Abstract
Biofilms are highly resistant to external forces, especially chemicals. Hence, alternative control strategies, like antimicrobial substances, are forced. Antimicrobial surfaces can inhibit and reduce microbial adhesion to surfaces, preventing biofilm formation. Thus, this research aimed to investigate the bacterial attachment and biofilm formation on different sealants and stainless steel (SS) surfaces with or without antimicrobials on two Gram-positive biofilm forming bacterial strains. Antimicrobial surfaces were either incorporated or coated with anti-microbial, -fungal or/and bactericidal agents. Attachment (after 3 h) and early-stage biofilm formation (after 48 h) of Staphylococcus capitis (S. capitis) and Microbacterium lacticum (M. lacticum) onto different surfaces were assessed using the plate count method. In general, bacterial adhesion on sealants was lower compared to adhesion on SS, for surfaces with and without antimicrobials. Antimicrobial coatings on SS surfaces played a role in reducing early-stage biofilm formation for S. capitis, however, no effects were observed for M. lacticum. S. capitis adhesion and biofilm formation were reduced by 8% and 25%, respectively, on SS coated with an antimicrobial substance (SS_4_M), compared to the same surface without the antimicrobial coating (SS_4_control). Incorporation of both antifungicidal and bactericidal agents (S_5_FB) significantly reduced (p ≤ 0.05) early-stage biofilm formation of M. lacticum, compared to the other sealants incoportating either solely antifungal agents (S_2_F) or no active compound (S_control). Furthermore, the thickness of the coating layer correlated weakly with the antimicrobial effect. Hence, equipment manufacturers and food producers should carefully select antimicrobial surfaces as their effects on bacterial adhesion and early-stage biofilm formation depend on the active agent and bacterial species.
1. Introduction
Biofilms in the food industry are a primary cause for cross-contamination and metal corrosion [1,2]. Spoilage and pathogenic bacteria can colonize a wide range of surfaces commonly found in the food industry, like rubber, polypropylene, plastic, glass, or stainless steel (SS) [3]. SS is widely used because of its high corrosion resistance and superior mechanical properties [4], while polymer materials, such as polyethylene (PE) and polyvinyl chloride (PVC), are cost-effective options known to inhibit biofilm growth and corrosion [5].
Nowadays, cleaning and disinfectant agents are typically used to reduce or eliminate bacterial biofilms [6,7]. However, biofilms show increased resistance against chemicals compared to planktonic cells [8]. Hence, alternative methods to control biofilm formation, like modification of surfaces to prevent bacterial adhesion, are needed [2,6]. Different techniques can be applied to develop antimicrobial surfaces: (i) incorporation of antimicrobial agents into the surface material; (ii) deposition of antimicrobial coatings on the surface (=surface coating), or (iii) changes in relevant surface characteristics (e.g., roughness, surface free energy, hydrophobicity) [9,10]. Furthermore, in the context of increased environmental awareness, antimicrobial surfaces have the potential to become sustainable, eco-friendly alternatives replacing chemical cleaning and disinfection agents [11,12].
Moreover, antimicrobial surfaces can be divided further into (i) antimicrobial (bactericidal or/and fungicidal) and (ii) antibiofouling surfaces. The antimicrobial effect is achieved by releasing biocides or by contact killing which is specifically targeting i.e., the cell membrane of bacteria for bactericidal substances [13,14]. In contrast, antibiofouling surfaces are created to resist microbial accumulation and adsorption, thus limiting the attachment of the microorganisms on the surface and the biofilm formation [14,15]. In the present study antimicrobial (bactericidal, fungicidal or a combined approach) surfaces are considered. More details on antibiofouling surfaces can be found in the following review [16].
In recent years, significant progress has been made, and many different types of antimicrobial surfaces proved effective against surface-associated biofilms [17,18]. The mode of action was mainly related to the smaller contact area between bacteria and coating or to the hydrophilic properties of coatings [19,20,21]. However, the variation in results is still high due to the type of antimicrobial agent used, mode of applying the coatings, biofilm assessment method, or bacterial strains tested [22,23,24].
The aim of this study was to assess the attachment and early-stage biofilm formation of two Gram-positive bacterial strains in a static environment on different sealants and SS surfaces, some of them incorporating or coated with active antimicrobial, -fungal and/or bactericidal agents. Hence, this study provides valuable insights for equipment manufacturers and food processors regarding the efficacy of different surfaces in reducing biofilm formation.
2. Material and Methods
2.1. Bacterial Strains and Culture Conditions
Two Gram-positive bacteria frequently found in the food industry, especially in the milk or meat industry were selected because of their strong biofilm forming ability [25]. Microbacterium lacticum (M. lacticum) D84 (EF 204392) was isolated from extended shelf-life (ESL) milk, and Staphylococcus capitis subsp. capitis (S. capitis) was isolated from an air decontamination step prior to packaging at a meat production facility. Bacterial stock and working cultures were maintained and prepared according to Zand et al. [25]. The isolates were preserved in a 50% (v/v) glycerol stock at −80 °C. To obtain a stock culture, bacteria were sub-cultured overnight in tryptic soy broth (TSB; Carl Roth, Karlsruhe, Germany) at 37 °C and in 0.8% (v/v) skimmed milk broth (MB; Carl Roth) at 30 °C, for S. capitis and M. lacticum, respectively. Subsequently, stock cultures were then streaked onto either tryptic soy agar (TSA) or milk agar (MA) (Carl Roth) and incubated at 37 °C or 30 °C overnight and stored at 4 °C. Before each experiment, one colony was inoculated in 10 mL fresh TSB or MB, and the optical density600 (OD) was standardized to 0.1 to obtain a working culture.
2.2. Surface Types
Six sealants and five SS surface coupons (4 cm2) were selected, considering surfaces with and without antibactericidal, -fungal, or -microbial agents according to the manufacturer’s data (Table 1), to test their efficiency against biofilm-forming bacteria.

Table 1.
Detailed information about the tested sealant and stainless steel surfaces with or without antimicrobial, -fungal or/and bactericidal components according to the manufacturer’s data; The different antimicrobial surfaces are marked as followed: F—antifungal, M—antimicrobial, FB—fungicidal and bactericidal. 1 Same surface type with and without antimicrobial coating.
A surface with 1K silyl-modified polyethers (MS) polymer-basis without fungicidal or bactericidal active ingredients, referred to as hybrid glue 007 (S_control, EVT Dichtstoffe GmbH, Korntal-Münchingen, Germany), was included as a control. Additionally, one-component silicon-based sealants incorporating active fungicidal agents: Joint HPA (S_1_F), clean room sanitary HPCR (S_2_F) and sanitary HPS (S_3_F) (all EVT Dichtstoffe GmbH, Korntal-Münchingen, Germany), were tested. Other sealant materials with silane-terminated hybrid-polymer (STP) or acetat base, one incorporating active antimicrobial agents, referred to as Novasil (S_4_M, Hermann Otto GmbH, Fridolfing, Germany) and one incorporating both fungicidal and bactericidal active ingredients named 450 sanitary (S_5_FB, Ramsauer GmbH & Co KG, Upper Austria, Austria), respectively, were included. All SS surfaces (Brucha GmbH, Michelhausen, Austria) were SS type AISI-304 and differ in respect to the type and size of the coating applied on the metal: Polyester foil with 25 µm thickness (SS_1), polyurethan-polyamide foil with 50 µm thickness (SS_2), Niro V2A standard alloy (SS_3) as well as PVC foil with 150 µm thickness (SS_4_control, control surface) and PVC foil with 150 µm thickness including an antimicrobial coating (SS_4_M).
All coupons were cleaned before use, according to Zand, et al. [25], with minor modifications. The coupons were soaked in acetone (Roth, Karlsruhe, Germany) for 30 s, rinsed with distilled water, and soaked again in 1N NaOH (Roth, Karlsruhe, Germany) for 1 h. A final rinse with distilled water was carried out prior to sterilization (121 °C for 15 min).
2.3. Bacterial Adhesion and Biofilm Formation
Bacterial adhesion and biofilm formation assays were performed as described by Zand, et al. [25]. Briefly, coupons were immersed in a bacterial culture at a level of ~2 log CFU cm−2 (~7.5 CFU mL−1) in a small petri dish (35 × 10 mm; Greiner Bio-One, Kremsmünster, Austria) and incubated at 30 °C for M. lacticum and at 37 °C for S. capitis. To assess adhesion, bacterial cells were enumerated after 3 h of incubation. Early-stage biofilm formation was examined after 48 h. A washing step was performed, after the first 24 h of incubation to remove non-adherent cells and provide fresh growth media. After 3 h and 48 h, the coupons were washed with phosphate-buffered saline solution (PBS, Carl Roth, Karlsruhe, Germany), bacterial cells were removed using sonication (3 × 1 min at 35 kHz; Ultrasonic bath, Sonorex RK100H; Bandelin electronic, Berlin, Germany) and drop plated (6 × 5 µL drops) with an electronic multi channel pipette onto TSA (Carl Roth, Germany) and milk agar (Carl Roth, Germany), for S. capitis and M. lacticum, respectively. Each experiment was repeated in at least triplicates.
2.4. Statistical Analysis
For statistical analysis, a multiple range test and Pearson correlation analysis were performed in Statgraphics Centurion XVIII (Statpoint Technologies, Inc., Warrenton, FL, USA). Each experiment was repeated in at least triplicates. All results (n ≥ 18) were used for statistical analysis. Statistical significance was considered for p ≤ 0.05.
3. Results and Discussion
3.1. Bacterial Adhesion and Biofilm Formation
Staphylococcus spp. and Microbacterium spp. are known as good biofilm formers, able to attach well to plastic surfaces and SS, depending on the growth conditions and bacterial strains used [26,27,28,29,30] and are frequently isolated from food contact surfaces in the industry [31,32,33,34]. Furthermore, different S. capitis strains showed high resistance against conventional disinfectants, such as chlorhexidine and quaternary ammonium compounds, while M. lacticum, adherent to abiotic surfaces, is resistant against cleaning and disinfection and against pasteurization [35,36]. Consequently, these two bacterial strains were selected to investigate the effects of different surface types on biofilm growth.
Adhesion and early-stage biofilm levels between 1.05 ± 0.06 and 2.59 ± 0.11 log CFU cm−2 were detected in this study for both bacterial strains. This is similar to levels reported in other studies assessing adhesion and early stage biofilm formation on SS or polymers [37,38]. In general, bacterial attachment to surfaces and subsequently biofilm formation varies with the bacterial strain used and the environmental parameters (i.e., pH, NaCl concentration and temperature) [39]. Similar to other studies published so far [5,40,41,42,43], our data show that differences in bacterial adhesion and early stage biofilm formation are determined by surface type, and bacterial strain tested. Onto sealant surfaces S. capitis displayed better biofilm formation capacity compared to M. lacticum, which is reflected in the higher number of cells adherent and present in the early-stage biofilm, except for adhesion on S_1_F surface. In the case of SS higher numbers of S. capitis cells were detected compared to M. lacticum only for adhesion.
On average, both bacterial strains attached in slightly lower numbers to sealant surfaces (Figure 1) compared to SS (Figure 2). All sealant surfaces, except for S_control, incorporated either antifungal, antimicrobial or both antifungal and bactericidal agents, while in the case of SS only the surface SS_4_M was coated with antimicrobials (Table 1). This might explain the differences in the number of attached cells detected. Previous studies [42,44,45] show higher attachment and/or biofilm formation onto SS than rubber and/or plasticwhile other studies show the contrary [25,46,47,48,49]. Furthermore, similar biofilm formation on PVC, PE, and SS surfaces was also reported by Zacheus, et al. [50]. Moreover, variation in the assessment method for biofilm formation and types of surfaces tested makes it often difficult to obtain consistent results. Microbial attachment and further biofilm formation are regulated by bacterial cell surface components and by several surface-specific characteristics, such as surface charge and hydrophobicity [6]. For Gram-negative and Gram-positive bacteria, lipopolysaccharides and teichoic acids, respectively, provide charges on the outer surface of bacteria with effect on adhesion and biofilm formation [51]. Most bacteria have a negatively charged and hydrophobic surface at neutral pH and tend to adhere to hydrophobic surfaces rather than hydrophilic ones. However, the degree of cell membrane hydrophobicity depends on the bacterial strain [52,53] and adapts with growth conditions [54]. The hydrophobicity of bacterial strains and surfaces was not characterized within the present study. Even though SS is generally considered hydrophilic, while polymers are known as hydrophobic materials [55], there are studies that found SS type 304 surfaces to be hydrophobic [25,56]. This supports the observations made in the present study. However, the role of hydrophobicity on the bacterial adhesion to a surface is still controversially discussed, as many studies found no relationship between surface properties and biofilm formation [43,45,57,58].
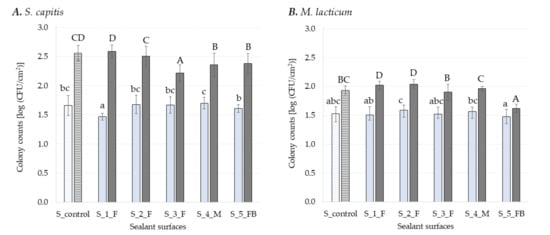
Figure 1.
Log CFU cm−2 of S. capitis (A) and M. lacticum (B) enumerated on different sealant surfaces after 3 h (light blue) and 48 h (dark grey) of incubation. Surfaces with antimicrobial coatings are colored with a solid fill and surfaces without antimicrobials are highlighted with a pattern fill. Different lowercase letters denote significant differences (multiple range test, p ≤ 0.05) between the respective samples during adhesion (3 h), while uppercase letters indicate differences in early-stage biofilm formation (48 h). Each experiment was repeated in at least in triplicates.
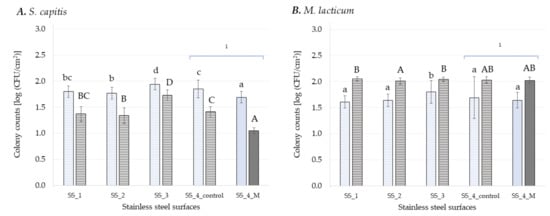
Figure 2.
Log CFU cm−2 of S. capitis (A) and M. lacticum (B) enumerated on different stainless steel surfaces after 3 h (light blue) and 48 h (dark grey) of incubation. Surfaces with antimicrobial coatings are colored with a solid fill and surfaces without antimicrobials are highlighted with a pattern fill. Different lowercase letters denote significant differences (multiple range test, p ≤ 0.05) between the respective samples during adhesion (3 h), while uppercase letters indicate differences in early-stage biofilm formation (48 h). Each experiment was repeated in at least triplicates. 1 Same SS surface with and without antimicrobial agent.
3.2. Antimicrobial Sealant Surfaces
Silicones are intensively used in the industry as sealants, being particularly appreciated for their elastic behavior and good resistance to weathering. The name silicone refers to poly-dimethylsiloxanes polymers (PDMS), and by substituting the methyl group along the chain with, e.g., phenyl, vinyl, or trifluoropropyl group, their properties change [59]. Figure 1 presents the number of bacterial cells attached and enumerated in early-stage biofilms subsequently formed onto sealant surfaces. Here, one control surface without antimicrobial agent and five sealants incorporating antimicrobial, antifungal and/or bactericidal agents were investigated. Surprisingly, the number of S. capitis cells attached to S_4_M (1.7 ± 0.14 log CFU cm−2), a sealant material incorporating an antimicrobial agent, displayed the highest number detected among all sealant surfaces, being significantly higher (p ≤ 0.05) compared to S_5_FB (1.61 ± 0.07 log CFU cm−2) and S_1_F (1.47 ± 0.17 log CFU cm−2), with 5% and 14% higher CFU cm−2 counts, respectively. However this was not significantly different (p > 0.05) when compared to the control (S_control, the sealant surface of MS polymer-basis without any antimicrobial agent). For M. lacticum, the highest number of adherent cells was found onto S_2_F (1.59 ± 0.09 log CFU cm−2), while the lowest number was found onto S_5_FB (1.48 ± 0.12 log CFU cm−2). In this case the presence of both fungicidal and bactericidal active ingredients reduced the number of bacterial cells attached to S_5 _FB by 7% when compared to S_2_F, while for S_4_M only 1% bacterial cell reduction was seen. Furthermore, none of the tested surfaces incorporationg antimicrobial and/or antifungal compounds was significantly different to the control surface (S_control).
Counts of bacterial cells in early-stage biofilms (48 h) formed on sealants ranged between 1.62 ± 0.07 log CFU cm−2 for S_5_FB and 2.04 ± 0.08 log CFU cm−2 for S_2_F in case of M. lacticum. Incorporation of antibacterial agents into S_5_FB and S_4_M, reduced, therefore, the early-stage biofilm formation of M. lacticum by 21% and 4% respectively as compared to S_2_F. However, compared to the control surface (S_control) a significant reduction of 16% (p ≤ 0.05) was seen in the number of cells in the early-stage biofilm formed onto S_5_FB, but not onto S_4_M surface. The S_5_FB surface is the only one incorporating both fungicidal and antibacterial agents, most likely resulting in synergic effects in reducing biofilm formation of M. lacticum. Previous reports described synergic interactions between antibacterial and antifungal compounds [60,61]. However, such effects of S_5_FB on S. capitis biofilm growth was not observed. Therefore, it is assumed that the effects are bacterial strains specific. In future research, it would be also of interest to assess fungi or fungal spores to obtain holistic information on the effectiveness of the surface agents. Early-stage biofilms formed onto S_1_F and S_2_F, both incorporationg an antifungal agent were including significantly (p ≤ 0.05) higher numbers of cells compared to the control (S_control). For S. capitis, the highest mean value in the early-stage biofilm (48 h) was observed onto S_1_F (2.59 ± 0.11 log CFU cm−2) and the lowest mean value onto S_3_F (2.22 ± 0.14 log CFU cm−2) (Figure 1).
Incorporation of an antibacterial agent into surfaces S_4_M and S_5_FB reduced the number of bacterial cells in the biofilm developed on these two surfaces by 7% compared to the control surface (S_control). Furthermore, a significant reduction (p ≤ 0.05), of 13%, could be seen also in the number of cells in the early-stage biofilm formed onto S_3_F compared to S_control. Previous studies already showed that different combinations of polymers with antimicrobial agents efficiently reduce biofilm formation [5,62,63]. For example, immobilization of polymyxins on PDMS was able to prevent the adhesion of Pseudomonas aeruginosa and inactivated a significant fraction of the adherent cells [62]. Moreover, it was found that surface coating with 2-methacryloyloxyethyl phosphorylcholine co-polymer significantly reduces retention of human pathogenic microorganisms [63]. For antimicrobial polymer surfaces, polymer charge and hydrophobicity have been identified as the leading parameters that affect antimicrobial activity. These surfaces bear cationic charges and kill or deactivate bacteria by interaction with negatively charged parts of their cell envelope [64]. According to Hyde, et al. [65], biofilm adherence to polymer-based surfaces is a function of both surface finish and surface chemistry. Assessment of surface-specific properties was not performed in this study. However, the substratum surface properties, like hydrophobicity or roughness, are generally impacting the late-stage rather than the early-stage biofilm formation [3], and surface roughness of material may hinder effective cleaning and sanitizing and not biofilm formation [66,67].
3.3. Antimicrobial Stainless Steel Surfaces
Stainless steel (SS) is a common food contact material, regularly used for process equipment because of its corrosion resistance, cleanability and high mechanical strength. Here, four SS surfaces with varying thickness in the coating layer (SS_1, SS_2 and SS_4; 25, 50 and 150 µm) and additional alloy (SS_3; Standard V2A) were tested. Additionally to the SS with 150 µm thick coating (SS_4_control; 150 µm) a surface with the same coating and additional antimicrobial agents was tested (SS_4_M).
The number of cells attached to the SS surfaces and the early stage biofilm formed onto these surfaces is depicted in Figure 2. The lowest adhesion rate of S. capitis on SS surfaces was detected for SS_4_M (1.69 ± 0.11 log CFU cm−2), which was significantly reduced (p ≤ 0.05) compared to the control surface (SS_4_control) and also compared to the other SS surfaces without antimicrobials with a percentage of 5% to 13%. The highest adhesion rate was seen for SS_3 (1.94 ± 0.11 log CFU cm−2). Biofilm formation of S. capitis showed a similar trend with significantly (p ≤ 0.05) lowest cell density on SS_4_M (1.05 ± 0.06 log CFU cm−2) and with significantly (p ≤ 0.05) highest cell population on SS_3 (1.73 ± 0.1 log CFU cm−2). Furthermore, the antimicrobial agent used to coat the SS_4_M reduced the number of bacterial cells in the early-stage biofilm by 26% in comparison to SS_4_control (=control surface with the same base but without antibacterial agent). Furthermore, the size of coating applied on SS_1 (25 µm) and SS_2 (50 µm) reduced significantly (p ≤ 0.05) the number of attached cell onto these surfaces when compared to SS_4_control (140 µm), but significantly increased the number of cells in the early-stage biofilm formed onto these surfaces. Surprisingly, the mean value of S. capitis cells detected in early stage biofilm (after 48 h) was significantly (p ≤ 0.05) lower compared to attachment rate (after 3 h) on SS surfaces (Figure 2A). This can be related to the static biofilm system used, where the availability of nutrients might be a limiting factor during biofilm growth [3], as S. capitis has a faster growth rate when compared to M. lacticum [25]. It is considered that under static conditions, adherent cells may be present in high numbers but do not always increase over the incubation time as a consequence of the cell division process and/or redistribution of adherent cells forming the biofilm [68]. Therefore, high levels of initial adherence do not necessarily lead to a thicker and stronger biofilm matrix [69]. This was also seen in the present study for sealants (Figure 1). The lowest adhesion rate of S. capitis on S_1_F (1.47 ± 0.17 log CFU cm−2) led to the highest cell population in early stage biofilm (2.59 ± 0.11 log CFU cm−2). Furthermore, some of the cells within a biofilm might be in the viable but non-culturable (VBNC) state and hence, cannot be detected by plating [70].
Despite some limitations, the plate count method used in this study is still the gold standard for microbiological measurements, especially for industrial uses, mainly because it is cost-efficient, easy to apply, and allows adaptation to a variety of conditions showing reliable results [3]. Overall, bacterial adhesion on sealants (Figure 1) was lower compared to adhesion on SS (Figure 2), for surfaces with and without antimicrobials, while antimicrobial coatings on SS surfaces played a role in reducing early-stage biofilm formation of S. capitis. Their different effects on the two Gram-positive bacteria are most likely related to the different mechanisms of action of incorporated antimicrobials vs. antimicrobial coatings [71].
No significant difference (p > 0.05) in the mean value of attached cells was found for M. lacticum among the SS surfaces, except for SS_3. M. lacticum cells in early-stage biofilm formed on SS_2 (2.01 ± 0.06 log CFU cm−2) were by 2% significantly lower (p ≤ 0.05) when compared to SS_1 (2.05 ± 0.04 log CFU cm−2) and SS_3 (2.04 ± 0.04 log CFU cm−2) (Figure 2). Because of cracks and crevices on its surface, SS is prone to bacterial attachment and biofilm formation. Several publications reported that antibiofilm coatings of SS are successful in preventing adhesion and biofilm formation of different bacterial strains [22,23,72,73]. In this study, the addition of an antimicrobial agent to the PVC foil (SS_4_M) significantly reduced (p ≤ 0.05) the number of S. capitis cells attached as well as the number of cells in early-stage biofilm compared to the control (SS_4_control, coated only with PVC foil), by 8% and by 25%, respectively (Figure 2). For M. lacticum, no significant (p > 0.05) difference could be detected, neither for adhesion nor early stage biofilm formation by the addition of antimicrobial agents in the PVC coating. Furthermore, maximum cell counts were recorded for both adhesion and biofilm formation on SS_3 (the standard SS Niro Duplo V2A) independent of the bacterial strain used. Flint, et al. [74] reported that the natural oxide coat on SS enhances adhesion of thermo-resistant streptococci, and this can be reduced by removing the oxide layer. Besides the antimicrobial agent and surface characteristics, growth conditions impact on the antimicrobial effect [75]. Furthermore, the thickness of coating layer and molecular weight of the coating polymer can cause differences in surface coverage or modulus and hence influences bacterial adhesion and biofilm formation onto surface [64]. In this study, weak positive correlations (0.30) between the thickness of the coating layer for SS_1 (25 µm), SS_2 (50 µm), and SS_4_control (150 µm) and rate of adhesion and cell population in early-stage biofilms, respectively, were seen only for S. capitis. Al-Ahmad, et al. [76] noted that antimicrobial activity of various antimicrobial polymer-coated silicones was about the same but, inactivation kinetics varied significantly with layer thickness. The 50 nm and 150 nm thick networks were able to kill 17% and 63% of the adherent S. aureus cells in 3 min, respectively. The difference could be related to variations in bacteria-surface contact area or to stiffness of substrate, on which the networks were built.
Moreover, it should be noted that antimicrobial surface agents can inhibit biofilm formation for example via quorum sensing [77] or cyclic di-GMP (c-di-GMP) signaling interference, of which the latter is an intracellular signaling molecule of bacteria [78]. Quorum sensing (QS) is an intra- and intercellular mechanism, which facilitates the communication between cells and the interaction between the environment and cells [79]. The QS mechanism differs between bacteria species but can be organized in three categories: 1) the autoinducer-2 (AI-2) system, observed in interspecies interactions of both Gram-positive and Gram-negative bacteria, 2) the acyl-homoserine lactone (AHL) or autoinducer-I (AI-I) system found in Gram-negative bacteria, and 3) the peptide-mediated QS system observed in Gram-positive bacteria [80]. Since these mechanisms were not investigated in the present study, they are not discussed in more detail at this point, but should be addressed in future research.
4. Conclusions
In the present study, surface conditioning by incorporating antimicrobial agents was more efficient in reducing bacterial adhesion compared to active antimicrobial coatings. In contrast, active antimicrobial coatings played a role in reducing early-stage biofilm formation. Based on the present findings, antimicrobial surfaces cannot replace cleaning and disinfection strategies, but can be used as an additional tool to reduce bacterial adhesion and biofilm formation. Therefore, food producers and equipment manufacturers should carefully select antimicrobial surfaces for their intended use.
However, this study is also limited to the effects of antimicrobial agents on two Gram-positive biofilm formers. Hence, future studies should consider mixed biofilms consisting of Gram-positive and Gram-negative bacteria as well as fungi, especially for antifungal agents, to validate the present results. Additionally, an in-depth analysis of the antibacterial effect on bacterial attachment and biofilm growth, including microscopic evaluation and detailed characterization of the surface properties (e.g., hydrophobicity) should be considered.
Author Contributions
L.C.: methodology, investigation, data generation, writing—original draft; E.Z.: conceptualization, data generation, methodology, writing—review and editing; C.N.: investigation, data generation; H.J.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Austrian Research Promotion Agency [FFG Project No. 866346, New strategies for decontamination and aseptic processing considering manufacturing and building services engineering and Project No. FO999888198, Biomitate] as well as by BOKU Core Facility Food & Bio Processing.
Acknowledgments
We would like to thank BRUCHA GmbH (Austria) for their cooperation and for providing the surfaces investigated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cappitelli, F.; Polo, A.; Villa, F. Biofilm Formation in Food Processing Environments is Still Poorly Understood and Controlled. Food Eng. Rev. 2014, 6, 29–42. [Google Scholar] [CrossRef]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Zaffora, A.; Di Franco, F.; Santamaria, M. Corrosion of stainless steel in food and pharmaceutical industry. Curr. Opin. Electrochem. 2021, 29, 100760. [Google Scholar] [CrossRef]
- Jing, H.; Sahle-Demessie, E.; Sorial, G.A. Inhibition of biofilm growth on polymer-MWCNTs composites and metal surfaces. Sci. Total Environ. 2018, 633, 167–178. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, B.; Gu, Q.; Yu, X. Elimination of the formation of biofilm in industrial pipes using enzyme cleaning technique. MethodsX 2014, 1, 130–136. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Junter, G.-A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Silva, E.; Ferreira, O.; Ramalho, P.; Azevedo, N.; Bayón, R.; Igartua, A.; Bordado, J.; Calhorda, M. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Sci. Total Environ. 2018, 650, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.-L.; Rittschof, D.; Maki, J.S.; Gu, J.-D. Redirecting marine antibiofouling innovations from sustainable horizons. Trends Ecol. Evol. 2022, 37, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and Application Perspectives on Designing an Antimicrobial Surface. ACS Appl. Mater. Interfaces 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.E.; Bonilla, J.C.; Sinisterra, R.D. Biofilm formation, control and novel strategies for eradication. Sci. Against Microbial. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 896–905. [Google Scholar]
- Balaure, P.C.; Grumezescu, A.M. Recent advances in surface nanoengineering for biofilm prevention and control. part i: Molecular basis of biofilm recalcitrance. passive anti-biofouling nanocoatings. Nanomaterials 2020, 10, 1230. [Google Scholar] [CrossRef]
- Cattò, C.; Villa, F.; Cappitelli, F. Recent progress in bio-inspired biofilm-resistant polymeric surfaces. Crit. Rev. Microbiol. 2018, 44, 633–652. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Zhong, L.J.; Pang, L.Q.; Che, L.M.; Wu, X.E.; Chen, X.D. Nafion coated stainless steel for anti-biofilm application. Colloids Surf. B Biointerfaces 2013, 111, 252–256. [Google Scholar] [CrossRef]
- Gu, J.; Su, Y.; Liu, P.; Yang, P. An Environmentally Benign Antimicrobial Coating Based on a Protein Supramolecular Assembly. ACS Appl. Mater. Interfaces 2016, 9, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, P.; Muro-Fraguas, I.; Múgica-Vidal, R.; Sainz-García, A.; Sainz-García, E.; González-Raurich, M.; Álvarez-Ordóñez, A.; Prieto, M.; López, M.; López, M.; et al. Development and characterization of anti-biofilm coatings applied by Non-Equilibrium Atmospheric Plasma on stainless steel. Food Res. Int. 2020, 152, 109891. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Li, W.-W.; Morris, A.R.; Horrocks, P.D.; Yuan, C.-Q.; Yang, Y. Investigation of the antibiofilm capacity of peptide-modified stainless steel. R. Soc. Open Sci. 2018, 5, 172165. [Google Scholar] [CrossRef] [PubMed]
- Muro-Fraguas, I.; Sainz-García, A.; Gómez, P.F.; López, M.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; López, M.; González-Raurich, M.; et al. Atmospheric pressure cold plasma anti-biofilm coatings for 3D printed food tools. Innov. Food Sci. Emerg. Technol. 2020, 64, 102404. [Google Scholar] [CrossRef]
- Zand, E.; Pfanner, H.; Domig, K.; Sinn, G.; Zunabovic-Pichler, M.; Jaeger, H. Biofilm-Forming Ability of Microbacterium lacticum and Staphylococcus capitis Considering Physicochemical and Topographical Surface Properties. Foods 2021, 10, 611. [Google Scholar] [CrossRef]
- Planchon, S.; Gaillard-Martinie, B.; Leroy, S.; Bellon-Fontaine, M.; Fadda, S.; Talon, R. Surface properties and behaviour on abiotic surfaces of Staphylococcus carnosus, a genetically homogeneous species. Food Microbiol. 2007, 24, 44–51. [Google Scholar] [CrossRef]
- Heilmann, C.; Thumm, G.; Chhatwal, G.S.; Hartleib, J.; Uekötter, A.; Peters, G. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 2003, 149, 2769–2778. [Google Scholar] [CrossRef]
- Wagner, E.M.; Fischel, K.; Rammer, N.; Beer, C.; Palmetzhofer, A.L.; Conrady, B.; Roch, F.-F.; Hanson, B.T.; Wagner, M.; Rychli, K. Bacteria of eleven different species isolated from biofilms in a meat processing environment have diverse biofilm forming abilities. Int. J. Food Microbiol. 2021, 349, 109232. [Google Scholar] [CrossRef]
- Yong, Y.Y.; Dykes, G.; Choo, W.S. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit. Rev. Microbiol. 2019, 45, 201–222. [Google Scholar] [CrossRef]
- Møretrø, T.; Hermansen, L.; Holck, A.L.; Sidhu, M.S.; Rudi, K.; Langsrud, S. Biofilm Formation and the Presence of the Intercellular Adhesion Locus ica among Staphylococci from Food and Food Processing Environments. Appl. Environ. Microbiol. 2003, 69, 5648–5655. [Google Scholar] [CrossRef]
- Cherif-Antar, A.; Moussa–Boudjemâa, B.; Didouh, N.; Medjahdi, K.; Mayo, B.; Flórez, A.B. Diversity and biofilm-forming capability of bacteria recovered from stainless steel pipes of a milk-processing dairy plant. Dairy Sci. Technol. 2016, 96, 27–38. [Google Scholar] [CrossRef]
- Marchand, S.; De Block, J.; De Jonghe, V.; Coorevits, A.; Heyndrickx, M.; Herman, L. Biofilm Formation in Milk Production and Processing Environments; Influence on Milk Quality and Safety. Compr. Rev. Food Sci. Food Saf. 2012, 11, 133–147. [Google Scholar] [CrossRef]
- Maes, S.; Heyndrickx, M.; Vackier, T.; Steenackers, H.; Verplaetse, A.; De Reu, K. Identification and Spoilage Potential of the Remaining Dominant Microbiota on Food Contact Surfaces after Cleaning and Disinfection in Different Food Industries. J. Food Prot. 2019, 82, 262–275. [Google Scholar] [CrossRef]
- Techer, C.; Jan, S.; Gonnet, F.; Grosset, N.; Gautier, M.; Baron, F. Bacterial diversity on stainless steel surfaces of egg processing companies and potential of selected isolates to spoil liquid whole egg products. J. Appl. Microbiol. 2019, 127, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Liedtke, J.; Plattes, S.; Lipski, A. Bacterial community composition of biofilms in milking machines of two dairy farms assessed by a combination of culture-dependent and –independent methods. PLoS ONE 2019, 14, e0222238. [Google Scholar] [CrossRef]
- Fagerlund, A.; Elangsrud, S.; Eheir, E.; Mikkelsen, M.I.; Emøretrø, T. Biofilm Matrix Composition Affects the Susceptibility of Food Associated Staphylococci to Cleaning and Disinfection Agents. Front. Microbiol. 2016, 7, 856. [Google Scholar] [CrossRef] [PubMed]
- Dygico, L.K.; Gahan, C.G.; Grogan, H.; Burgess, C.M. The ability of Listeria monocytogenes to form biofilm on surfaces relevant to the mushroom production environment. Int. J. Food Microbiol. 2019, 317, 108385. [Google Scholar] [CrossRef]
- Veluz, G.A.; Pitchiah, S.; Alvarado, C.Z. Attachment ofSalmonella serovars andListeria monocytogenes to stainless steel and plastic conveyor belts. Poult. Sci. 2012, 91, 2004–2010. [Google Scholar] [CrossRef]
- Molobela, I.P.; Ilunga, M. Impact of bacterial biofilms: The importance of quantitative biofilm studies. Ann. Microbiol. 2011, 62, 461–467. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Tsuneda, S.; Hirata, A.; Katakai, A.; Tamada, M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf. B Biointerfaces 2005, 43, 99–107. [Google Scholar] [CrossRef]
- Oliveira, K.; Oliveira, T.; Teixeira, P.; Azeredo, J.; Henriques, M.; Oliveira, R. Comparison of the Adhesion Ability of Different Salmonella Enteritidis Serotypes to Materials Used in Kitchens. J. Food Prot. 2006, 69, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Goulter, R.; McMeekin, T.; Dykes, G.; Fegan, N. Attachment of different Salmonella serovars to materials commonly used in a poultry processing plant. Food Microbiol. 2009, 26, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Meira, Q.G.D.S.; Barbosa, I.D.M.; Athayde, A.J.A.A.; de Siqueira-Júnior, J.P.; de Souza, E.L. Influence of temperature and surface kind on biofilm formation by Staphylococcus aureus from food-contact surfaces and sensitivity to sanitizers. Food Control 2012, 25, 469–475. [Google Scholar] [CrossRef]
- Beresford, M.; Andrew, P.; Shama, G. Listeria monocytogenes adheres to many materials found in food-processing environments. J. Appl. Microbiol. 2001, 90, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.; Lima, J.; Azeredo, J.; Oliveira, R. Adhesion of Listeria monocytogenes to materials commonly found in domestic kitchens. Int. J. Food Sci. Technol. 2008, 43, 1239–1244. [Google Scholar] [CrossRef]
- Gazula, H.; Scherm, H.; Li, C.; Takeda, F.; Wang, P.; Chen, J. Ease of biofilm accumulation, and efficacy of sanitizing treatments in removing the biofilms formed, on coupons made of materials commonly used in blueberry packing environment. Food Control 2019, 104, 167–173. [Google Scholar] [CrossRef]
- Pagedar, A.; Singh, J.; Batish, V.K. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J. Basic Microb. 2010, 50, S98–S106. [Google Scholar] [CrossRef]
- Pawar, D.; Rossman, M.; Chen, J. Role of curli fimbriae in mediating the cells of enterohaemorrhagic Escherichia coli to attach to abiotic surfaces. J. Appl. Microbiol. 2005, 99, 418–425. [Google Scholar] [CrossRef]
- Sinde, E.; Carballo, J. Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: The influence of free energy and the effect of commercial sanitizers. Food Microbiol. 2000, 17, 439–447. [Google Scholar] [CrossRef]
- Zacheus, O.M.; Iivanainen, E.K.; Nissinen, T.K.; Lehtola, M.J.; Martikainen, P.J. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000, 34, 63–70. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Giaouris, E.; Chapot-Chartier, M.-P.; Briandet, R. Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int. J. Food Microbiol. 2009, 131, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ukuku, D.O.; Fett, W.F. Relationship of Cell Surface Charge and Hydrophobicity to Strength of Attachment of Bacteria to Cantaloupe Rind. J. Food Prot. 2002, 65, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, F.; Asserne, F.; EL Abed, S.; Bensouda, S.; Mabrouki, M.; Latrache, H. Adhesion of Staphylococcus aureus on stainless steel treated with three types of milk. Food Control 2014, 38, 104–108. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Azelmad, K.; Hamadi, F.; Mimouni, R.; Amzil, K.; Latrache, H.; Mabrouki, M.; El Boulani, A. Adhesion of Staphylococcus aureus and Staphylococcus xylosus to materials commonly found in catering and domestic kitchens. Food Control 2017, 73, 156–163. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Gutiérrez-Lomelí, M.; Guerrero-Medina, P.J.; Avila-Novoa, M.G. Biofilm formation by Staphylococcus aureus and Salmonella spp. under mono and dual-species conditions and their sensitivity to cetrimonium bromide, peracetic acid and sodium hypochlorite. Braz. J. Microbiol. 2017, 49, 310–319. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Gutiérrez-Lomelí, M.; Avila-Novoa, M.G. Kinetics of biofilm formation by pathogenic and spoilage microorganisms under conditions that mimic the poultry, meat, and egg processing industries. Int. J. Food Microbiol. 2019, 303, 32–41. [Google Scholar] [CrossRef]
- de Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Shakhatreh, M.A.K.; Al-Smadi, M.L.; Khabour, O.F.; Shuaibu, F.A.; Hussein, E.I.; Alzoubi, K.H. Study of the antibacterial and antifungal activities of synthetic benzyl bromides, ketones, and corresponding chalcone derivatives. Drug Des. Dev. Ther. 2016, 10, 3653–3660. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Santos, R.; Silva, A.; Cruz, L.; Ricardo, E.; Pina-Vaz, C.; Rodrigues, A.G. The effect of antibacterial and non-antibacterial compounds alone or associated with antifugals upon fungi. Front. Microbiol. 2015, 6, 669. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Pereira, M.O. Bio-Inspired Coating Strategies for the Immobilization of Polymyxins to Generate Contact-Killing Surfaces. Macromol. Biosci. 2016, 16, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Murakami, K.; Nemoto, K.; Miyake, Y. Coating of a surface with 2-methacryloyloxyethyl phosphorylcholine (MPC) co-polymer significantly reduces retention of human pathogenic microorganisms. FEMS Microbiol. Lett. 2005, 248, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications. Macromol. Rapid Commun. 2017, 38, 1700216. [Google Scholar] [CrossRef]
- Hyde, F.W.; Alberg, M.; Smith, K. Comparison of fluorinated polymers against stainless steel, glass and polypropylene in microbial biofilm adherence and removal. J. Ind. Microbiol. Biotechnol. 1997, 19, 142–149. [Google Scholar] [CrossRef]
- Chaturongkasumrit, Y.; Takahashi, H.; Keeratipibul, S.; Kuda, T.; Kimura, B. The effect of polyesterurethane belt surface roughness on Listeria monocytogenes biofilm formation and its cleaning efficiency. Food Control 2011, 22, 1893–1899. [Google Scholar] [CrossRef]
- Frank, J.F.; Chmielewski, R. Influence of Surface Finish on the Cleanability of Stainless Steel. J. Food Prot. 2001, 64, 1178–1182. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Cerca, N.; Pier, G.; Vilanova, M.; Oliveira, R.; Azeredo, J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res. Microbiol. 2005, 156, 506–514. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Elashnikov, R.; Ulbrich, P.; Vokatá, B.; Pavlíčková, V.S.; Švorčík, V.; Lyutakov, O.; Rimpelová, S. Physically switchable antimicrobial surfaces and coatings: General concept and recent achievements. Nanomaterials 2021, 11, 3083. [Google Scholar] [CrossRef]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized grafting of antimicrobial peptides on stainless steel surface and biofilm resistance tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.; Nir, S.; Reches, M.; Shemesh, M. Preventing Biofilm Formation by Dairy-Associated Bacteria Using Peptide-Coated Surfaces. Front. Microbiol. 2019, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Brooks, J.; Bremer, P. Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J. Food Eng. 2000, 43, 235–242. [Google Scholar] [CrossRef]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Zou, P.; Guevara-Solarte, D.L.; Hellwig, E.; Steinberg, T.; Lienkamp, K. Development of a Standardized and Safe Airborne Antibacterial Assay, and Its Evaluation on Antibacterial Biomimetic Model Surfaces. PLoS ONE 2014, 9, e111357. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Liu, H.; Ahmad, N.; Lv, B.; Li, C. Advances in production and structural derivatization of the promising molecule ursolic acid. Biotechnol. J. 2021, 16, 2000657. [Google Scholar] [CrossRef]
- Machado, I.; Silva, L.R.; Giaouris, E.; Melo, L.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2019, 127, 108754. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).