Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow Mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Chemical Composition and Antioxidant Activity of Yellow Mombin
2.2.1. Sample Preparation and Proximate Composition
2.2.2. Antioxidant Activity
2.2.3. Determination of Carotenoids and Ascorbic Acid
2.2.4. Sugars, Organic Acids and Phenolic Compounds
2.2.5. Mineral Profile
2.3. Study Design, Diets, and Yellow Mombin Supplementation Administered to Wistar Rats
2.4. Weight Gain and Food INTAKE Evaluation
2.5. Glucose Tolerance Test (GTT)
2.6. Euthanasia, Somatic Parameters, and Biological Material Collection
2.7. Lipid Profile
2.8. Quantification of the Total Lipid, Cholesterol, and Triglyceride in the Faeces and in the Liver
2.9. Liver Antioxidant Status
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Antioxidant Activity of Yellow Mombin
3.2. Effect on Weight Gain, Food, and Lipid Intake
3.3. Effect on Somatic Parameters
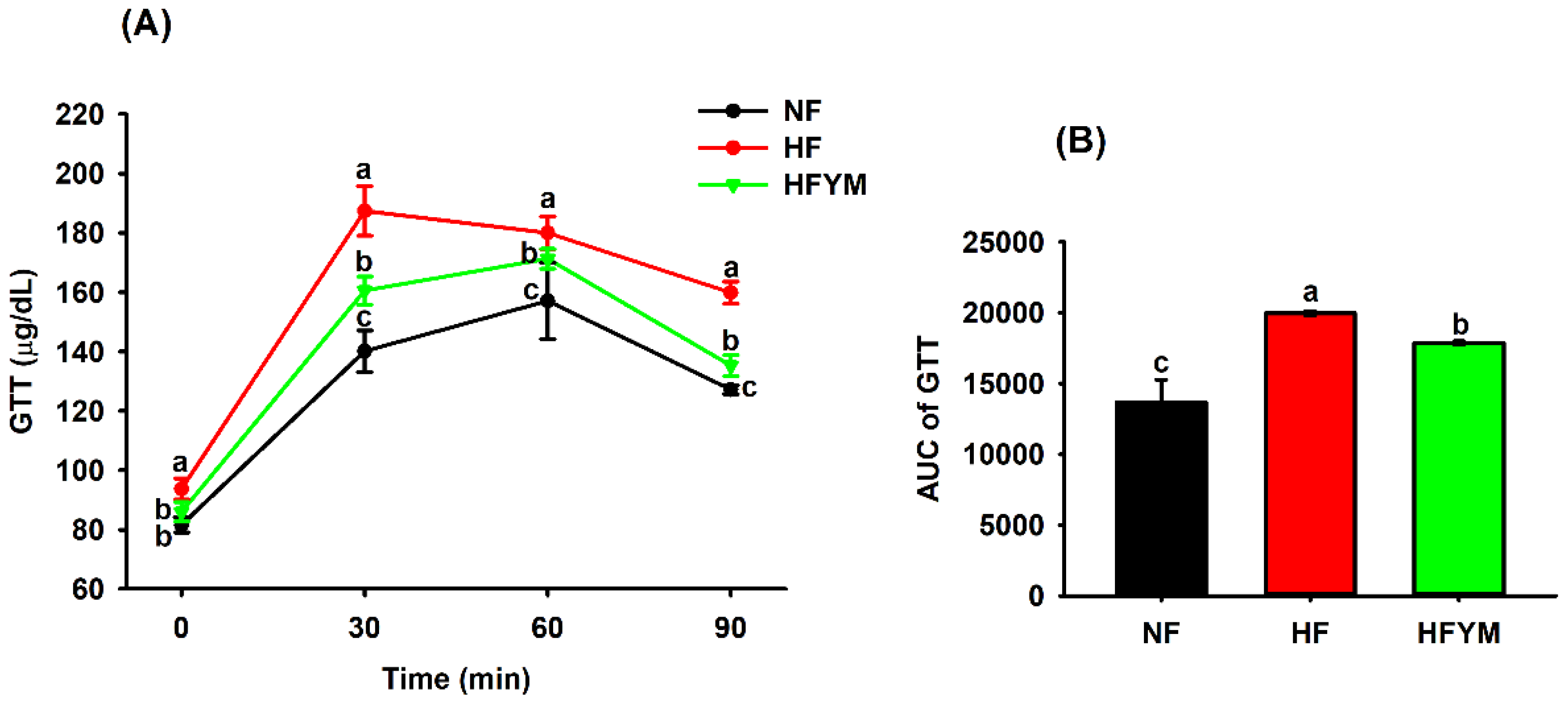
3.4. Effect of YM Supplementation on Glucose Tolerance
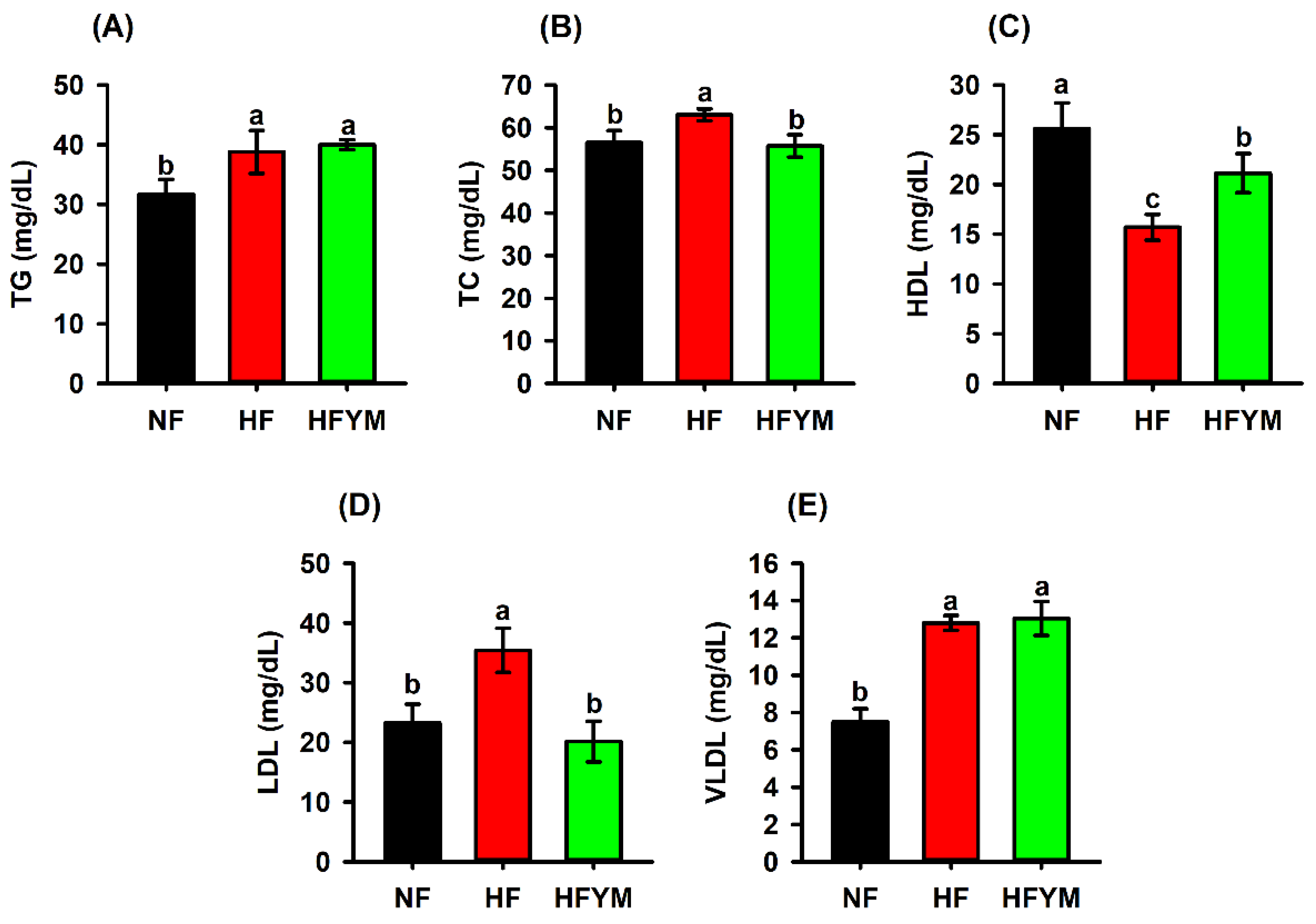
3.5. Effect of YM Supplementation on the Serum Lipid Profile, Triglycerides, and Cholesterol Quantified in the Faeces and Liver
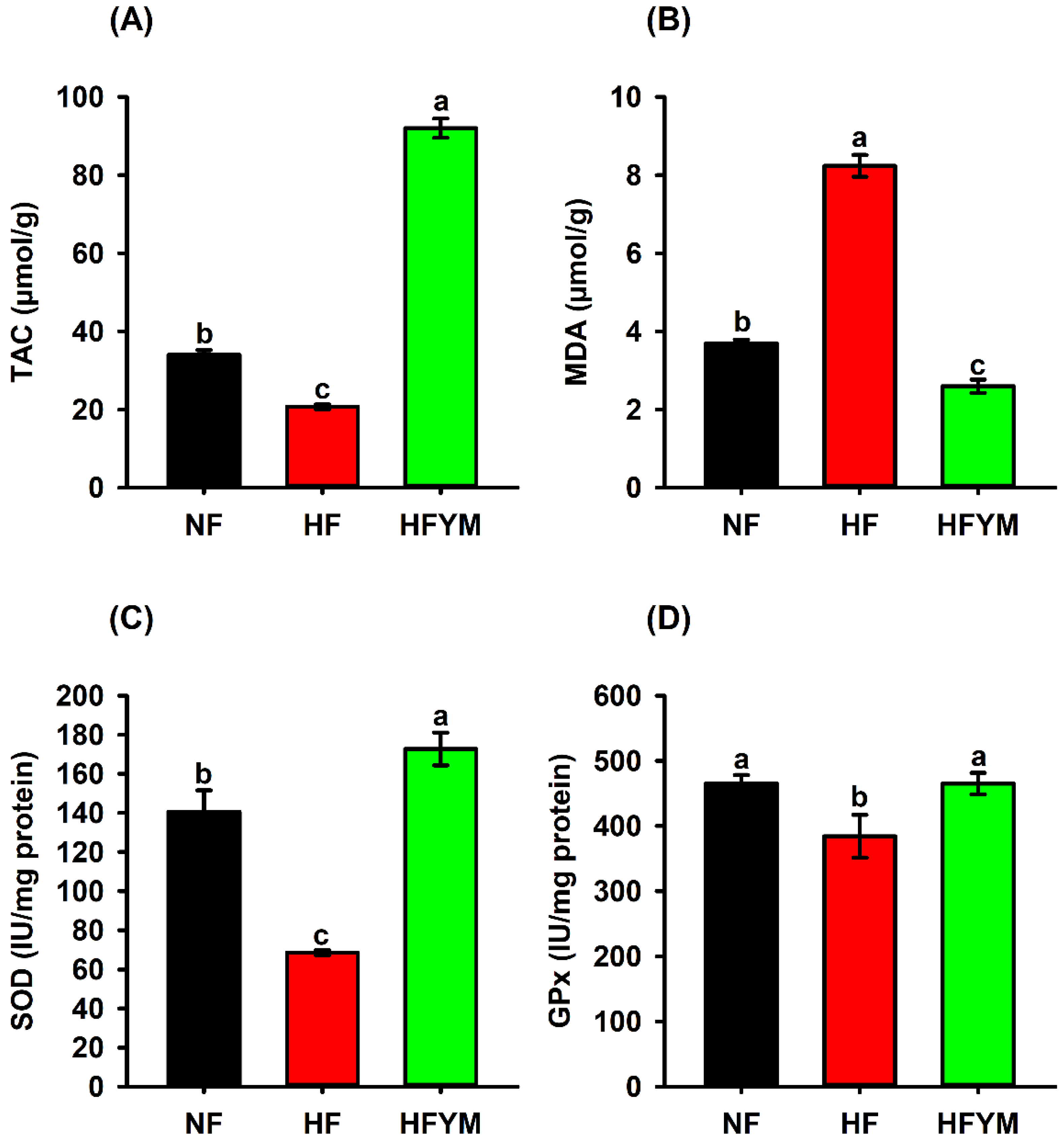
3.6. Antioxidant Status in the Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 5 August 2022).
- Reamy, B.V.; Williams, P.M.; Kuckel, D.P. Prevention of Cardiovascular Disease. Prim. Care—Clin. Off. Pract. 2018, 45, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-Alcoholic Fatty Liver Disease and Dyslipidemia: An Update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, D.H.; Iqbal, U.; Vazquez-Montesino, L.M.; Dennis, B.B.; Ahmed, A. Pathogenesis of Insulin Resistance and Atherogenic Dyslipidemia in Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2019, 7, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of High Fat Diets on Rodent Liver Bioenergetics and Oxidative Imbalance. Redox Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef]
- Azzimato, V.; Jager, J.; Chen, P.; Morgantini, C.; Levi, L.; Barreby, E.; Sulen, A.; Oses, C.; Willerbrords, J.; Xu, C.; et al. Liver Macrophages Inhibit the Endogenous Antioxidant Response in Obesity-Associated Insulin Resistance. Sci. Transl. Med. 2020, 12, eaaw9709. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Santos, R.D.; Sirtori, C.R.; Corsini, A. Lipid Lowering Drugs: Present Status and Future Developments. Curr. Atheroscler. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Laffin, L.J.; Davidson, M.H. Overcoming Toxicity and Side-Effects of Lipid-Lowering Therapies. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 439–452. [Google Scholar] [CrossRef]
- Astrup, A.; Bertram, H.C.S.; Bonjour, J.P.; De Groot, L.C.P.; De Oliveira Otto, M.C.; Feeney, E.L.; Garg, M.L.; Givens, I.; Kok, F.J.; Krauss, R.M.; et al. WHO Draft Guidelines on Dietary Saturated and Trans Fatty Acids: Time for a New Approach? BMJ 2019, 366, l4137. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.S.; Alves, A.F.; dos Lima, M.S.; da Silva, L.A.; Lins, P.P.; de Gomes, J.A.S.; Silva, A.S.; Toscano, L.T.; de Meireles, B.R.L.A.; de Cordeiro, A.M.T.M.; et al. Beneficial Effects of Consumption of Acerola, Cashew or Guava Processing by-Products on Intestinal Health and Lipid Metabolism in Dyslipidaemic Female Wistar Rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.C.V.; Ribeiro, M.d.A.; de Oliveira, K.A.; de Freitas, P.A.; dos Santos, N.S.; Magalhães, L.A.; Magalhães, S.C.; da Cruz Fonseca, S.G.; de Souza Aquino, J.; de Souza, E.L.; et al. Effects of Consumption of Acerola, Cashew and Guava by-Products on Adiposity and Redox Homeostasis of Adipose Tissue in Obese Rats. Clin. Nutr. ESPEN 2021, 43, 283–289. [Google Scholar] [CrossRef]
- Nunes, P.C.; Barbosa, F.K.S.; Silva, A.K.C.d.A.; Lima, M.d.S.; Alves, A.F.; Cordeiro, A.M.T.d.M.; Alcântara, M.A.; Meireles, B.R.L.d.A.; Melo, N.F.C.B.; Aquino, J.d.S.; et al. Malay Apple (Syzygium malaccense) Promotes Changes in Lipid Metabolism and a Hepatoprotective Effect in Rats Fed a High-Fat Diet. Food Res. Int. 2022, 155, 110994. [Google Scholar] [CrossRef]
- Alimentos Regionais Brasileiros, 2nd ed.; Ministério da Saúde: Brasília, Brasil, 2015; ISBN 9788533421455.
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N. Genus Spondias: A Phytochemical and Pharmacological Review. Evid.-Based Complement. Altern. Med. 2018, 2018, 5382904. [Google Scholar] [CrossRef]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional Properties of Yellow Mombin (Spondias mombin L.) Pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- Adediwura, F.J.; Kio, A. Antidiabetic Activity of Spondias mombin Extract in Niddm Rats. Pharm. Biol. 2009, 47, 215–218. [Google Scholar] [CrossRef]
- Pereira, B.L.B.; Rodrigue, A.; Arruda, F.C.d.O.; Bachiega, T.F.; Lourenço, M.A.M.; Correa, C.R.; Azevedo, P.S.; Polegato, B.F.; Okoshi, K.; Fernandes, A.A.H.; et al. Spondias mombin L. Attenuates Ventricular Remodelling after Myocardial Infarction Associated with Oxidative Stress and Inflammatory Modulation. J. Cell Mol. Med. 2020, 24, 7862–7872. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A Comparative Study on the Phenolic Acids Identified and Quantified in Dry Beans Using HPLC as Affected by Different Extraction and Hydrolysis Methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Silani, I.D.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced from New Brazilian Varieties Planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, H.J.; Ponting, J.D. Ascorbic Acid: Rapid Determination in Fresh, Frozen, or Dehydrated Fruits and Vegetables. Ind. Eng. Chem. Anal. Ed. 1942, 14, 846–849. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Coelho, E.M.; Padilha, C.V.d.S.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; Lima, M.d.S. Simultaneous Analysis of Sugars and Organic Acids in Wine and Grape Juices by HPLC: Method Validation and Characterization of Products from Northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Plaza, M.; Marina, M.L. High-Performance Thin-Layer Chromatography and Direct Analysis in Real Time-High Resolution Mass Spectrometry of Non-Extractable Polyphenols from Tropical Fruit Peels. Food Res. Int. 2021, 147, 110455. [Google Scholar] [CrossRef]
- Padilha, C.V.d.S.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.d.S. Rapid Determination of Flavonoids and Phenolic Acids in Grape Juices and Wines by RP-HPLC/DAD: Method Validation and Characterization of Commercial Products of the New Brazilian Varieties of Grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- Tavares, R.L.; Silva, A.S.; Campos, A.R.N.; Schuler, A.R.P.; de Souza Aquino, J. Nutritional Composition, Phytochemicals and Microbiological Quality of the Legume, Mucuna Pruriens. African J. Biotechnol. 2015, 14, 676–682. [Google Scholar] [CrossRef][Green Version]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bezerra, M.L.R.; De Souza, E.L.; De Sousa, J.M.B.; Lima, M.d.S.; Alves, A.F.; Almeida, M.d.G.; Coutinho Alves, R.; Veríssimo de Araújo, E.; Soares, N.L.; Da Silva, G.A.; et al. Effects of Honey from Mimosa quadrivalvis L. (Malícia) Produced by the Melipona Subnitida D. (Jandaíra) Stingless Bee on Dyslipidaemic Rats. Food Funct. 2018, 9, 4480–4492. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey Jr, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical Parameters and Markers of Obesity in Rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.F.; Luvizotto, R.A.M.; Leopoldo, A.S.; Lima-Leopoldo, A.P.; Seiva, F.R.; Justulin, L.A.; Silva, M.D.P.; Okoshi, K.; Wang, X.D.; Cicogna, A.C. Long-Term High-Fat Diet-Induced Obesity Decreases the Cardiac Leptin Receptor without Apparent Lipotoxicity. Life Sci. 2011, 88, 1031–1038. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and Their Bioactive Components: Adding Variety to the Fruit Plate for Health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Gault, V.A.; Bhat, V.K.; Irwin, N.; Flatt, P.R. A Novel Glucagon-like Peptide-1 (GLP-1)/Glucagon Hybrid Peptide with Triple-Acting Agonist Activity at Glucose-Dependent Insulinotropic Polypeptide, GLP-1, and Glucagon Receptors and Therapeutic Potential in High Fat-Fed Mice. J. Biol. Chem. 2013, 288, 35581–35591. [Google Scholar] [CrossRef]
- Warrilow, A.; Mellor, D.; McKune, A.; Pumpa, K. Dietary Fat, Fibre, Satiation, and Satiety—A Systematic Review of Acute Studies. Eur. J. Clin. Nutr. 2019, 73, 333–344. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing Obesity through Natural Polyphenols: A Review. Futur. Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Patterson, B.D. Correlation between “Lee Index” and Carcass Fat Content in Weanling and Adult Female Rats with Hypothalamic Lesions. J. Endocrinol. 1968, 40, 527–528. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Silva, L.M.A.; Alves Filho, E.G.; Santos, F.A.; de Lima, R.P.; Viana, A.F.S.C.; Nunes, P.I.G.; da Fonseca, S.G.C.; de Melo, T.S.; de Viana, D.A.; et al. Cashew Apple Fiber Prevents High Fat Diet-Induced Obesity in Mice: An NMR Metabolomic Evaluation. Food Funct. 2019, 10, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Mariosa, L.S.S.; Ribeiro-Filho, F.F.; Batista, M.C.; Hirota, A.H.; Borges, R.L.; Ribeiro, A.B.; Zanella, M.T. Potassium Depletion and Changes in Glucose Homeostasis During Diuretic Therapy. J. Clin. Hypertens. 2008, 10, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and Immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
- Sayahi, M.; Shirali, S. The Antidiabetic and Antioxidant Effects of Carotenoids: A Review. Asian J. Pharm. Res. Health Care 2017, 9, 186–191. [Google Scholar] [CrossRef]
- Santosh, H.N.; David, C.M. Role of Ascorbic Acid in Diabetes Mellitus: A Comprehensive Review. J. Med. Radiol. Pathol. Surg. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Yong, B.P.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in Db/Db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef]
- Sai, K.; Chhetri, S.B.B.; Devkota, S.R.; Khatri, D. Evaluation of the Hypoglycemic Potential of Leaves Extract of Spondias pinnata (L.f.) Kurz. From Nepal. Sci. World J. 2021, 2021, 3230351. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Modulation of Hepatic Steatosis by Dietary Fatty Acids. World J. Gastroenterol. 2014, 20, 1746–1755. [Google Scholar] [CrossRef]
- Ma, Z.; Chu, L.; Liu, H.; Wang, W.; Li, J.; Yao, W.; Yi, J.; Gao, Y. Beneficial Effects of Paeoniflorin on Non-Alcoholic Fatty Liver Disease Induced by High-Fat Diet in Rats. Sci. Rep. 2017, 7, 44819. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, F.; Liu, Z.; Xie, H.; Xu, Y.; Zhou, D.; Zhu, B. Acerola Polysaccharides Ameliorate High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease through Reduction of Lipogenesis and Improvement of Mitochondrial Functions in Mice. Food Funct. 2020, 11, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, S.M.; Do, H.J.; Cho, Y.; Chung, J.H.; Shin, M.J. Quercetin Up-Regulates LDL Receptor Expression in HepG2 Cells. Phyther. Res. 2012, 26, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Kadar, N.N.M.A.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Adelabu, A.A.; Saliu, I.O.; Adetuyi, A.R.; Olaleye, M.T. Protective Properties of Spondias mombin Linn Leaves on Redox Status, Cholinergic Dysfunction and Electrolyte Disturbance in Cyanide-Intoxicated Rats. Sci. Prog. 2021, 104, 00368504211011866. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty Acid Metabolism: Target for Metabolic Syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Janhavi, P.; Divyashree, S.; Sanjailal, K.P.; Muthukumar, S.P. DoseCal: A Virtual Calculator for Dosage Conversion between Human and Different Animal Species. Arch. Physiol. Biochem. 2022, 128, 426–430. [Google Scholar] [CrossRef]

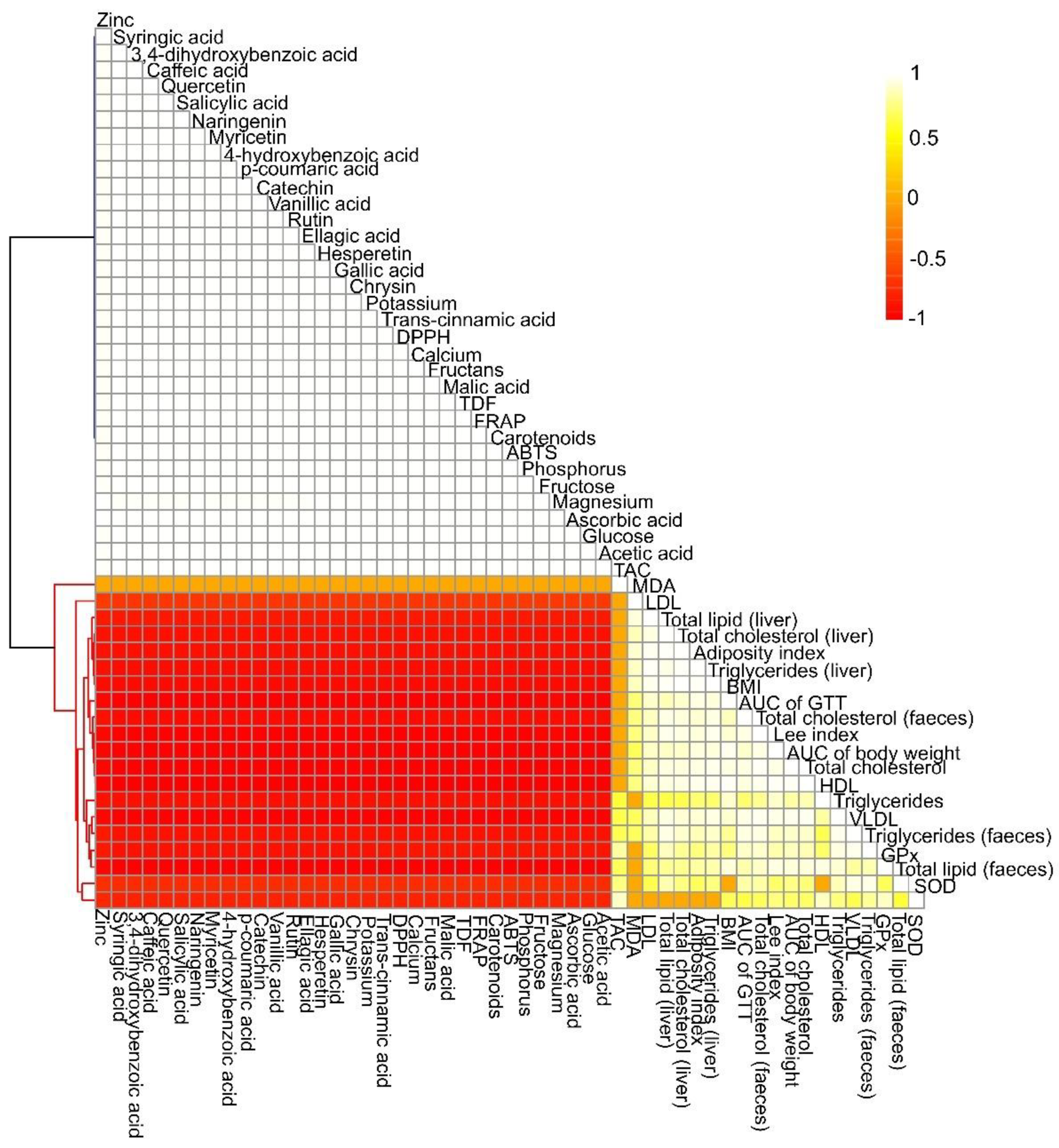


| Parameters (Dry Basis) | Yellow Mombin | Parameters (Dry Basis) | Yellow Mombin |
|---|---|---|---|
| Ash (g/100g) | 5.21 ± 0.06 | Phenolics compounds | |
| Moisture (g/100g) | 13.51 ± 0.20 | Hydroxybenzoic acids (µg/g) | |
| Protein (g/100g) | 7.12 ± 0.10 | Gallic acid | 16.02 ± 0.02 |
| Lipid (g/100g) | 1.43 ± 0.25 | Syringic acid | 80.18 ± 0.02 |
| Glucose (mg/g) | 0.16 ± 0.01 | Salicylic acid | 618.89 ± 0.12 |
| Fructose (mg/g) | 0.17 ± 0.02 | Vanillic acid | 528.78 ± 0.26 |
| Fructans (g/100g) | 0.99 ± 0.02 | Hydroxycinnamic acids (µg/g) | |
| SDF (g/100g) | 6.20 ± 0.04 | Trans-cinnamic acid | 2.23 ± 0.02 |
| IDF(g/100g) | 8.91 ± 0.07 | Caffeic acid | 764.41 ± 0.21 |
| TDF(g/100g) | 15.11 ± 0.11 | p-coumaric acid | 216.56 ± 0.09 |
| IDF:SDF ratio | 1.44 | * Sum of non-flavonoids (µg/g) | 4153.59 |
| Organic acids (mg/g) | Mineral elements (mg/g) | ||
| Acetic acid | 3.34 ± 0.21 | Potassium | 83.06 ± 0.12 |
| Malic acid | 6.00 ± 0.12 | Calcium | 9.35 ± 0.11 |
| Phenolics compounds | Chlorine | 3.53 ± 0.07 | |
| Flavanol (µg/g) | Phosphorus | 2.65 ± 0.08 | |
| Catechin | 38.18 ± 0.02 | Magnesium | 1.82 ± 0.17 |
| Flavanone (µg/g) | Sulphur | 1.06 ± 0.03 | |
| Hesperetin | 28.02 ± 0.03 | Iron | 0.22 ± 0.02 |
| Naringenin | 28.05 ± 0.02 | Calcium | 0.12 ± 0.02 |
| Chrysin | 12.04 ± 0.02 | Copper | 0.06 ± <0.01 |
| Flavonols (µg/g) | Zinc | 0.05 ± <0.01 | |
| Myricetin | 322.01 ± 0.19 | Cobalt | 0.02 ± <0.01 |
| Quercetin | 774.09 ± 0.10 | Other antioxidant compounds (mg/100 g) | |
| Rutin | 325.29 ± 0.15 | Ascorbic acid | 238.06 ± 15.60 |
| * Sum of flavonoids (µg/g) | 1527.62 | Carotenoids | 17.81 ± 0.37 |
| Hydroxybenzoic acids (µg/g) | Antioxidant activity | ||
| 3,4-dihydroxybenzoic acid | 1390.20 ± 0.40 | DPPH (µmol TE /g) | 669.61 ± 7.90 |
| 4-hydroxybenzoic acid | 420.65 ± 0.17 | ABTS (µmol TE /g) | 498.76 ± 16.14 |
| Ellagic acid | 105.67 ± 0.05 | FRAP (μmol ferrous sulfate/g) | 1205.24 ± 25.17 |
| Somatic Parameters | NF | HF | HFYM |
|---|---|---|---|
| Abdominal circumference (cm) | 16.10 ± 0.42 b | 17.29 ± 0.76 a | 15.07 ± 0.45 b |
| Thoracic circumference (cm) | 14.28 ± 0.68 b | 15.13 ± 0.35 a | 13.75 ± 0.60 b |
| Final body weight (g) | 304.00 ± 24.85 a | 330.00 ± 15.81 a | 268.13 ± 20.86 b |
| Body length (cm) | 22.81 ± 0.75 a | 23.44 ± 0.78 a | 22.94 ± 0.90 a |
| BMI (g/cm2) | 0.62 ± 0.06 a | 0.62 ± 0.03 a | 0.51 ± 0.03 b |
| Lee index | 0.24 ± 0.01 a | 0.24 ± 0.03 a | 0.23 ± 0.04 a |
| Adiposity index (%) | 2.82 ± 0.64 b | 3.70 ± 0.15 a | 2.53 ± 0.28 b |
| Carcass weight (g) | 184.17 ± 24.38 b | 207.50 ± 9.35 a | 163.14 ± 17.39 b |
| Liver weight (g) | 8.33 ± 0.89 b | 10.25 ± 0.91 a | 8.63 ± 0.49 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena, T.L.C.; Batista, K.S.; Pinheiro, R.O.; Cavalcante, H.C.; Gomes, J.A.d.S.; Silva, L.A.d.; Lins, P.P.; Ferreira, F.S.; Lima, R.F.; Lima, M.d.S.; et al. Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow Mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet. Foods 2022, 11, 3064. https://doi.org/10.3390/foods11193064
Lucena TLC, Batista KS, Pinheiro RO, Cavalcante HC, Gomes JAdS, Silva LAd, Lins PP, Ferreira FS, Lima RF, Lima MdS, et al. Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow Mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet. Foods. 2022; 11(19):3064. https://doi.org/10.3390/foods11193064
Chicago/Turabian StyleLucena, Tatiana Luiza Costa, Kamila Sabino Batista, Rafael Oliveira Pinheiro, Hassler Clementino Cavalcante, Jéssyca Alencar de Sousa Gomes, Laiane Alves da Silva, Priscilla Paulo Lins, Fabrícia Souza Ferreira, Rafael Ferreira Lima, Marcos dos Santos Lima, and et al. 2022. "Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow Mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet" Foods 11, no. 19: 3064. https://doi.org/10.3390/foods11193064
APA StyleLucena, T. L. C., Batista, K. S., Pinheiro, R. O., Cavalcante, H. C., Gomes, J. A. d. S., Silva, L. A. d., Lins, P. P., Ferreira, F. S., Lima, R. F., Lima, M. d. S., & Aquino, J. d. S. (2022). Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow Mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet. Foods, 11(19), 3064. https://doi.org/10.3390/foods11193064









