonate and melatonin treatments on the sensory quality and bioactive compounds of harvested broccoli. RSC Adv. 2018, 8, 41422–41431. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Luo, F.; Cai, J.-H.; Kong, X.-M.; Zhou, Q.; Zhou, X.; Zhao, Y.-B.; Ji, S.-J. Transcriptome profiling reveals the roles of pigment mechanisms in postharvest broccoli yellowing. Hortic. Res. 2019, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Cheng, S.-C.; Cai, J.-H.; Wei, B.-D.; Zhou, X.; Zhou, Q.; Zhao, Y.-B.; Ji, S.-J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Choudhary, P.; Jain, V. Effects of chitosan and selenium treatments on retention of membrane integrity of guava (Psidium guajava L.) fruits during storage. J. Food Process. Preserv. 2021, 45, e15843. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Zhu, Z.; Liu, Y.; Yuan, L.; Li, M. Effect of selenium on control of postharvest gray mould of tomato fruit and the possible mechanisms involved. Front. Microbiol. 2016, 6, 14–41. [Google Scholar] [CrossRef] [Green Version]
- Carlos, G.-M.F.; Maribel, R.-M.; María, C.-G.A.; Iris, T.-T.L. Lanthanum Prolongs Vase Life of Cut Tulip Flowers by Increasing Water Consumption and Concentrations of Sugars, Proteins and Chlorophylls. Sci. Rep. 2020, 10, 4209. [Google Scholar]
- Lu, N.; Dai, L.; Luo, Z.; Wang, S.; Wen, Y.; Duan, H.; Hou, R.; Sun, Y.; Li, Y. Characterization of the Transcriptome and Gene Expression of Tetraploid Black Locust Cuttings in Response to Etiolation. Genes 2017, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanga, D.; Capell, T.; Slafer, G.A.; Christou, P.; Savin, R. A carotenogenic mini-pathway introduced into white corn does not affect development or agronomic performance. Sci. Rep. 2016, 6, 38288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Zhu, C.; Zhao, S.; Zhang, S.; Wang, W.; Fu, H.; Li, X.; Zhou, C.; Chen, L.; Lin, Y.; et al. De novo transcriptome and phytochemical analyses reveal differentially expressed genes and characteristic secondary metabolites in the original oolong tea (Camellia sinensis) cultivar ‘Tieguanyin’ compared with cultivar ‘Benshan’. BMC Genom. 2019, 20, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Han, M.; Wang, R.; Gao, M. Comparative transcriptome analysis identifies genes associated with chlorophyll levels and reveals photosynthesis in green flesh of radish taproot. PLoS ONE 2021, 16, e0252031. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Cheng, Y.; Feng, L.; Wu, X.; Fan, Y.; Ali, R.M.; Wang, X.; Yong, T.; Liu, W.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- Spano, A.J.; He, Z.; Michel, H.; Hunt, D.F.; Timko, M.P. Molecular cloning, nuclear gene structure, and developmental expression of NADPH: Protochlorophyllide oxidoreductase in pea (Pisum sativum L.). Plant Mol. Biol. 1992, 18, 967–972. [Google Scholar] [CrossRef]
- Oosawa, N.; Masuda, T.; Awai, K.; Fusada, N.; Shimada, H.; Ohta, H.; Takamiya, K. Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Lett. 2000, 474, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Saori, N.; Hisashi, I.; Ryouichi, T.; Ayumi, T. Chlorophyll b reductase plays an essential role in maturation and storability of Arabidopsis seeds. Plant Physiol. 2012, 160, 261–273. [Google Scholar]
- Li, W.-X.; Yang, S.-B.; Lu, Z.; He, Z.-C.; Ye, Y.-L.; Zhao, B.-B.; Wang, L.; Jin, B. Erratum: Publisher Correction: Cytological, physiological, and transcriptomic analyses of golden leaf coloration in Ginkgo biloba L. Hortic. Res. 2018, 5, 32. [Google Scholar] [CrossRef]
- Shen, J.; Zou, Z.; Zhang, X.; Zhou, L.; Wang, Y.; Fang, W.; Zhu, X. Metabolic analyses reveal different mechanisms of leaf color change in two purple-leaf tea plant (Camellia sinensis L.) cultivars. Hortic. Res. 2018, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-García, M.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Carotenoid Biosynthesis in Chili Peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeknić, Z.; Morré, J.T.; Jeknić, S.; Jevremović, S.; Subotić, A.; Chen, T.H. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium Thunb. ‘Splendens’). Plant Cell Physiol. 2012, 53, 899–912. [Google Scholar]
- Ren, Y.; Yang, J.; Lu, B.; Jiang, Y.; Chen, H.; Hong, Y.; Wu, B.; Miao, Y. Structure of Pigment Metabolic Pathways and Their Contributions to White Tepal Color Formation of Chinese Narcissus tazetta var. chinensis cv Jinzhanyintai. Int. J. Mol. Sci. 2017, 18, 1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Zhang, J.; Xu, J.; Ye, J.; Yun, Z.; Xu, Q.; Xu, J.; Deng, X. Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J. Exp. Bot. 2012, 63, 4403–4417. [Google Scholar] [CrossRef] [PubMed]
- Sestili, F.; Garcia-Molina, M.D.; Gambacorta, G.; Beleggia, R.; Botticella, E.; Vita, P.D.; Savatin, D.V.; Masci, S.; Lafiandra, D. Provitamin A Biofortification of Durum Wheat through a TILLING Approach. Int. J. Mol. Sci. 2019, 20, 5703. [Google Scholar] [CrossRef] [Green Version]
- Christian, F.; Diana, S.; Tomas, M. Identification of key residues for pH dependent activation of violaxanthin de-epoxidase from Arabidopsis thaliana. PLoS ONE 2012, 7, e35669. [Google Scholar]
- Ebrahim, D.; Ali, S.; Hossein, J.; Juan, D.D.A.; Abbas, B.; Mehrshad, Z.; Alireza, S.S. Differential expression of genes in olive leaves and buds of ON-versus OFF-crop trees. Sci. Rep. 2020, 10, 15762. [Google Scholar]
- Roberts, W.R.; Roalson, E. Co-expression clustering across flower development identifies modules for diverse floral forms in Achimenes (Gesneriaceae). PeerJ 2020, 8, e8778. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-F.; Zhang, G.-J.; Zhang, X.-J.; Yuan, J.-H.; Deng, C.-L.; Gao, W.-J. Comparative transcriptome analysis reveals differentially expressed genes associated with sex expression in garden asparagus (Asparagus officinalis). BMC Plant Biol. 2017, 17, 143. [Google Scholar] [CrossRef] [Green Version]
- Proveniers, M.C.; van Zanten, M. High temperature acclimation through PIF4 signaling. Trends Plant Sci. 2013, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, C.; Gao, S.; Zhang, W.; Li, L.; Kuai, B. Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol. Plant 2014, 7, 76–87. [Google Scholar] [CrossRef] [PubMed]
| Gene | Gene Function | Primer Sequences (5′-3′) | Gene Bank Accession |
|---|---|---|---|
| ACT2 | Internal reference | F: 5′-TTCAAGCTGGAGCCAAGAAGGTTC-3′ R: 5′-ACGAATGGTGCGAGACAGTTAGTG-3′ | AF044573 |
| BoHO | Involvement in chlorophyll metabolism | F: 5′-CCACAACTCACCAGCTTCCACTTC-3′ R: 5′-GCCGTCGTAGCTGCAATCACC-3′ | LOC106295105 |
| BoCAO | F: 5′-ACCATCATGCTCCTTCACGACAAG-3′ R: 5′-TCGCCAACTCTCCGCCTCTG-3′ | LOC106350863 | |
| BoNYC1 | F: 5′-ACATTGTGACGATGACGAGCACTG-3′ R: 5′-TAACTCCACCGAGCCAGCTATACC-3′ | LOC106341846 | |
| BoCHLI | F: 5′-CGGCGAGACTGACGAAGTGAAC-3′ R: 5′-AGCAAGCGGTATCGAGAGCAATC-3′ | LOC106305850 | |
| BoPOR | F: 5′-TTCAGGCTGCTTACTCGCTTCTTC-3′ R: 5′-CAACCATGGCATTGGCGTGAAG-3′ | LOC106307221 | |
| BoHYD | Involvement in carotenoid synthesis | F: 5′-AGAGGCTTCTCGGTCTGCTACG-3′ R: 5′-GCTCGACATCACTGCGGCTATTAG-3′ | LOC106302033 |
| BoZEP | F: 5′-AGACGGCGGCGGAGAGTAAG-3′ R: 5′-GCTCAAGTCCTTCTCGAACACCAG-3′ | LOC106390346 | |
| BoCCS | F: 5′-CTGGCTATCGCTGATCCTTGGC-3′ R: 5′-GTTCTCCGCCGTACTTCTCATCG-3′ | LOC106296637 | |
| BoLCYE | F: 5′-AAGGCAGCGAAAAGCAGGAA-3′ R: 5′-TGACCATCCATGTAGTTTCTCCG-3′ | LOC106296030 | |
| BoVDE | F: 5′-GATACGGCGGTGCGGTTGTG-3′ R: 5′-CTCTCCACCAGAGGAGGTTCAGG-3′ | LOC106415200 | |
| BoAPL | Transcription factors for pigment metabolism | F: 5′-GCAGCCGCACAAGGAGTATGG-3′ R: 5′-AGCTGTTCGTGCAACCTTCTCTG-3′ | LOC106402384 |
| BoNAC92 | F: 5′-AACGACAAGACCTCAAGCACATCC-3′ R: 5′-ACACTCACAAGAGAACGCTCCAAC-3′ | LOC106294382 | |
| BoPIF4 | F: 5′-GTGATGACCGTTGGACCGAACC-3′ R: 5′-ACCAGAGGAGCCACCTGATGATG-3′ | LOC106328266 | |
| BobHLH66 | F: 5′-CCGCCTCCGTCCTCAGATGG-3′ R: 5′-ATTCATCATTCCACCTGCCGTTCC-3′ | LOC106308180 | |
| BoZIPPER | F: 5′-CTACCACGTCCGATGAAGCAACTG-3′ R: 5′-GAAGCCAAGCAACCTGCGAGAG-3′ | LOC106325179 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Liu, Y.; Deng, B.; Wang, Y.; Lin, W.; Zhang, Y.; Di, J.; Yang, J. Gene Expression Analyses Reveal Mechanisms of Inhibited Yellowing by Applying Selenium-Chitosan on Fresh-Cut Broccoli. Foods 2022, 11, 3123. https://doi.org/10.3390/foods11193123
Ren G, Liu Y, Deng B, Wang Y, Lin W, Zhang Y, Di J, Yang J. Gene Expression Analyses Reveal Mechanisms of Inhibited Yellowing by Applying Selenium-Chitosan on Fresh-Cut Broccoli. Foods. 2022; 11(19):3123. https://doi.org/10.3390/foods11193123
Chicago/Turabian StyleRen, Gang, Yaping Liu, Bing Deng, Yu Wang, Wenyan Lin, Yulei Zhang, Jianbing Di, and Jiali Yang. 2022. "Gene Expression Analyses Reveal Mechanisms of Inhibited Yellowing by Applying Selenium-Chitosan on Fresh-Cut Broccoli" Foods 11, no. 19: 3123. https://doi.org/10.3390/foods11193123
APA StyleRen, G., Liu, Y., Deng, B., Wang, Y., Lin, W., Zhang, Y., Di, J., & Yang, J. (2022). Gene Expression Analyses Reveal Mechanisms of Inhibited Yellowing by Applying Selenium-Chitosan on Fresh-Cut Broccoli. Foods, 11(19), 3123. https://doi.org/10.3390/foods11193123






