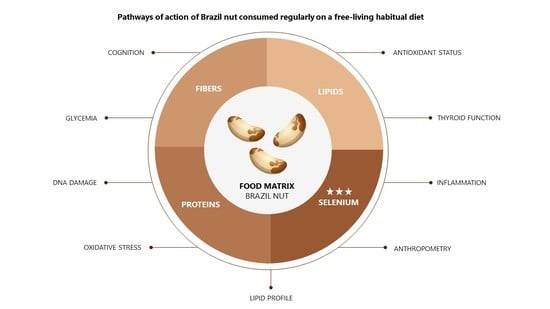
Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
2.3. Eligibility Criteria
- (i).
- Original clinical trials, randomized or not, controlled or not.
- (ii).
- Studies that evaluated the effects of regular BN consumption on any human health marker.
- (iii).
- Studies with any dose and time of intervention.
- (iv).
- Studies in which BNs were consumed as whole seed.
- (v).
- Studies evaluating any health marker, anthropometrics, or body composition and those examining lipid, glucose, kidney, liver, and other markers.
- (vi).
- Studies with any subject profile (healthy subjects or subjects with comorbidities), and with any sex.
- (i).
- Postprandial studies.
- (ii).
- Studies with children or animals, observational designs, reviews, congress abstracts, letters, protocol articles, notes, and in vitro analyses.
- (iii).
- Studies that did not investigate the effects of the BN on human health markers
- (iv).
- Interventions that supplemented BNs with minerals/vitamins or other nutritional enhancements.
- (v).
- Interventions with BN oil or flour.
- (vi).
- Interventions that included behavioral modifications, such as physical activity.
2.4. Selecting Studies and Data Extraction
- (i).
- Name of the first author, year of publication, and country.
- (ii).
- Sample characteristics (number of participants, presence of diseases, age, and body mass index (BMI).
- (iii).
- Characteristics of the intervention (description of each intervention group and the doses of BNs used with their Se contents).
- (iv).
- Study design and duration.
- (v).
- Markers evaluated in the study.
- (vi).
- Observed results.
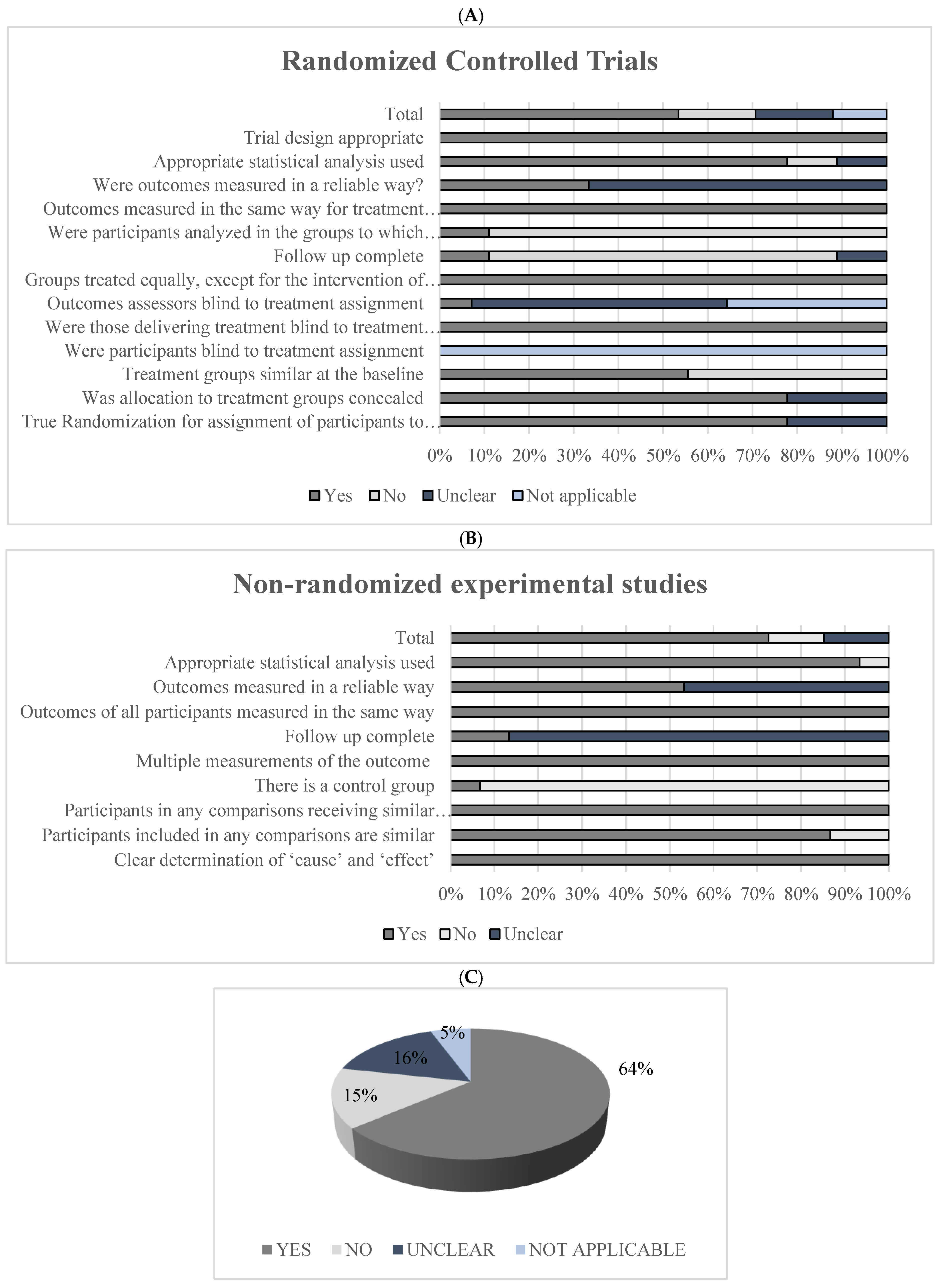
2.5. Bias Risk Assessment
3. Results and Discussion
3.1. Study Selection
3.2. Studies’ Characteristics
3.3. BNs Consumed by Healthy Subjects (Subjects without Diagnosed Diseases)
3.4. BN Consumption by Subjects with Obesity
3.5. BN Consumption by Subjects with Dyslipidemia, Type 2 Diabetes, or Coronary Artery Disease
3.6. BN Consumption by Subjects Undergoing Hemodialysis
3.7. BN Consumption by Subjects with MCIs
3.8. Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| PubMed | Search: ((((Brazil nut[Title/Abstract]) OR (Brazil nuts[Title/Abstract])) OR (Bertholletia excelsa[Title/Abstract])) OR (Bertholletia excelsa Humn. Bonpl.[Title/Abstract])) OR (Bertholletia excelsa H.B.K[Title/Abstract]) Filters: Clinical Trial, Humans Sort by: Most Recent (“brazil nut”[Title/Abstract] OR “brazil nuts”[Title/Abstract] OR “bertholletia excelsa”[Title/Abstract] OR ((“Bertholletia”[MeSH Terms] OR “Bertholletia”[All Fields] OR (“Bertholletia”[All Fields] AND “excelsa”[All Fields]) OR “bertholletia excelsa”[All Fields]) AND “humn”[All Fields] AND “bonpl”[Title/Abstract]) OR “bertholletia excelsa h b k”[Title/Abstract]) AND ((clinicaltrial[Filter]) AND (humans[Filter])) Translations Bertholletia excelsa: “bertholletia”[MeSH Terms] OR “bertholletia”[All Fields] OR (“bertholletia”[All Fields] AND “excelsa”[All Fields]) OR “bertholletia excelsa”[All Fields] |
| Embase | ‘brazil nut’/exp OR ‘brazil nut’ OR ‘para nut’ OR ‘paranut’ OR ‘brazil nuts’ OR ‘bertholletia excelsa’ OR ‘bertholletia excelsa humn. bonpl.’ OR ‘bertholletia excelsa h.b.k’ AND (‘clinical trial’/de OR ‘human’/de) |
| Scielo | (ti:(Brazil nut )) OR (ti:(Brazil nuts )) OR (ti:(Bertholletia excelsa )) OR (ti:(Bertholletia excelsa Humn. Bonpl. )) OR (ti:(Bertholletia excelsa H.B.K)) OR (ti:(castanha-do-brasil)) OR (ti:(castanha-do-pará)) |
| Studies | Quantity of BN | Selenium (µg) | Calories (Kcal) | Carbohydrates (g) | Proteins (g) | Lipids (g) | PUFA (g) | MUFA (g) | SFA (g) | Fiber (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| [14] | 2 units (~8.2 g) | 53 (20–84) | ||||||||
| [15] | 3 to 5 units (15–25 g) | 108.5 (27) | 124 (31) | 5.2 (1.3) | 16.8 (0.2) | 10.1 (2.5) | 4.2 (1.0) | 5.5 (1.4) | 3.0 (0.7) | |
| [16,17] | 1 unit (~5 g) | 288.75 | 35.74 | 0.54 | 0.81 | 3.27 | ||||
| [18] | 6 units | 48 | ||||||||
| [19] | 1 unit (~5 g) | 1261.4 | 35.31 | 0.79 | 0.78 | 3.25 | ||||
| [20] | 1 unit (~5 g) | 1261.4 | 35.31 | 0.79 | 0.78 | 3.25 | ||||
| [21] | 1 unit (5 g) | 290.5 | 36.7 | 0.45 | 0.75 | 3.53 | ||||
| [22] | 1 unit (5 g) | 290.5 | 36.7 | 0.45 | 0.75 | 3.53 | ||||
| [24] | 11 units (45 g) | 862.65 | 295.17 | 5.52 | 6.44 | 29.89 | 9.26 | 11.06 | 6.81 | 3.38 |
| [25,28,29,31] | 1 unit (5 g) | 290.5 | 36.7 | 0.45 | 0.75 | 3.53 | ||||
| [26,27] | 1 unit (5 g) | 290 | ||||||||
| [23] | 1 unit (5 g) | 290.5 | 36.7 | 0.45 | 0.75 | 3.53 | ||||
| [32,33,34] | 1 unit (3–4 g) | 300–400 | 21.99–29.32 | 0.45–0.6 | 0.39–0.52 | 2.07–2.76 | ||||
| [35] | 1 unit (3.7 g) | 213.67 | 26.45 | 0.4 | 0.6 | 2.49 | ||||
| [36,37] | 1 unit (5 g) | 290,00 | 34.8 | 0.65 | 0.55 | 3.58 | ||||
| USDA [67] | 1 unit (5 g) | 96,00 | 33 | 0.585 | 0.715 | 3.36 | 1.22 | 1.2 | 0.805 | 0.375 |
References
- INC Global Statistical Review, Brazil Nuts. Available online: https://www.nutfruit.org/industry/news/detail/global-statistical-review-brazil-nuts (accessed on 3 September 2022).
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil Nuts: Nutritional Composition, Health Benefits and Safety Aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Raai, M.N.; Zain, N.A.M.; Massawe, F.; Singh, A.; Wan-Mohtar, W.A.A.Q.I. In Search of Alternative Proteins: Unlocking the Potential of Underutilized Tropical Legumes. Food Secur. 2019, 11, 1205–1215. [Google Scholar] [CrossRef]
- Alehagen, U.; Opstad, T.B.; Alexander, J.; Larsson, A.; Aaseth, J. Impact of Selenium on Biomarkers and Clinical Aspects Related to Ageing. A Review. Biomolecules 2021, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Pacheco, A.M.; Lucas, A.C.S.; Andrello, A.C.; Appoloni, C.R.; Xavier, J.J.M. Brazil Nuts: Determination of Natural Elements and Aflatoxin. Acta Amaz. 2012, 42, 157–164. [Google Scholar] [CrossRef]
- Li, Y.; Clark, C.; Abdulazeeme, H.M.; Salehisahlabadi, A.; Rahmani, J.; Zhang, Y. The Effect of Brazil Nuts on Selenium Levels, Glutathione Peroxidase, and Thyroid Hormones: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. King Saud Univ.-Sci. 2020, 32, 1845–1852. [Google Scholar] [CrossRef]
- Hou, L.; Rashid, M.; Chehabra, M.; Chandrasekhar, B.; Amirthalingam, P.; Ray, S.; Li, Z. The Effect of Bertholletia Excelsa on Body Weight, Cholestrol, and c-Reactive Protein: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 102636. [Google Scholar] [CrossRef]
- da Silva, A.C.T.; Cardozo, L.F.M.F.; da Cruz, B.O.; Mafra, D.; Stockler-Pinto, M.B. Nuts and Cardiovascular Diseases: Focus on Brazil Nuts. Int. J. Cardiovasc. Sci. 2019. [Google Scholar] [CrossRef]
- Alcântara, D.B.; Dionísio, A.P.; Artur, A.G.; Silveira, B.K.S.; Lopes, A.F.; Guedes, J.A.C.; Luz, L.R.; Nascimento, R.F.; Lopes, G.S.; Hermsdorff, H.H.M.; et al. Selenium in Brazil Nuts: An Overview of Agronomical Aspects, Recent Trends in Analytical Chemistry, and Health Outcomes. Food Chem. 2022, 372, 131207. [Google Scholar] [CrossRef]
- Godos, J.; Giampieri, F.; Micek, A.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L.; Paladino, N.; Falzone, L.; Grosso, G. Effect of Brazil Nuts on Selenium Status, Blood Lipids, and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants 2022, 11, 403. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L.; Mattis, P.; Lisy, K.; Mu, P. Chapter 3: Systematic Reviews of Effectiveness. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2017; pp. 1–9. [Google Scholar]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil Nuts: An Effective Way to Improve Selenium Status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Maranhao, P.A.; Kraemer-aguiar, L.G.; Oliveira, C.L.; Kuschnir, M.C.C.; Vieira, Y.R.; Souza, M.G.C.; Koury, J.C. Brazil Nuts Intake Improves Lipid Profile, Oxidative Stress and Microvascular Function in Obese Adolescents: A Randomized Controlled. Nutr. Metab. 2011, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rita Cardoso, B.; Apolinário, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Effects of Brazil Nut Consumption on Selenium Status and Cognitive Performance in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Pilot Trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Busse, A.L.; Hare, D.J.; Cominetti, C.; Horst, M.A.; McColl, G.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Pro198Leu Polymorphism Affects the Selenium Status and GPx Activity in Response to Brazil Nut Intake. Food Funct. 2016, 7, 825–833. [Google Scholar] [CrossRef]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Somashekar, R.; Meng, X.Q.; Gopalsamy, G.; Bambaca, L.; McKinnon, R.A.; Young, G.P. Supplementation with Brazil Nuts and Green Tea Extract Regulates Targeted Biomarkers Related to Colorectal Cancer Risk in Humans. Br. J. Nutr. 2016, 116, 1901–1911. [Google Scholar] [CrossRef]
- Duarte, G.B.S.; Reis, B.Z.; Rogero, M.M.; Vargas-Mendez, E.; Júnior, F.B.; Cercato, C.; Cozzolino, S.M.F. Consumption of Brazil Nuts with High Selenium Levels Increased Inflammation Biomarkers in Obese Women: A Randomized Controlled Trial. Nutrition 2019, 63–64, 162–168. [Google Scholar] [CrossRef]
- Reis, B.Z.; Duarte, G.B.S.; Vargas-Mendez, E.; Ferreira, L.R.P.; Barbosa, F.; Cercato, C.; Rogero, M.M.; Cozzolino, S.M.F. Brazil Nut Intake Increases Circulating MiR-454-3p and MiR-584-5p in Obese Women. Nutr. Res. 2019, 67, 40–52. [Google Scholar] [CrossRef]
- de Cardozo, L.F.M.F.; Mafra, D.; Tavares da Silva, A.C.; Ermida Barbosa, J.; Oliveira da Cruz, B.; Tinoco Mesquita, C.; Barcza Stockler-Pinto, M. Effects of a Brazil Nut-enriched Diet on Oxidative Stress and Inflammation Markers in Coronary Artery Disease Patients: A Small and Preliminary Randomised Clinical Trial. Nutr. Bull. 2021, 46, 139–148. [Google Scholar] [CrossRef]
- da Cruz, B.O.; de Cardozo, L.F.M.; Coutinho-Wolino, K.S.; Mesquita, C.T.; Leal, V.O.; Mafra, D.; Stockler-Pinto, M.B. Brazil Nut Supplementation Does Not Regulate PPARβ/δ Signaling Pathway in Peripheral Blood Mononuclear Cells from Coronary Artery Disease Patients. J. Am. Coll. Nutr. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Stockler-Pinto, M.B.; Mafra, D. Brazil Nut Consumption Modulates Nrf2 Expression in Hemodialysis Patients: A Pilot Study. Mol. Nutr. Food Res. 2016, 60, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Strunz, C.C.; Oliveira, T.V.; Vinagre, J.C.M.; Lima, A.; Cozzolino, S.; Maranhão, R.C. Brazil Nut Ingestion Increased Plasma Selenium but Had Minimal Effects on Lipids, Apolipoproteins, and High-Density Lipoprotein Function in Human Subjects. Nutr. Res. 2008, 28, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Stockler-Pinto, M.B.; Mafra, D.; Farage, N.E.; Boaventura, G.T.; Cozzolino, S.M.F. Effect of Brazil Nut Supplementation on the Blood Levels of Selenium and Glutathione Peroxidase in Hemodialysis Patients. Nutrition 2010, 26, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Cominetti, C.; de Bortoli, M.C.; Purgatto, E.; Ong, T.P.; Moreno, F.S.; Garrido, A.B.; Cozzolino, S.M.F. Associations between Glutathione Peroxidase-1 Pro198Leu Polymorphism, Selenium Status, and DNA Damage Levels in Obese Women after Consumption of Brazil Nuts. Nutrition 2011, 27, 891–896. [Google Scholar] [CrossRef]
- Cominetti, C.; De Bortoli, M.C.; Garrido, A.B.; Cozzolino, S.M.F. Brazilian Nut Consumption Improves Selenium Status and Glutathione Peroxidase Activity and Reduces Atherogenic Risk in Obese Women. Nutr. Res. 2012, 32, 403–407. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Lobo, J.; Moraes, C.; Leal, V.O.; Farage, N.E.; Rocha, A.V.; Boaventura, G.T.; Cozzolino, S.M.F.; Malm, O.; Mafra, D. Effect of Brazil Nut Supplementation on Plasma Levels of Selenium in Hemodialysis Patients: 12 Months of Follow-Up. J. Ren. Nutr. 2012, 22, 434–439. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Mafra, D.; Moraes, C.; Lobo, J.; Boaventura, G.T.; Farage, N.E.; Silva, W.S.; Cozzolino, S.F.; Malm, O. Brazil Nut (Bertholletia Excelsa, H.B.K.) Improves Oxidative Stress and Inflammation Biomarkers in Hemodialysis Patients. Biol. Trace Elem. Res. 2014, 158, 105–112. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Malm, O.; Moraes, C.; Farage, N.E.; Silva, W.S.; Cozzolino, S.M.F.; Mafra, D. A Follow-up Study of the Chronic Kidney Disease Patients Treated with Brazil Nut: Focus on Inflammation and Oxidative Stress. Biol. Trace Elem. Res. 2015, 163, 67–72. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Carrero, J.J.; de Weide, L.C.C.; Cozzolino, S.M.F.; Mafra, D. Efecto de La Suplementación de Selenio a Través de La Nuez de Brasil (Bertholletia Excelsa, HBK) En Los Niveles de Hormonas Tiroideas En Pacientes de Hemodiálisis: Un Estudio Piloto. Nutr. Hosp. 2015, 32, 1808–1812. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; Rogero, M.M.; Guerra-Shinohara, E.M.; Desmarchelier, C.; Borel, P.; Cozzolino, S.M.F. SEPP1 Polymorphisms Modulate Serum Glucose and Lipid Response to Brazil Nut Supplementation. Eur. J. Nutr. 2018, 57, 1873–1882. [Google Scholar] [CrossRef]
- Donadio, J.; Rogero, M.; Cockell, S.; Hesketh, J.; Cozzolino, S. Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts. Nutrients 2017, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.L.S.; Rogero, M.M.; Guerra-Shinohara, E.M.; Barbosa, F.; Desmarchelier, C.; Borel, P.; Sneddon, A.A.; Hesketh, J.E.; Cozzolino, S.M.F. Genetic Variants in Selenoprotein Genes Modulate Biomarkers of Selenium Status in Response to Brazil Nut Supplementation (the SU.BRA.NUT Study). Clin. Nutr. 2019, 38, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Macan, T.P.; de Amorim, T.A.; Damiani, A.P.; da Beretta, Â.C.L.; Magenis, M.L.; Vilela, T.C.; Teixeira, J.P.; de Andrade, V.M. Brazil Nut Prevents Oxidative DNA Damage in Type 2 Diabetes Patients. Drug Chem. Toxicol. 2020, 1–7. [Google Scholar] [CrossRef]
- Watanabe, L.M.; Fernandes de Lima, L.; Ferraz-Bannitz, R.; Takaara, D.; Coimbra Romano, B.; Braga Costa, T.M.; Foss de Freitas, M.C.; Bueno, A.C.; Barbosa Júnior, F.; Marliere Navarro, A. Association between Creatine Kinase Activity, Oxidative Stress and Selenoproteins MRNA Expression Changes after Brazil Nut Consumption of Patients Using Statins. Clin. Nutr. 2020, 39, 3175–3181. [Google Scholar] [CrossRef]
- Watanabe, L.; Bueno M, A.C.; de Lima, L.F.; Ferraz-Bannitz, R.; Dessordi, R.; Guimarães, M.P.; Foss-Freitas, M.C.; Barbosa, F.; Navarro, A.M. Genetically Determined Variations of Selenoprotein P Are Associated with Antioxidant, Muscular, and Lipid Biomarkers in Response to Brazil Nut Consumption by Patients Using Statins. Br. J. Nutr. 2022, 127, 679–686. [Google Scholar] [CrossRef]
- Colpo, E.; Dalton, D.A.; Vilanova, C.; Reetz, L.G.B.; Duarte, M.M.M.F.; Farias, I.L.G.; Meinerz, D.F.; Mariano, D.O.C.; Vendrusculo, R.G.; Boligon, A.A.; et al. Brazilian Nut Consumption by Healthy Volunteers Improves Inflammatory Parameters. Nutrition 2014, 30, 459–465. [Google Scholar] [CrossRef]
- Colpo, E.; de Vilanova, C.D.A.; Brenner Reetz, L.G.; Medeiros Frescura Duarte, M.M.; Farias, I.L.G.; Irineu Muller, E.; Muller, A.L.H.; Moraes Flores, E.M.; Wagner, R.; da Rocha, J.B.T. A Single Consumption of High Amounts of the Brazil Nuts Improves Lipid Profile of Healthy Volunteers. J. Nutr. Metab. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of Selenium Supplementation on Antioxidant Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hormones 2019, 18, 451–462. [Google Scholar] [CrossRef]
- Dhingra, S.; Bansal, M.P. Modulation of Hypercholesterolemia-Induced Alterations in Apolipoprotein B and HMG-CoA Reductase Expression by Selenium Supplementation. Chem.-Biol. Interact. 2006, 161, 49–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of Oral Selenium and Magnesium Co-Supplementation on Lipid Metabolism, Antioxidative Status, Histopathological Lesions, and Related Gene Expression in Rats Fed a High-Fat Diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef]
- Jablonska, E.; Reszka, E.; Gromadzinska, J.; Wieczorek, E.; Krol, M.; Raimondi, S.; Socha, K.; Borawska, M.; Wasowicz, W. The Effect of Selenium Supplementation on Glucose Homeostasis and the Expression of Genes Related to Glucose Metabolism. Nutrients 2016, 8, 772. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Braat, S.; Graham, R.M. Selenium Status Is Associated With Insulin Resistance Markers in Adults: Findings From the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Ribeiro, P.V.M.; Silva, A.; Almeida, A.P.; Hermsdorff, H.H.; Alfenas, R.C. Effect of Chronic Consumption of Pistachios (Pistacia vera L.) on Glucose Metabolism in Pre-Diabetics and Type 2 Diabetics: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1115–1123. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High–Monounsaturated Fatty Acid Diets Lower Both Plasma Cholesterol and Triacylglycerol Concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Stevens, R.; Bain, J.; Ikayeva, O.; Crain, K.; Koves, T.R.; Muoio, D.M. Dietary Intake of Palmitate and Oleate Has Broad Impact on Systemic and Tissue Lipid Profiles in Humans. Am. J. Clin. Nutr. 2014, 99, 436–445. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Lai, K.S.; Hee, C.-W.; Loh, J.Y.; Lim, S.H.E.; Sathiya, M. Oxidative Stress Parameters as Biomarkers of Cardiovascular Disease towards the Development and Progression. Antioxidants 2022, 11, 1175. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.-J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.-R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Natoli, S.; McCoy, P. A Review of the Evidence: Nuts and Body Weight. Asia Pac. J. Clin. Nutr. 2007, 16, 588–597. [Google Scholar]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium Stimulates the Antitumour Immunity: Insights to Future Research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Thomson, C.D. Assessment of Requirements for Selenium and Adequacy of Selenium Status: A Review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Selenoproteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively High Mortality Risk in Elderly Swedish Subjects with Low Selenium Status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef]
- Watanabe, L.M.; Navarro, A.M.; Seale, L.A. Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil. Nutrients 2021, 13, 2027. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Selenoprotein Synthesis and Side-Effects of Statins. Lancet 2004, 363, 892–894. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.-C.; Lei, X.G.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef]
- Cavedon, E.; Manso, J.; Negro, I.; Censi, S.; Serra, R.; Busetto, L.; Vettor, R.; Plebani, M.; Pezzani, R.; Nacamulli, D.; et al. Selenium Supplementation, Body Mass Composition, and Leptin Levels in Patients with Obesity on a Balanced Mildly Hypocaloric Diet: A Pilot Study. Int. J. Endocrinol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium Status and Cardiovascular Diseases: Meta-Analysis of Prospective Observational Studies and Randomized Controlled Trials. Eur. J. Clin. Nutr. 2016, 70, 162–169. [Google Scholar] [CrossRef]
- Nowrouzi-Sohrabi, P.; Hassanipour, S.; Sisakht, M.; Daryabeygi-Khotbehsara, R.; Savardashtaki, A.; Fathalipour, M. The Effectiveness of Pistachio on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes, Prediabetes and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1589–1595. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Tsimihodimos, V. Dyslipidemia Associated with Chronic Kidney Disease. Open Cardiovasc. Med. J. 2011, 5, 41–48. [Google Scholar] [CrossRef]
- Berr, C.; Arnaud, J.; Akbaraly, T.N. Selenium and Cognitive Impairment: A Brief-Review Based on Results from the EVA Study. BioFactors 2012, 38, 139–144. [Google Scholar] [CrossRef]
- Cardoso, B.; Szymlek-Gay, E.; Roberts, B.; Formica, M.; Gianoudis, J.; O’Connell, S.; Nowson, C.; Daly, R. Selenium Status Is Not Associated with Cognitive Performance: A Cross-Sectional Study in 154 Older Australian Adults. Nutrients 2018, 10, 1847. [Google Scholar] [CrossRef]
- Caldas, A.P.S.; Rocha, D.M.U.P.; Dionísio, A.P.; Hermsdorff, H.H.M.; Bressan, J. Brazil and Cashew Nuts Intake Improve Body Composition and Endothelial Health in Women at Cardiometabolic Risk (Brazilian Nuts Study): A Randomised Controlled Trial. Br. J. Nutr. 2022, 23, 1–38. [Google Scholar] [CrossRef]
- U.S. DEPARTMENT OF AGRICULTURE. Nuts, Brazilnuts, Dried, Unblanched. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170569/nutrients (accessed on 3 September 2022).



| Reference Author, Year, Country | Sample Characteristics | Intervention Characteristics | Study Design and Duration | Evaluated Markers | Results |
|---|---|---|---|---|---|
| Placebo-controlled clinical trials | |||||
| [14] New Zealand | n: 59 healthy subjects G1: Age: 49.5 (SD 8.6) y BMI: 28.2 (SD 5.3) kg/m2 G2: Age: 45.6 (SD 11) y BMI: 26 (SD 3.6) kg/m2 G3: Age: 42.5 (SD 9.9) y BMI: 25.9 (SD 4.2) kg/m2 | G1: two units/day of BN (~53 µg of Se) G2: selenomethionine supplement (97.5 ± 11.1 µg of Se) G3: placebo (0.038 ± 0.036 µg of Se) | Randomized, controlled clinical trial 12 weeks | Antioxidant status | G1, G2: ↑ Se and GPx activity in plasma vs. G3 G1: ↑ GPx activity in whole blood vs. G2, G3 |
| [15] Brazil | n: 17 obese female adolescents Age: 15.4 (SD 2) y BMI: 35.6 (SD 3.3) kg/m2 | G1: 15–25 g/day (three to five units) of BN (108.5 ± 27 µg of Se) G2: placebo (one capsule/day containing lactose) | Randomized, non-blinded pilot trial 16 weeks | Antioxidant status Lipid and glucose metabolism markers Inflammation Anthropometry Oxidative stress | G1: ↓ total cholesterol, triglycerides, and ox-LDL vs. G2 G1: ↑ RBCV vs. G2 G1: ↑ Se and RBCV max vs. baseline ↔ BMI, waist circumference, insulin, glycemia, HOMA-IR, CRP, HDL-c, GPx-3, and 8-epi-PGF2α |
| Controlled clinical trials | |||||
| [16] Brazil | n: 20 older adults with mild cognitive impairment Age: 77.7 (SD 5.3) y | G1: one unit/day of BN (288.75 µg of Se) G2: control | Randomized, controlled clinical trial 24 weeks | Antioxidant status Oxidative stress Cognition | G1: ↑ Se in plasma and erythrocytes vs. G2 G1: ↑ GPx activity in erythrocytes, verbal fluency, construction praxis vs. G2 ↔ ORAC, MDA, CERAD total score, Boston naming test, word list learning test, word list recall |
| [17] Brazil | n: 20 older adults with mild cognitive impairment Age: 77.7 (SD 5.3) y | One unit/day of BN (288.75 µg of Se) | Secondary analysis of a randomized controlled clinical trial, which evaluated only the group that received BN 24 weeks | Antioxidant status Oxidative stress | ↔ Se in plasma and erythrocytes, GPx, ORAC, and MDA activity among genotypes ↑ expression of GPx1 mRNA and selenoprotein P in CT + TT allele carriers for rs1050450 over time ↑ selenoprotein mRNA expression and ↓ GPX1 mRNA expression in A-carriers for rs7579 and GG-carriers for rs3877899 |
| [18] Australia | n: 32 healthy subjects Age: 60 (52–76) y | G1: six units/day of BN (~48 µg of Se) G2: four capsules containing 800 mg of (-) epigallocatechin-3-gallate/day G3: combination of G1 and G2 interventions | Randomized, controlled clinical trial 6 weeks | Antioxidant status Kidney function marker Glucose Inflammation Thyroid function markers Genes/proteins related to the colorectal cancer oncogenesis | G1: ↑ Se and plasma urea vs. G2 G3: ↑ Se and ↓ plasma creatinine vs. G2 ↔ glycemia, CRP, TSH, T3 and T4, mRNA expression of Ac-H3 histones, Ki-67, SELENOP, NF-κB, β-catenin, c-Myc, cyclin D1, and DNNT1 between groups G1 and G2: ↑ SELENOP mRNA expression vs. baseline G1: ↔ DNMT1, NF-κB, c-Myc, and cyclin D1 mRNA expression vs. baseline G1 and G2: ↓ β-catenin vs. baseline |
| [19] Brazil | n: 55 women with obesity G1: Age: 40.4 (SD 9) y BMI: 34.6 (30.8–37.4) kg/m2 G2: Age: 39.4 (SD 9.5) y BMI: 34.8 (33.1–40.2) kg/m2 | G1: one unit/day of BN (~1261 µg of Se) G2: control | Randomized, controlled clinical trial 8 weeks | Antioxidant status Inflammation Endothelial function markers | G1: ↑ Se in plasma and erythrocytes, GPx1 activity, selenoprotein P, gene expression for selenoproteins, TNF-α, IL-6, IL-10, TLR2, TLR4 and ↓ GPx1 gene expression vs. G2 ↔ CRP, MCP-1, IL-6, IL-10, IL-1 β, TNF-α, IFN-γ, fibrinogen |
| [20] Brazil | n: 54 women with obesity G1: Age: 40.4 (SD 9) y BMI: 34.9 (SD 4.7) kg/m2 G2: Age: 39.4 (SD 9.5) y BMI: 36.6 (SD 6.5) kg/m2 | G1: one unit/day of BN (~1261 µg of Se) G2: control | Randomized, controlled clinical trial 8 weeks | Antioxidant status | G1: ↑ Se in plasma and erythrocytes, expression of miR-454-3p and miR-584-5p vs. G2 |
| [21] Brazil | n: 42 subjects with coronary artery disease G1: Age: 63.3 (SD 6.7) y BMI: 29.3 (SD 5.6) kg/m2 G2: Age: 63.3 (SD 8) y BMI: 28.6 (SD 4.8) kg/m2 | G1: one unit/day of BN (~290.5 µg of Se) G2: control | Randomized, controlled clinical trial 12 weeks | Lipid metabolism markers Inflammation Oxidative stress | ↔ Nrf2, NF-κB, and NQO1 mRNA expression, TC, HDL-c, LDL-c, TG, TC/HDL-c, LDL-c/HDL-c, and TBARS G1: ↓ TBARS (?) vs. G2 |
| [22] Brazil | n: 36 subjects with coronary artery disease G1: Age: 63 (SD 6.7) y BMI: 28.5 (SD 4.5) kg/m2 G2: Age: 64.6 (SD 7.2) y BMI: 29.4 (SD 5.3) kg/m2 | G1: one unit/day of BN (~290.5 µg of Se) G2: control | Randomized, controlled clinical trial 12 weeks | Lipid metabolism markers Inflammation Oxidative stress | ↔ PPARβ/δ and NF-κB mRNA expression, CRP, TC, HDL-c, LDL-c, TG, and TNF G2: ↓ TBARS vs. G1 |
| Reference | Sample Characteristics | Intervention Characteristics | Study Design and Duration | Evaluated Markers | Results |
|---|---|---|---|---|---|
| Controlled clinical trials | |||||
| [23] Brazil | n: 25 subjects on hemodialysis G1 (n = 13): Age: 57.1 (SD 12) y BMI: 24.4 (SD 3.2) kg/m2 G2 (n = 12): Age: 52 (SD 15.5) y BMI: 26.1 (SD 5.8) kg/m2 | G1: one unit/day of BN (~5 g with 290.5 µg of Se) G2: control | Controlled clinical trial 12 weeks | Antioxidant status Inflammation Oxidative stress | G1: ↑ mRNA expression of Nrf2, NAD(P)H: quinone oxidoreductase 1 (NQO1) and ↓ mRNA expression of NF-κB vs. G2 G1: ↓ MDA, IL-6 vs. baseline |
| Uncontrolled clinical trial | |||||
| [24] Brazil | n: 15 normolipidemic subjects Age: 27.3 (SD 3.9) y BMI: 23.8 (SD 2.8) kg/m2 | 45 g/day of BN (11 units with 862.65 µg of Se) | Clinical trial 15 days | Antioxidant status Anthropometry Lipid metabolism markers | ↑ Se in plasma and reception of cholesteryl esters by HDL-c ↔ weight, total cholesterol, LDL-c, HDL-c, TG, Apo A-I, Apo B, HDL-c diameter, PON 1 activity, % cholesterol, TG, and phospholipid transfer |
| [25] Brazil | n: 81 hemodialysis patients Age: 52 (SD 15.2) y BMI: 24.9 (SD 4.4) kg/m2 | One unit/day of BN (~5 g with 290.5 µg Se) | Clinical trial 12 weeks | Antioxidant status | ↑ Se in plasma and erythrocytes ↑ GSH-Px activity in erythrocytes (began to be within normal) |
| [26] Brazil | n: 37 morbidly obese women Age: 34.5 (SD 6.8) y BMI: 45.2 (SD 4.2) kg/m2 | One unit/day of BN (~290 µg of Se) | Clinical trial 8 weeks | Antioxidant status Glucose Anthropometry DNA damage | ↑ Se in plasma and erythrocytes and GPx activity in all genotypes ↓ DNA damage in those with the Pro/Pro genotype. vs. baseline ↑ DNA damage in those with genotype Leu/Leu vs. Pro/Read ↔ weight, BMI, blood glucose |
| [27] Brazil | n: 37 morbidly obese women Age: 34.5 (SD 6.8) y BMI: 45.2 (SD 4.2) kg/m2 | One unit/day of BN (~290 µg of Se) | Clinical trial 8 weeks | Antioxidant status Lipid profile markers Glucose Anthropometry | ↑ Se in plasma and erythrocytes ↑ GPx activity ↑ HDL-c ↓ TC/HDL-c and LDL-c/HDL-c ratio ↔ weight, BMI, total cholesterol, LDL-c, VLDL-c, TC, fasting glucose |
| [28] Brazil | n: 21 hemodialysis patients Age: 54.2 (SD 15.2) y BMI: 24.4 (SD 3.8) kg/m2 | One unit/day of BN (~5 g with 290.5 µg Se) | Clinical trial 12 weeks | Antioxidant status Anthropometry, body fat, Kidney function markers Minerals | ↑ Se in plasma ↓ urea nitrogen Follow-up after 12 months: ↓ Se in plasma and urea nitrogen ↔ BMI, body fat, WC, creatinine, minerals |
| [29] Brazil | n: 40 hemodialysis patients Age: 53.3 (SD 16.1) y | One unit/day of BN (~5 g with 290.5 µg Se) | Clinical trial 12 weeks | Antioxidant status Lipid metabolism markers Inflammation Oxidative stress and DNA damage | ↑ Se, GPx activity and HDL-c in plasma ↓ TNF, IL-6, 8-OHdG, 8-isoprostane, LDL-c, Castelli index I and II ↔ total cholesterol, TG |
| [30] Brazil | n: 29 hemodialysis patients Age: 51 (SD 3.3) y BMI: 23.6 (17.7–40.3) kg/m2 | One unit/day of BN (~5 g with 290.5 µg Se) | 12-month follow-up after 3 months of BN consumption | Antioxidant status Lipid metabolism markers Inflammation Oxidative stress and DNA damage | ↓ Se and GPx activity in plasma ↑ TNF, IL-6, 8-OHdG, 8-isoprostane ↔ total cholesterol, TG, LDL-c, HDL-c |
| [31] Brazil | n: 40 hemodialysis patients Age: 53.3 (SD 16.1) y | One unit/day of BN (~5 g with 290.5 µg Se) | Clinical trial 12 weeks | Antioxidant status Thyroid function markers | ↑ Se in plasma, GPx activity, T3 and T4 levels ↔ TSH |
| [32] Brazil | n: 130 healthy subjects Age: 29.8 (SD 9.2) y BMI: 23.3 (SD 3.3) kg/m2 | One unit/day of BN (3 to 4 g with ~300 µg of Se) | Clinical trial 8 weeks | Glucose and lipid metabolism markers | ↓ glucose at 4 and 8 weeks and total cholesterol at 8 weeks |
| [33] Brazil | n: 130 healthy subjects Age: 29.8 (SD 9.2) y BMI: 23.3 (SD 3.3) kg/m2 | One unit/day of BN (3 to 4 g with ~300 µg of Se) | Clinical trial 8 weeks | Antioxidant status | ↑ mRNA expression of GPX1 in subjects with genotype in rs713041 ↑ Selenoprotein P mRNA expression in A allele carriers in rs7579 before and after consumption |
| [34] Brazil | n: 130 healthy subjects Age: 29.8 (SD 9.2) y BMI: 23.3 (SD 3.3) kg/m2 | One unit/day of BN (3 to 4 g with ~300 µg of Se) | Clinical trial 8 weeks | Antioxidant status | GPx1 activity: ↓ at 4 weeks but did not differ from baseline at 8 weeks GPx3 activity: ↑ at 4 weeks but did not differ from baseline at 8 weeks Se in plasma and erythrocytes: ↑ at 4 and 8 weeks Selenoprotein P: ↑ in 8 weeks |
| [35] Brazil | n: 60 subjects with type 2 diabetes Men: Age: 62 (SD 9) y BMI: 30.2 (SD 3.2) Women: Age: 66 (SD 8) BMI: 32.6 (SD 4.1) | One unit of BN/day (~3.7 g with 213.67 µg of Se) | Clinical trial 24 weeks | Antioxidant status Anthropometry DNA damage Glucose metabolism markers | ↑ Se, waist circumference, glycemia ↓ DNA damage, both basal and cell-induced oxidative damage ↔ BMI, HbA1c |
| [36] Brazil | n: 32 patients using statins | G1: one unit/day of BN (~5 g with 290 µg of Se) for subjects classified as having high concentrations of creatine kinase G1: one unit/day of BN (~5 g with 290 µg of Se) for subjects classified as having normal creatine kinase concentration | Clinical trial 12 weeks | Antioxidant status Oxidative stress Lipid metabolism marker | G1, G2: ↓ concentrations of protein kinase, MDA, SOD vs. baseline G1, G2: ↑ Se in plasma and erythrocytes, GPx vs. baseline ↔ total cholesterol and mRNA expression of selenoproteins |
| [37] Brazil | n: 32 patients using statins Age: 50.1 (SEM 7.6) y BMI: 31.1 (SEM 3.8) kg/m2 | One unit/day of BN (~5 g with 290 µg of Se) | Clinical trial 12 weeks | Antioxidant status Anthropometry Oxidative stress Lipid metabolism marker | ↑ erythrocyte GPx activity in all genotypes for the rs1050450 polymorphism in the GPx ↑ erythrocyte GPx activity for those with CC genotype for the rs3877899 polymorphism in the SELENOP and all genotypes for rs7579 polymorphism in the SELENOP ↓ creatine kinase in all genotypes for the rs1050450 polymorphism in the GPx ↓ creatine kinase for those with CC genotype for the rs3877899 polymorphism in the SELENOP and GG genotype for rs7579 polymorphism in the SELENOP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.; Silveira, B.K.S.; de Freitas, B.V.M.; Hermsdorff, H.H.M.; Bressan, J. Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods 2022, 11, 2925. https://doi.org/10.3390/foods11182925
da Silva A, Silveira BKS, de Freitas BVM, Hermsdorff HHM, Bressan J. Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods. 2022; 11(18):2925. https://doi.org/10.3390/foods11182925
Chicago/Turabian Styleda Silva, Alessandra, Brenda Kelly Souza Silveira, Brenda Vieira Machado de Freitas, Helen Hermana M. Hermsdorff, and Josefina Bressan. 2022. "Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials" Foods 11, no. 18: 2925. https://doi.org/10.3390/foods11182925
APA Styleda Silva, A., Silveira, B. K. S., de Freitas, B. V. M., Hermsdorff, H. H. M., & Bressan, J. (2022). Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods, 11(18), 2925. https://doi.org/10.3390/foods11182925