A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment
2.3. Sampling
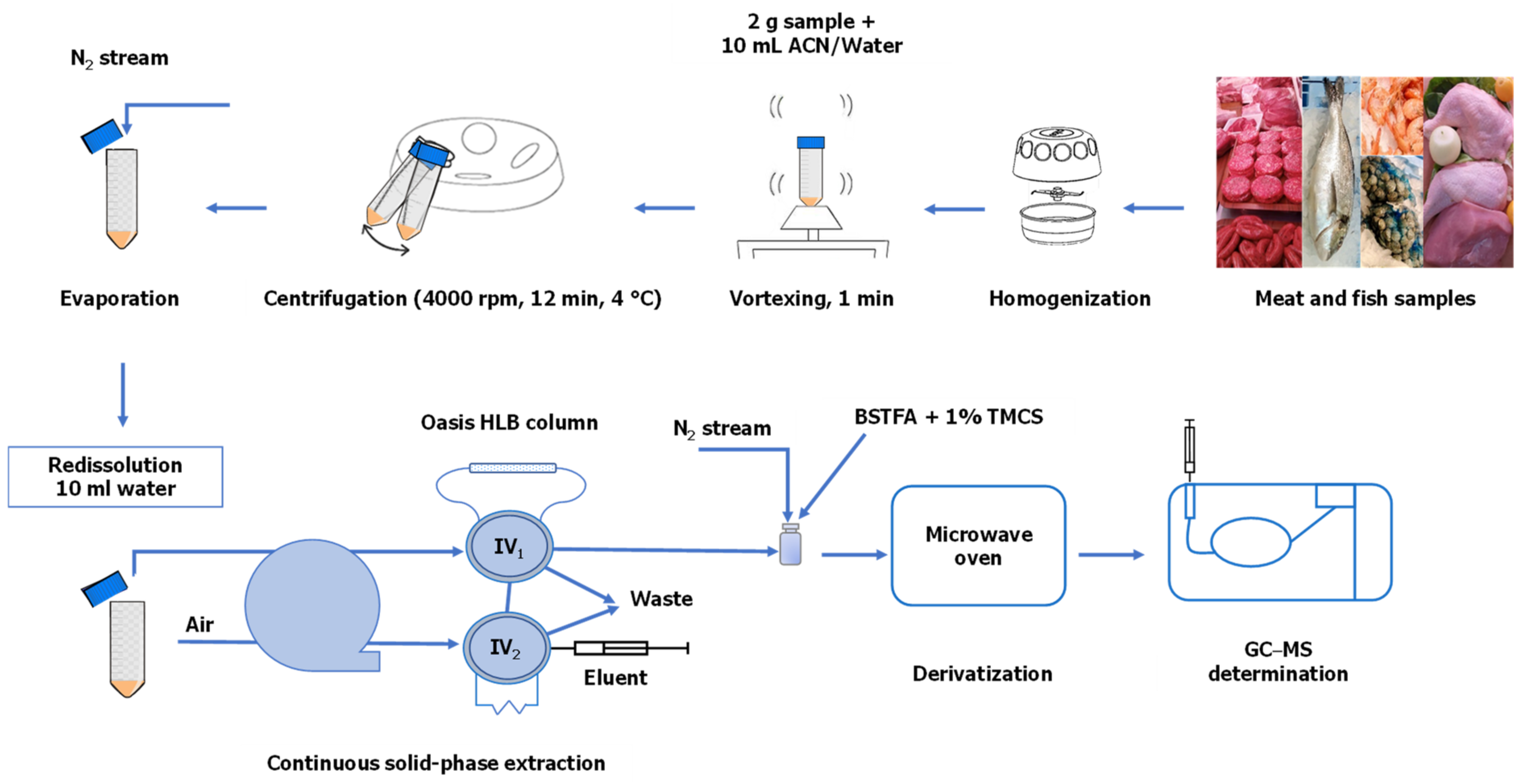
2.4. Sample Preparation
3. Results and Discussion
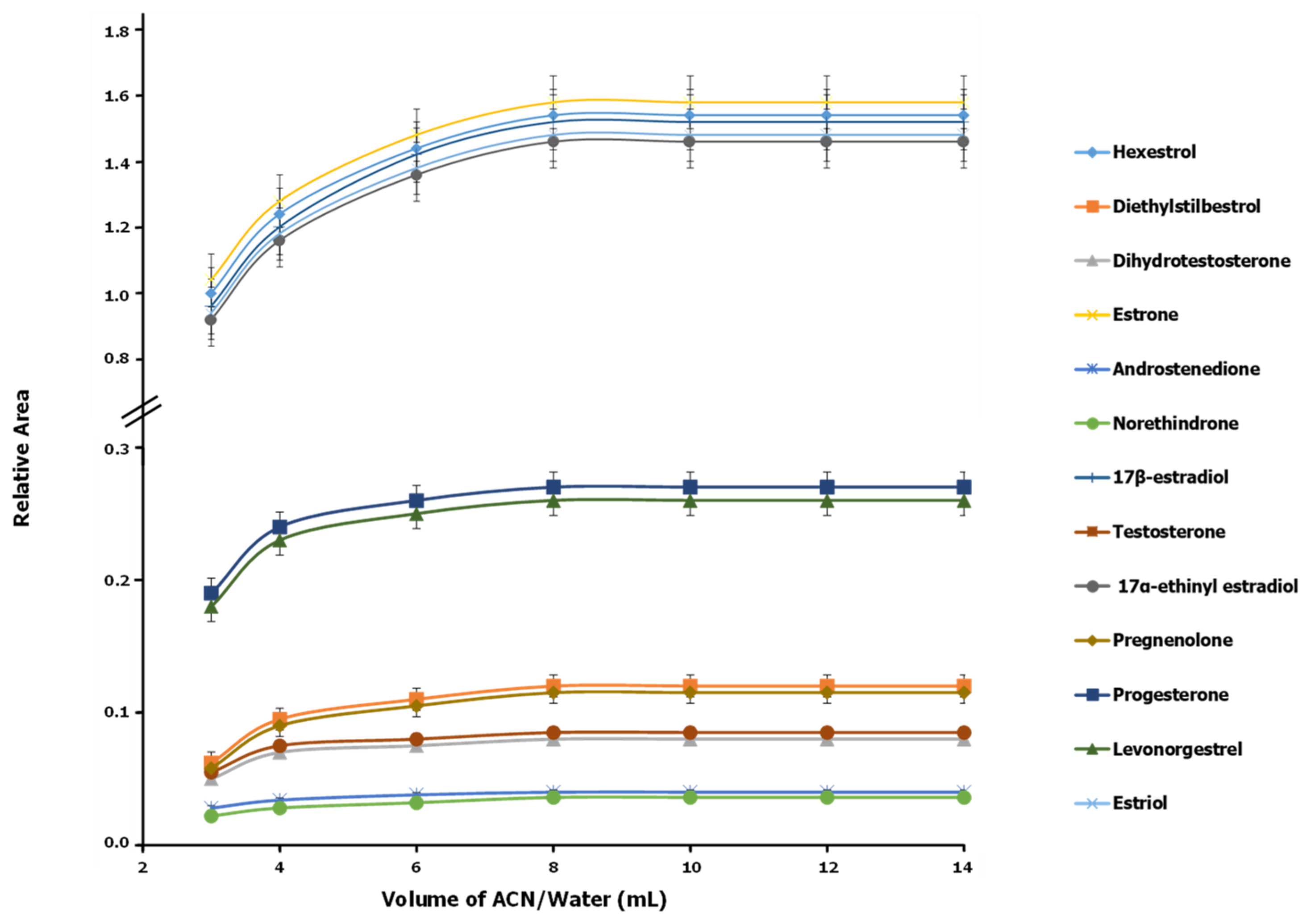
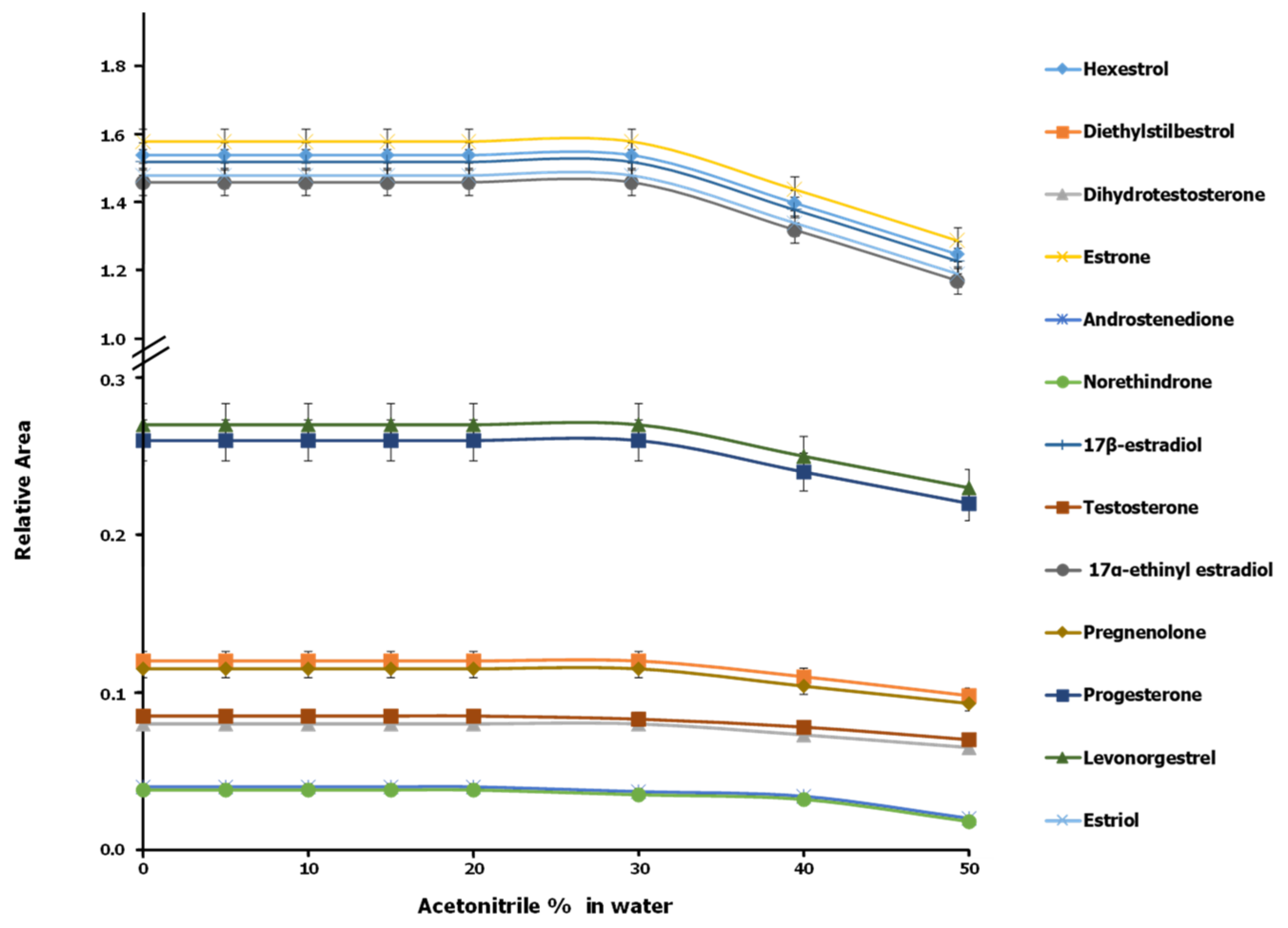
3.1. Optimization of Sample Treatment
3.2. Matrix Effects
3.3. Validation of the Proposed Method
3.4. Determination of Hormones in Meat and Fish Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.B.; Yin, Y.M.; Jiang, W.B.; Chen, Y.P.; Yang, J.W.; Wu, J.; Xie, M.X. Simultaneous determination of ten steroid hormones in animal origin food by matrix solid-phase dispersion and liquid chromatography-electrospray tandem mass spectrometry. Food Chem. 2014, 142, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Overall evaluations of carcinogenicity: An updating of IARC monographs, volumes 1 to 42. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Sydney, Australia, 1987; Volume 7, pp. 1–440. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-Supplements/Overall-Evaluations-Of-Carcinogenicity-An-Updating-Of-IARC-Monographs-Volumes-1%E2%80%9342-1987 (accessed on 5 September 2022).
- SCVPH (Scientific Committee on Veterinary Measures Relating to Public Health). Review of Previous SCVPH Opinions of 30 April 1999 and 3 May 2000 on the Potential Risks to Human Health from Hormone Residues in Bovine Meat and Meat Products, Adopted on 10 April 2002; EU Council: Brussels, Belgium, 2002; Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scv_out50_en.pdf (accessed on 5 September 2022).
- Ros, O.; Izaguirre, J.K.; Olivares, M.; Bizarro, C.; Ortiz-Zarragoitia, M.; Cajaraville, M.P.; Etxebarria, N.; Prieto, A.; Vallejo, A. Determination of endocrine disrupting compounds and their metabolites in fish bile. Sci. Total Environ. 2015, 536, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, Z.; Luo, Z.; Li, H.; Chen, G. Endocrine disrupting chemicals in wild freshwater fishes: Species, tissues, sizes and human health risks. Environ. Pollut. 2019, 244, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, B.; Zhang, J.; Wu, Y.; Duan, H. Determination of the residues of 50 anabolic hormones in muscle, milk and liver by very-high-pressure liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2009, 877, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hoga, C.A.; Almeida, F.L.; Reyes, F.G.R. A review on the use of hormones in fish farming: Analytical methods to determine their residues. CyTA J. Food 2018, 16, 679–691. [Google Scholar] [CrossRef]
- Rocha, D.G.; Lana, M.A.G.; Augusti, R.; Faria, A.F. Simultaneous identification and quantitation of 38 hormonally growth promoting agent residues in bovine muscle by a highly sensitive HPLC-MS/MS Method. Food Anal. Methods 2019, 12, 1914–1926. [Google Scholar] [CrossRef]
- Moussa, F.; Mokh, S.; Doumiati, S.; Barboni, B.; Bernabò, N.; Al Iskandarani, M. LC-MS/MS method for the determination of hormones: Validation, application and health risk assessment in various bovine matrices. Food Chem. Toxicol. 2020, 138, 111204. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of steroid hormones in fish tissues by microwave-assisted extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 237, 1012–1020. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Xu, X.R.; Hao, Q.W.; Zhao, J.L.; Ying, G.G. Three classes of steroids in typical freshwater aquaculture farms: Comparison to marine aquaculture farms. Sci. Total Environ. 2017, 609, 942–950. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, J.; Li, H.; Tu, Y.; Wan, Y.; Yang, Z. Air-assisted liquid-liquid microextraction integrated with QuEChERS for determining endocrine-disrupting compounds in fish by high-performance liquid chromatography–tandem mass spectrometry. Food Chem. 2018, 260, 174–182. [Google Scholar] [CrossRef]
- European Commission. Directive 2003/74/EC of The Europe-An Parliament and of the Council of 22 September 2003 Amending Council Directive 96/22/EC Concerning the Prohibition on the Use in Stockfarming of Certain Substances Having a Hormonal or Thyrostatic Action and of Beta-Agonists. Off. J. Eur. Comm. 2003, L262, 17–21. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Veterinary Drug Residues in Food World Health Organization; WHO Technical Report Series no. 893; World Health Organization: Geneva, Switzerland, 2000; Available online: https://apps.who.int/iris/bitstream/handle/10665/42251/WHO_TRS_893.pdf?sequence=1&isAllowed=y (accessed on 5 September 2022).
- Codex Alimentarius. Index of Veterinary Drugs. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Food CX/MRL 2-2018. 2018. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/veterinary-drugs/en/ (accessed on 5 September 2022).
- European Commission. European Community Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Off. J. Eur. Comm. 1996, L125, 10–32. [Google Scholar]
- CRL (Guidance Paper). CRLs View on State of the Art Analytical Methods for National Residue Control Plans. 2007. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/07_Untersuchungen/EURL_Empfehlungen_Konzentrationsauswahl_Methodenvalierungen_EN.html?nn=11011448 (accessed on 5 September 2022).
- Seo, J.; Kim, H.Y.; Chul, B.C.; Hong, J. Simultaneous determination of anabolic steroids and synthetic hormones in meat by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2005, 1067, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Dévier, M.H.; Labadie, P.; Togola, A.; Budzinski, H. Simple methodology coupling microwave-assisted extraction to SPE/GC/MS for the analysis of natural steroids in biological tissues: Application to the monitoring of endogenous steroids in marine mussels Mytilus sp. Anal. Chim. Acta 2010, 657, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Colapicchioni, V.; Piovesana, S.; Samperi, R.; Laganà, A. Analytical strategies based on chromatography–mass spectrometry for the determination of estrogen-mimicking compounds in food. J. Chromatogr. A 2013, 1313, 62–77. [Google Scholar] [CrossRef]
- Wolecki, D.; Caban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P. Simultaneous determination of non-steroidal anti-inflammatory drugs and natural estrogens in the mussels Mytilus edulis trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Azzouz, A.; Souhail, B.; Ballesteros, E. Determination of residual pharmaceuticals in edible animal tissues by continuous solid-phase extraction and gas chromatography–mass spectrometry. Talanta 2011, 84, 820–828. [Google Scholar] [CrossRef]
- Vanhaecke, L.; Van Meulebroek, L.; De Clercq, N.; Bussche, J.V. High resolution orbitrap mass spectrometry in comparison with tandem mass spectrometry for confirmation of anabolic steroids in meat. Anal. Chim. Acta 2013, 767, 118–127. [Google Scholar] [CrossRef]
- López-García, M.; Romero-González, R.; Garrido Frenich, A. Determination of steroid hormones and their metabolite in several types of meat samples by ultra high performance liquid chromatography—Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2018, 1540, 21–30. [Google Scholar] [CrossRef]
- Zhao, C.; Yue, Z.; Wu, H.; Lai, F. Simultaneous determination of fourteen steroid hormone residues in beef samples by liquid chromatography-tandem mass spectrometry. Anal. Methods 2014, 6, 8030–8038. [Google Scholar] [CrossRef]
- Di Donna, L.; Benabdelkamel, H.; Taverna, D.; Indelicato, S.; Aiello, D.; Napoli, A.; Sindona, G.; Mazzotti, F. Determination of ketosteroid hormones in meat by liquid chromatography tandem mass spectrometry and derivatization chemistry. Anal. Bioanal. Chem. 2015, 407, 5835–5842. [Google Scholar] [CrossRef] [PubMed]
- Van Tricht, F.; Essers, M.; Groot, M.; Sterk, S.; Blokland, M.; van Ginkel, L. A fast quantitative multi-analyte method for growth promoters in bovine meat using bead-disruption, 96-well SPE clean-up and narrow-bore UHPLC-MS/MS Analysis. Food Anal. Methods 2018, 11, 2206–2217. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Yuan, L.; Xiao, Y.; Wang, S.; Wang, X. Dummy molecularly imprinted matrix solid-phase dispersion for selective extraction of seven estrogens in aquatic products. Food Anal. Methods 2019, 12, 2241–2249. [Google Scholar] [CrossRef]
- Xiong, X.; Li, D.; Du, Z.; Xiong, C.; Jiang, H. Magnetic solid-phase extraction modified Quick, Easy, Cheap, Effective, Rugged and Safe method combined with pre-column derivatization and ultra-high performance liquid chromatography-tandem mass spectrometry for determination of estrogens and estrogen mimics in pork and chicken samples. J. Chromatogr. A 2020, 1622, 461137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, X.; Su, S.; Guo, Z.; Wang, J.; Ding, L.; Liu, Y.; Zhu, J. A multi-Class, multi-residue method for detection of veterinary drugs in multiple meat using a pass-through cleanup SPE technique and UPLC-MS/MS analysis. Food Anal. Methods 2018, 11, 2865–2884. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Aris, A.Z.; Wee, S.Y.; Nasir, H.M.; Razak, M.R.; Kamarulzaman, N.H.; Omar, T.F.T. Occurrence and distribution of endocrine- disrupting chemicals in mariculture fish and the human health implications. Food Chem. 2021, 345, 128806. [Google Scholar] [CrossRef]
- Wu, H.; Li, G.; Liu, S.; Hu, N.; Geng, D.; Chen, G.; Sun, Z.; Zhao, X.; Xia, L.; You, J. Monitoring the contents of six steroidal and phenolic endocrine disrupting chemicals in chicken, fish and aquaculture pond water samples using pre-column derivatization and dispersive liquid—Liquid microextraction with the aid of experimental design methodology. Food Chem. 2016, 192, 98–106. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Zhang, Y.; Chen, H.; Li, G.; Xu, Y.; Zhao, Q.; Song, W.; Jin, H.; Ding, L. Dynamic microwave-assisted extraction coupled with salting-out liquid-liquid extraction for determination of steroid hormones in fish tissues. J. Agric. Food Chem. 2012, 60, 10343–10351. [Google Scholar] [CrossRef]
- Chafi, S.; Ballesteros, E. A sensitive, robust method for determining natural and synthetic hormones in surface and wastewaters by continuous solid-phase extraction–gas chromatography–mass spectrometry. Environ. Sci. Pollut. Res. 2022, 29, 53619–53632. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Fernández, D.; Moreno-González, D.; Bouza, M.; Saez-Gómez, A.; Ballesteros, E.; García-Reyes, J.F.; Molina-Díaz, A. Assessment of a specific sample cleanup for the multiresidue determination of veterinary drugs and pesticides in salmon using liquid chromatography/tandem mass spectrometry. Food Control 2021, 130, 108311. [Google Scholar] [CrossRef]
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence and level of emerging organic contaminant in fish and mollusk from Klang River estuary, Malaysia and assessment on human health risk. Environ. Pollut. 2019, 248, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, A.; Hofer, R.; Schiemer, F. Fish as bioindicators. In Bioindicators and Biomonitors; Markert, B.A., Breuve, A.M., Zechmeisster, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 639–676. [Google Scholar]
| Compounds | Linear Range (ng/kg) | a r | b LOD (ng/kg) | c Precision RSD (%) | d tR | em/z | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meat | Fish | M+ | [M-15]+ | Additional Ions | |||||||
| Intra-day | Inter-day | Intra-day | Inter-day | ||||||||
| Estrogens | |||||||||||
| Hexestrol | 1.5–35,000 | 0.999 | 0.4 | 3.5 | 6.8 | 4.3 | 5.9 | 7.08 | 414 | 399 | 207, 179 |
| Diethylstilbestrol | 20–35,000 | 0.997 | 5.2 | 3.5 | 5.9 | 5.0 | 5.4 | 7.17 | 412 | 397 | 383, 217 |
| Estrone | 1.5–35,000 | 0.996 | 0.4 | 6.3 | 6.4 | 3.1 | 5.9 | 9.94 | 342 | 327 | 218, 257 |
| 17β-estradiol | 1.5–35,000 | 0.998 | 0.4 | 6.0 | 6.1 | 3.4 | 5.3 | 10.27 | 416 | 401 | 285, 326 |
| 17α-ethinyl estradiol | 1.5–35,000 | 0.996 | 0.4 | 4.3 | 5.4 | 6.0 | 6.5 | 11.46 | 440 | 425 | 232, 300 |
| Estriol | 1.5–35,000 | 0.996 | 0.4 | 4.5 | 3.4 | 4.9 | 5.7 | 12.44 | 504 | 489 | 147, 311 |
| Androgens | |||||||||||
| Testosterone | 25–35,000 | 0.996 | 7.5 | 4.4 | 5.4 | 3.2 | 3.5 | 10.46 | 360 | 345 | 270, 226 |
| Dihydrotestosterone | 25–35,000 | 0.996 | 7.6 | 5.3 | 6.4 | 3.5 | 5.0 | 9.75 | 362 | 347 | 129, 272 |
| Androstenedione | 50–35,000 | 0.999 | 15 | 5.5 | 6.8 | 3.9 | 4.4 | 10.37 | 286g | – | 244, 148 |
| Progestogens | |||||||||||
| Progesterone | 10–35,000 | 0.999 | 2.5 | 6.2 | 6.1 | 5.3 | 5.5 | 12.14 | 314 g | – | 124, 272 |
| Norethindrone | 50–35,000 | 0.997 | 15 | 6.2 | 6.3 | 5.7 | 6.3 | 10.41 | 370 | 355 | 231, 298 |
| Levonorgestrel | 10–35,000 | 0.998 | 2.6 | 6.3 | 6.8 | 4.8 | 6.5 | 11.60 | 384 | 369 | 355, 281 |
| Others | |||||||||||
| Pregnenolone | 20–35,000 | 0.999 | 5.3 | 5.1 | 6.5 | 4.9 | 5.3 | 11.12 | 388 | 373 | 129, 298 |
| Percent Recoveries (±SD, n = 3)/Matrix Effect (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormones | Fish Sample | Meat Sample | ||||||||||||
| Sea Bass | Salmon | Cod | Mussels | Hake | Shrimp | Squid Rings | Anchovies | Chicken Sausage | Beef Hamburger | Pork Loin | Chicken Breast | Turkey Hamburger | Lamb | |
| Hexestrol | 95 ± 6 a 10% b | 96 ± 5 7% | 101 ± 6 8% | 95 ± 4 8% | 104 ± 5 10% | 95 ± 4 10% | 91 ± 4 −4% | 94 ± 4 −6% | 91 ± 5 13% | 102 ± 4 12% | 100 ± 6 −14% | 102 ± 5 −12% | 93 ± 6 −4% | 103 ± 5 −16% |
| Diethylstilbestrol | 96 ± 5 −6% | 102 ± 5 3% | 100 ± 5 11% | 99 ± 6 −9% | 103 ± 6 8% | 93 ± 5 −2% | 103 ± 6 6% | 102 ± 6 11% | 94 ± 6 18% | 94 ± 4 5% | 99 ± 4 11% | 99 ± 4 −5% | 95 ± 6 5% | 94 ± 6 −19% |
| Estrone | 103 ± 4 2% | 97 ± 5 −9% | 94 ± 6 −2% | 90 ± 5 −5% | 98 ± 5 −12% | 98 ± 6 9% | 102 ± 5 7% | 97 ± 5 −4% | 96 ± 5 −3% | 100 ± 5 4% | 92 ± 5 12% | 91 ± 5 −14% | 96 ± 5 5% | 100 ± 4 4% |
| 17β-estradiol | 101 ± 5 −10% | 96 ± 5 −3% | 90 ± 6 4% | 104 ± 6 −1% | 99 ± 5 4% | 101 ± 4 5% | 99 ± 5 −4% | 92 ± 6 −1% | 101 ± 5 3% | 92 ± 6 9% | 97 ± 4 7% | 102 ± 6 −3% | 103 ± 6 3% | 92 ± 6 8% |
| 17α-ethinylestradiol | 92 ± 4 4% | 97 ± 4 10% | 94 ± 6 10% | 91 ± 5 4% | 94 ± 4 −10% | 100 ± 5 5% | 103 ± 6 −8% | 105 ± 4 −8% | 102 ± 5 −11% | 92 ± 4 −7% | 101 ± 6 6% | 101 ± 4 −7% | 99 ± 5 2% | 92 ± 7 −17% |
| Estriol | 96 ± 4 −8% | 100 ± 5 6% | 99 ± 4 6% | 100 ± 6 −10% | 99 ± 6 −6% | 104 ± 6 4% | 101 ± 5 3% | 93 ± 5 −8% | 101 ± 5 3% | 97 ± 4 5% | 93 ± 6 −9% | 95 ± 5 −6% | 94 ± 5 10% | 103 ± 6 5% |
| Testosterone | 100 ± 5 −13% | 103 ± 6 −7% | 102 ± 6 −14% | 91 ± 4 −2% | 100 ± 5 −1% | 92 ± 4 −10% | 97 ± 4 −14% | 100 ± 4 2% | 103 ± 6 1% | 93 ± 6 −19% | 96 ± 5 7% | 94 ± 4 −10% | 101 ± 6 −2% | 96 ± 6 −5% |
| Dihydrotestosterone | 100 ± 4 −12% | 96 ± 4 −3% | 104 ± 5 4% | 94 ± 5 −7% | 100 ± 6 2% | 92 ± 6 −2% | 93 ± 5 −10% | 94 ± 6 −5% | 102 ± 4 7% | 98 ± 5 −17% | 100 ± 4 −1% | 101 ± 5 −8% | 104 ± 6 −3% | 95 ± 5 −7% |
| Androstenedione | 102 ± 6 7% | 97 ± 5 4% | 92 ± 5 −11% | 93 ± 5 3% | 101 ± 5 −13% | 101 ± 6 2% | 94 ± 6 −4% | 98 ± 5 4% | 98 ± 4 −11% | 99 ± 5 −13% | 95 ± 6 2% | 97 ± 6 −19% | 97 ± 4 5% | 105 ± 6 −6% |
| Progesterone | 101 ± 6 −3% | 99 ± 4 10% | 93 ± 4 2% | 100 ± 6 10% | 94 ± 4 −5% | 100 ± 6 −1% | 93 ± 4 8% | 103 ± 5 −13% | 96 ± 6 −14% | 96 ± 6 5% | 93 ± 6 −3% | 94 ± 4 3% | 96 ± 6 2% | 91 ± 4 −9% |
| Norethindrone | 99 ± 7 −3% | 103 ± 4 11% | 97 ± 6 6% | 92 ± 4 −5% | 91 ± 6 6% | 104 ± 5 −7% | 98 ± 4 −10% | 101 ± 6 −9% | 94 ± 6 −2% | 104 ± 6 −7% | 97 ± 6 7% | 101 ± 6 8% | 90 ± 7 8% | 95 ± 5 2% |
| Levonorgestrel | 91 ± 4 9% | 104 ± 6 4% | 92 ± 5 −16% | 93 ± 4 −8% | 104 ± 4 3% | 101 ± 5 −3% | 93 ± 4 5% | 98 ± 6 6% | 101 ± 5 −9% | 94 ± 4 −19% | 93 ± 6 −18% | 95 ± 6 8% | 99 ± 4 8% | 100 ± 4 12% |
| Pregnenolone | 103 ± 5 3% | 96 ± 6 10% | 100 ± 5 3% | 95 ± 6 −4% | 99 ± 5 −11% | 98 ± 6 −2% | 92 ± 6 −5% | 97 ± 5 −12% | 91 ± 6 −3% | 103 ± 6 5% | 101 ± 4 −19% | 102 ± 4 −9% | 97 ± 4 2% | 102 ± 5 −7% |
| Compound a | Hexestrol | Estrone | 17β-Estradiol | Testosterone | Dihydrotestosterone | Androstenedione | Progesterone | Levonorgestrel | Pregnenolone | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fish sample | Sea bass (S) | nd b | nd | nd | nd | nd | nd | 130 ± 10 | nd | nd |
| Salmon (N) | nd | nd | nd | nd | nd | nd | 640 ± 40 | 30 ± 2 | nd | |
| Cod (N) | nd | 60 ± 4 | nd | nd | 26 ± 2 | nd | 550 ± 30 | 20 ± 1 | nd | |
| Mussels (I) | nd | 97 ± 5 | 470 ± 30 | nd | 1900 ± 100 | nd | 91 ± 5 | nd | 810 ± 50 | |
| Hake (S) | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Anchovies (M) | nd | 140 ± 10 | nd | nd | nd | nd | nd | nd | nd | |
| Sea Bream (G) | 20 ± 3 | 420 ± 20 | nd | nd | nd | nd | nd | nd | nd | |
| Prawns (S) | nd | nd | nd | nd | nd | nd | 190 ± 10 | 400 ± 20 | nd | |
| Shrimp (M) | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Turbot (S) | nd | nd | nd | nd | nd | nd | 460 ± 30 | nd | nd | |
| Croaker (S) | nd | nd | nd | nd | nd | nd | 310 ± 20 | nd | nd | |
| Squid rings (P) | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Meat sample | Chicken sausage (Ge) | nd | nd | nd | nd | nd | nd | 160 ± 10 | nd | nd |
| Turkey sausage (S) | nd | nd | nd | nd | nd | 320 ± 20 | 580 ± 30 | nd | nd | |
| Beef hamburger (USA) | nd | 70 ± 4 | 440 ± 30 | 30 ± 2 | nd | 160 ± 10 | 780 ± 50 | nd | nd | |
| Pork hamburger (S) | nd | 390 ± 20 | 170 ± 10 | 200 ± 10 | nd | 270 ± 20 | 700 ± 40 | nd | 630 ± 40 | |
| Turkey hamburger (S) | nd | nd | nd | nd | nd | nd | 410 ± 30 | nd | 380 ± 20 | |
| Pork loin (S) | nd | 410 ± 20 | 550 ± 30 | nd | nd | nd | 440 ± 30 | nd | nd | |
| Chicken breast (S) | nd | 72 ± 4 | nd | nd | nd | nd | 460 ± 30 | nd | nd | |
| Chicken breast (P) | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Lamb (S) | nd | 970 ± 60 | nd | nd | nd | nd | 290 ± 20 | nd | nd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chafi, S.; Ballesteros, E. A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry. Foods 2022, 11, 3095. https://doi.org/10.3390/foods11193095
Chafi S, Ballesteros E. A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry. Foods. 2022; 11(19):3095. https://doi.org/10.3390/foods11193095
Chicago/Turabian StyleChafi, Safae, and Evaristo Ballesteros. 2022. "A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry" Foods 11, no. 19: 3095. https://doi.org/10.3390/foods11193095
APA StyleChafi, S., & Ballesteros, E. (2022). A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry. Foods, 11(19), 3095. https://doi.org/10.3390/foods11193095






