Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Cell Culture and Adipocyte Differentiation
2.3. Cytotoxicity Assay
2.4. Assessment of Cellular Lipid Content
2.5. Cell Proliferation Assay
2.6. Western Blotting
2.7. Quantification of Gene Expression Using Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Suppressive Effect of Pinostrobin on Adipogenesis in 3T3-L1 Pre-Adipocytes
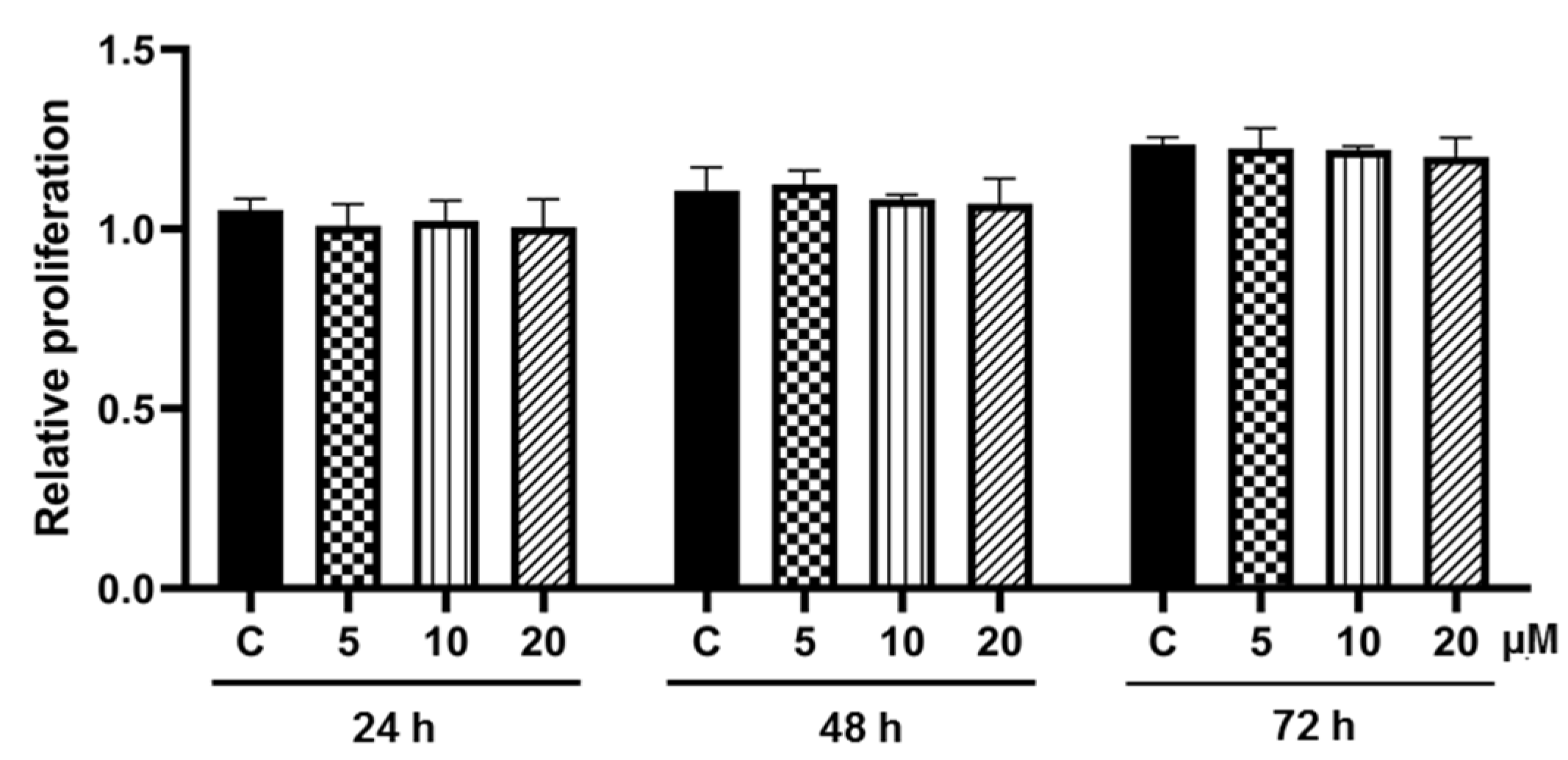
3.2. Effect of Pinostrobin on Cell Proliferation during Adipogenesis
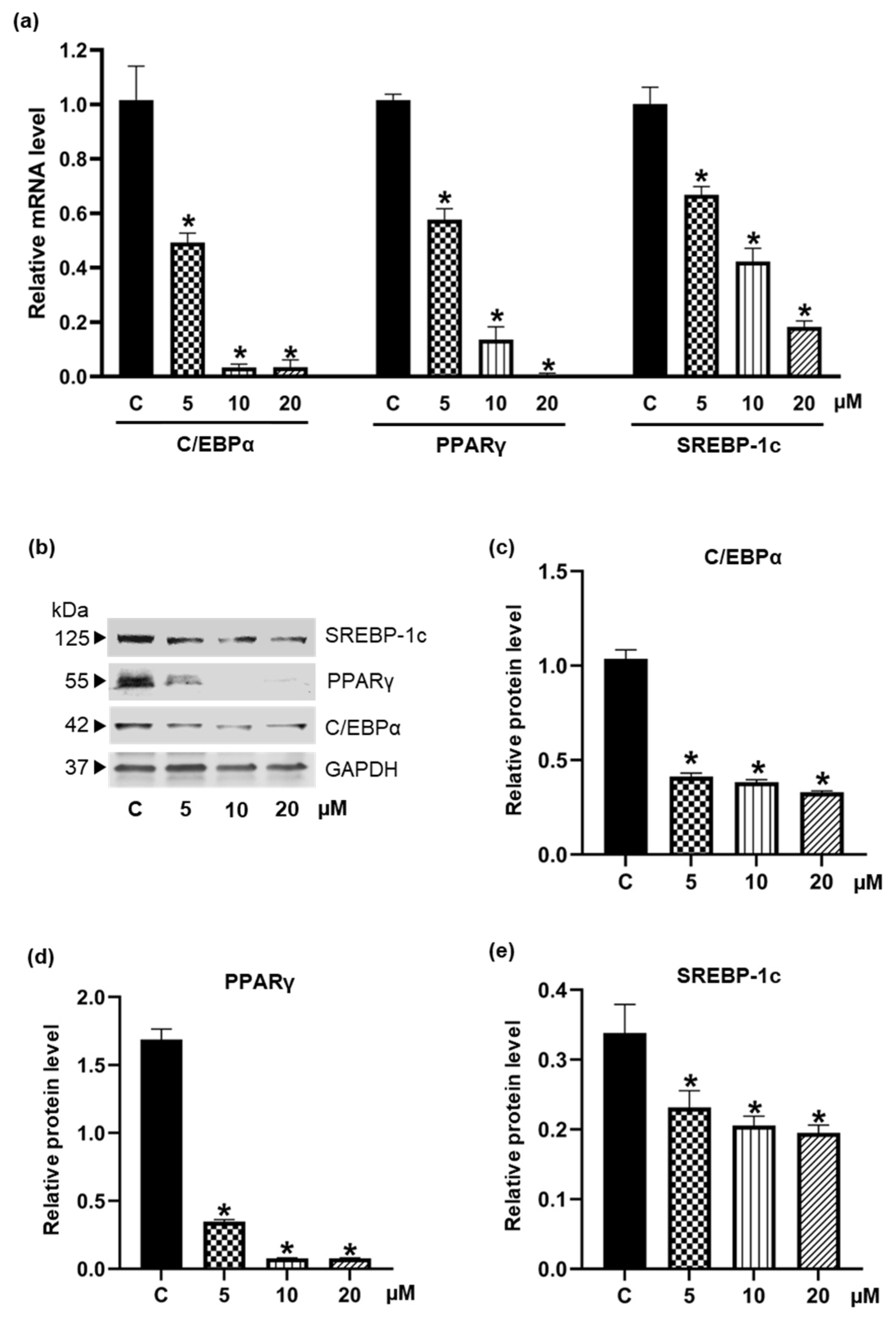
3.3. Inhibiton of the Expression of Adipogenic Transcription Factors by Pinostrobin
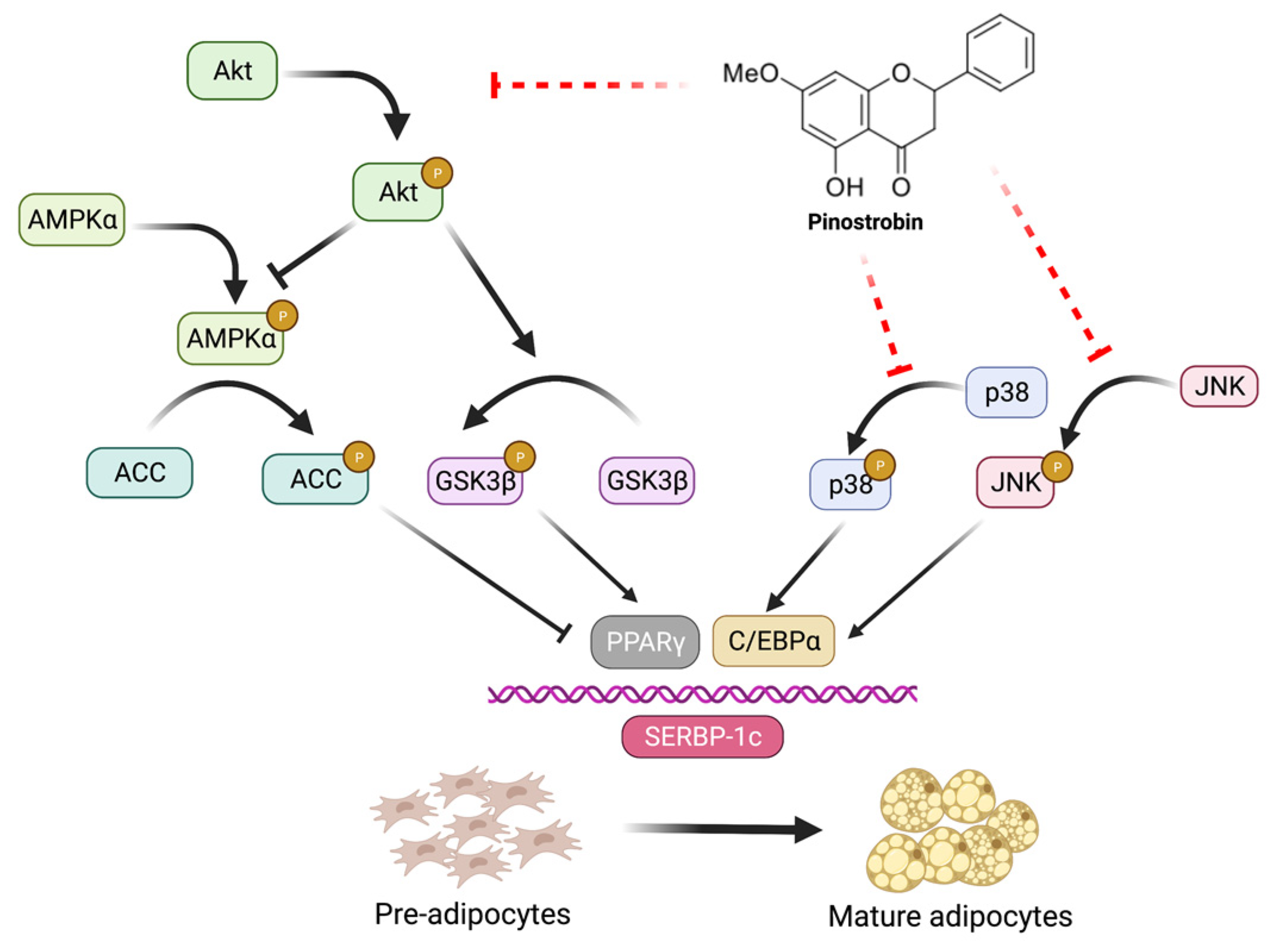
3.4. Pinostrobin Downregulates Upstream Akt and MAPK Signaling Pathways
3.5. Pinostrobin Inhibits Adipocyte Maturation in Human Pre-Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease (GBDx). Number of Deaths and Death Rates from Obesity. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 26 July 2022).
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2022).
- Chang, T.H.; Chou, C.C.; Chang, L.Y. Effect of obesity and body mass index on coronavirus disease 2019 severity: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13089. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body mass index and risk for COVID-19–related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March–December 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 355. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Anti-obesity drugs: Long-term efficacy and safety: An updated review. World J. Men’s Health 2021, 39, 208. [Google Scholar] [CrossRef]
- 2020 Drug Safety Communications. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/2020-drug-safety-communications (accessed on 22 July 2022).
- Feng, S.; Reuss, L.; Wang, Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal medicine for the treatment of obesity: An overview of scientific evidence from 2007 to 2017. Evid. -Based Complementary Altern. Med. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Sun, N.-N.; Wu, T.-Y.; Chau, C.-F. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/β-catenin signaling. Am. J. Physiol. -Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Singh, D.P.; Sarma, S.M.; Kaur, J.; Sandhir, R.; Boparai, R.K.; Kondepudi, K.K.; Bishnoi, M. Capsaicin induces “brite” phenotype in differentiating 3T3-L1 preadipocytes. PLoS ONE 2014, 9, e103093. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B. Energy balance and obesity: What are the main drivers? Cancer Causes Control. 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Chuakul, W.; Boonpleng, A. Ethnomedical uses of Thai Zingiberaceous plant (1). Thai Herbal J. 2003, 10, 33–39. [Google Scholar]
- Chahyadi, A.; Hartati, R.; Wirasutisna, K.R. Boesenbergia pandurata Roxb., an Indonesian medicinal plant: Phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014, 13, 13–37. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew, Plants of the World. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:872224-1 (accessed on 18 July 2022).
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A. Boesenbergia rotunda: From ethnomedicine to drug discovery. Evid. -Based Complement. Altern. Med. 2012, 2012, 1–25. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, M.-S.; Sa, B.-K.; Kim, M.-B.; Hwang, J.-K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef]
- Saah, S.; Siriwan, D.; Trisonthi, P. Biological activities of Boesenbergia rotunda parts and extracting solvents in promoting osteogenic differentiation of pre-osteoblasts. Food Biosci. 2021, 41, 101011. [Google Scholar] [CrossRef]
- Isa, N.; Abdelwahab, S.; Mohan, S.; Abdul, A.; Sukari, M.; Taha, M.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.)(fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Kim, D.U. Use of Panduratin Derivative or Boesenbergia pandurata Extract. US Patent 8653143B2, 18 February 2014. [Google Scholar]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Myoung, K.-S.; Ahn, Y.-T.; Lee, M.-H.; Park, D.-Y.; Ahn, Y.-M.; Huh, C.-S. Fingerroot (Boesenbergia pandurata) extract inhibits the accumulation of visceral fat in C57BL/6J mice. J. Korean Soc. Food Sci. Nutr. 2013, 42, 26–32. [Google Scholar] [CrossRef]
- Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New biflavonoids with α-glucosidase and pancreatic lipase inhibitory activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. [Google Scholar] [CrossRef]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Sritularak, B.; Tantrakarnsakul, K.; Likhitwitayawuid, K.; Lipipun, V. New 2-arylbenzofurans from the root bark of Artocarpus lakoocha. Molecules 2010, 15, 6548–6558. [Google Scholar] [CrossRef]
- Choi, B.-H.; Ahn, I.-S.; Kim, Y.-H.; Park, J.-W.; Lee, S.-Y.; Hyun, C.-K.; Do, M.-S. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp. Mol. Med. 2006, 38, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087379. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Iris, M.; Li, E.T.; Wang, M. Inhibitory effects of oxyresveratrol and cyanomaclurin on adipogenesis of 3T3-L1 cells. J. Funct. Foods 2015, 15, 207–216. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Ross, S.E.; Erickson, R.L.; Hemati, N.; MacDougald, O.A. Glycogen synthase kinase 3 is an insulin-regulated C/EBPα kinase. Mol. Cell. Biol. 1999, 19, 8433–8441. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea-Alonso, M.T.; Ericsson, J. A phosphorylation cascade controls the degradation of active SREBP1. J. Biol. Chem. 2009, 284, 5885–5895. [Google Scholar] [CrossRef]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, S.; Soltys, C.-L.M.; Barr, A.J.; Shiojima, I.; Walsh, K.; Dyck, J.R. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J. Biol. Chem. 2003, 278, 39422–39427. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.R.; Washington, T.B.; Chhabria, S.; Wang, E.H.-C.; Czepiel, K.; Reyes, K.J.C.; Stanford, F.C. Food as Medicine for Obesity Treatment and Management. Clin. Ther. 2022, 44, 671–681. [Google Scholar] [CrossRef]
- Trigueros, L.; Peña, S.; Ugidos, A.; Sayas-Barberá, E.; Pérez-Álvarez, J.; Sendra, E. Food ingredients as anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, X.-B.; Zhao, W.; Zeng, Y.-Y.; Shen, H.; Xiang, H.; Xiao, H. Anti-obesity effect of an isoflavone fatty acid ester on obese mice induced by high fat diet and its potential mechanism. Lipids Health Dis. 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Gathercole, L.L.; Morgan, S.A.; Tomlinson, J.W. Chapter One-Hormonal Regulation of Lipogenesis. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 91, pp. 1–27. [Google Scholar]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2010, 425, 215–224. [Google Scholar] [CrossRef]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef]
- Huang, W.-P.; Huang, Y.-F.; Chen, J.-Z.; Jin, B.; Tan, J.-N.; Ding, Z.-S. Effect of pinostrobin chalcone on adipogenic differentiation of mesenchymal stem cell C3H10T1/2. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2017, 42, 2339–2344. [Google Scholar] [CrossRef]
- Peng, X.-d.; Xu, P.-Z.; Chen, M.-L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-g.; Qu, X.-h.; Yang, Y.; Han, X.-g.; Wang, L.; Qiao, H.; Fan, Q.-m.; Tang, T.-t.; Dai, K.-r. AMPK promotes osteogenesis and inhibits adipogenesis through AMPK-Gfi1-OPN axis. Cell. Signal. 2016, 28, 1270–1282. [Google Scholar] [CrossRef]
- Gormand, A.; Berggreen, C.; Amar, L.; Henriksson, E.; Lund, I.; Albinsson, S.; Göransson, O. LKB1 signalling attenuates early events of adipogenesis and responds to adipogenic cues. J. Mol. Endocrinol. 2014, 53, 117–130. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Prusty, D.; Park, B.-H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, H.T.; Khine, H.E.E.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.-T.; Likhitwitayawuid, K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods 2022, 11, 3024. https://doi.org/10.3390/foods11193024
San HT, Khine HEE, Sritularak B, Prompetchara E, Chaotham C, Che C-T, Likhitwitayawuid K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods. 2022; 11(19):3024. https://doi.org/10.3390/foods11193024
Chicago/Turabian StyleSan, Htoo Tint, Hnin Ei Ei Khine, Boonchoo Sritularak, Eakachai Prompetchara, Chatchai Chaotham, Chun-Tao Che, and Kittisak Likhitwitayawuid. 2022. "Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms" Foods 11, no. 19: 3024. https://doi.org/10.3390/foods11193024
APA StyleSan, H. T., Khine, H. E. E., Sritularak, B., Prompetchara, E., Chaotham, C., Che, C.-T., & Likhitwitayawuid, K. (2022). Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods, 11(19), 3024. https://doi.org/10.3390/foods11193024








