In vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of P. Vulgaris Extracts
2.2. HPLC-DAD Analysis of Main Polyphenolic Constituents
2.3. Cell Culture
2.4. MTT Assay
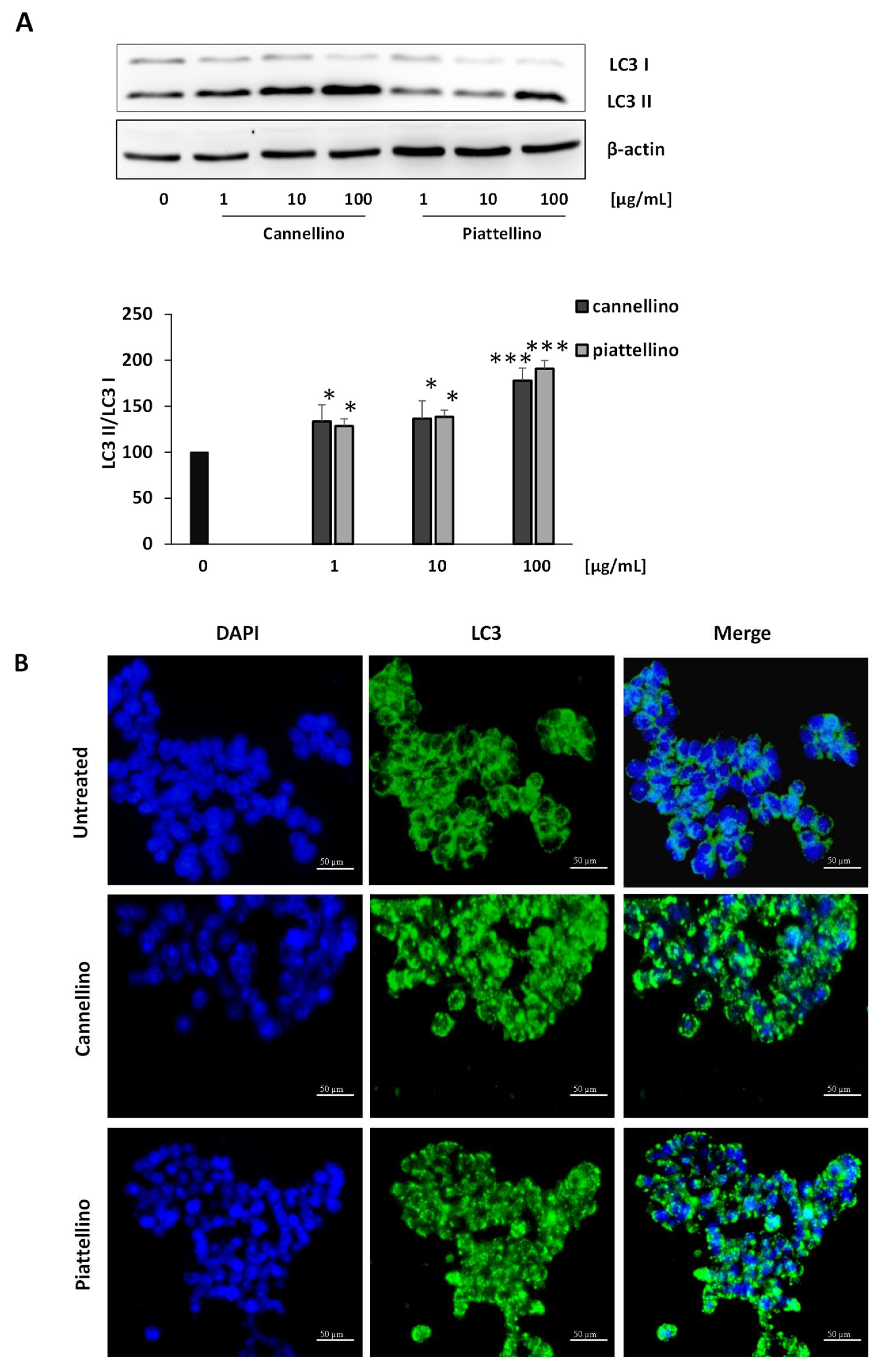
2.5. Western Blotting Analysis
2.6. Clonogenic Assay
2.7. Trypan Blue Assay
2.8. Immunofluorescence Assay
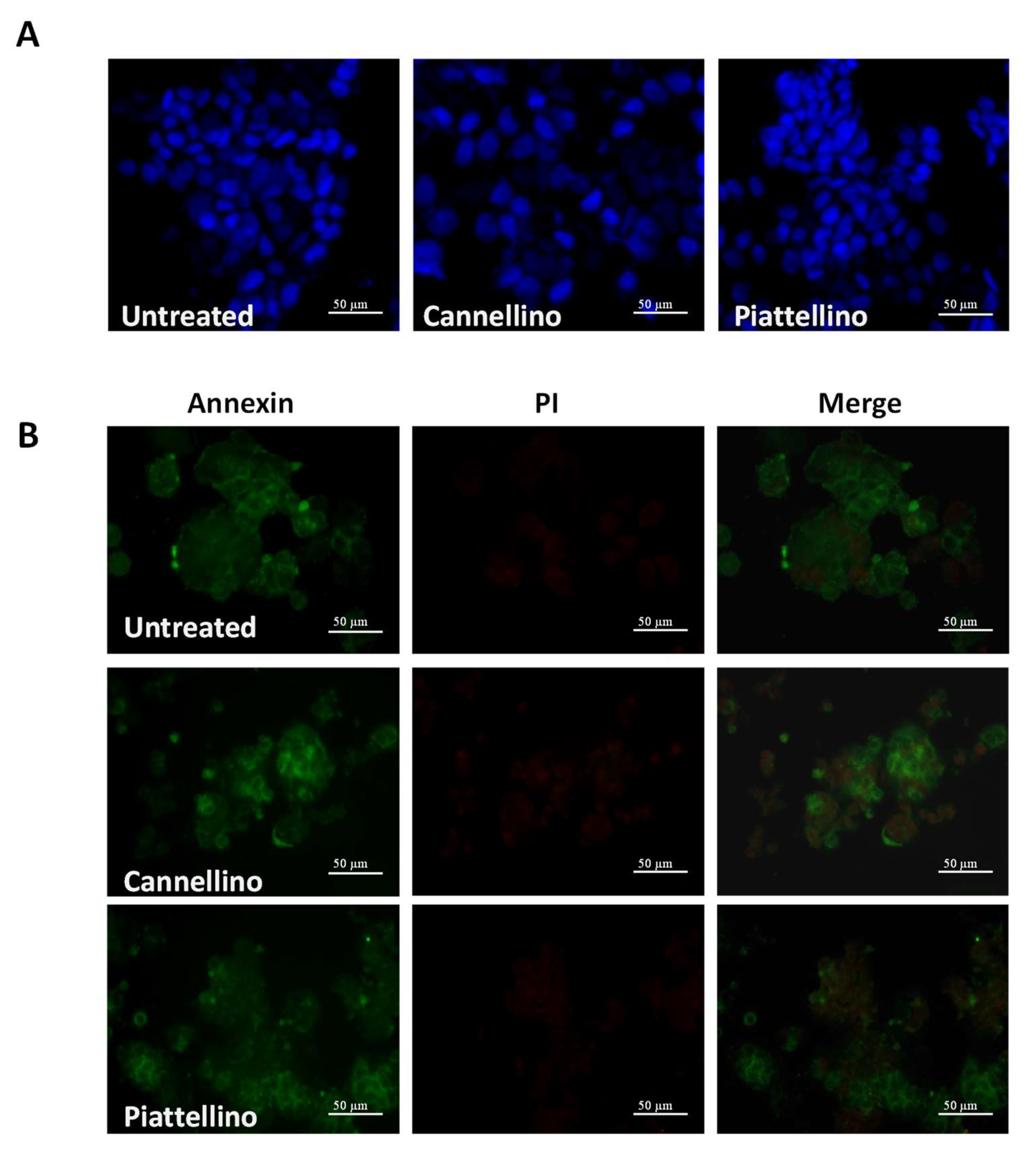
2.9. Annexin V-FITC Staining
2.10. DAPI
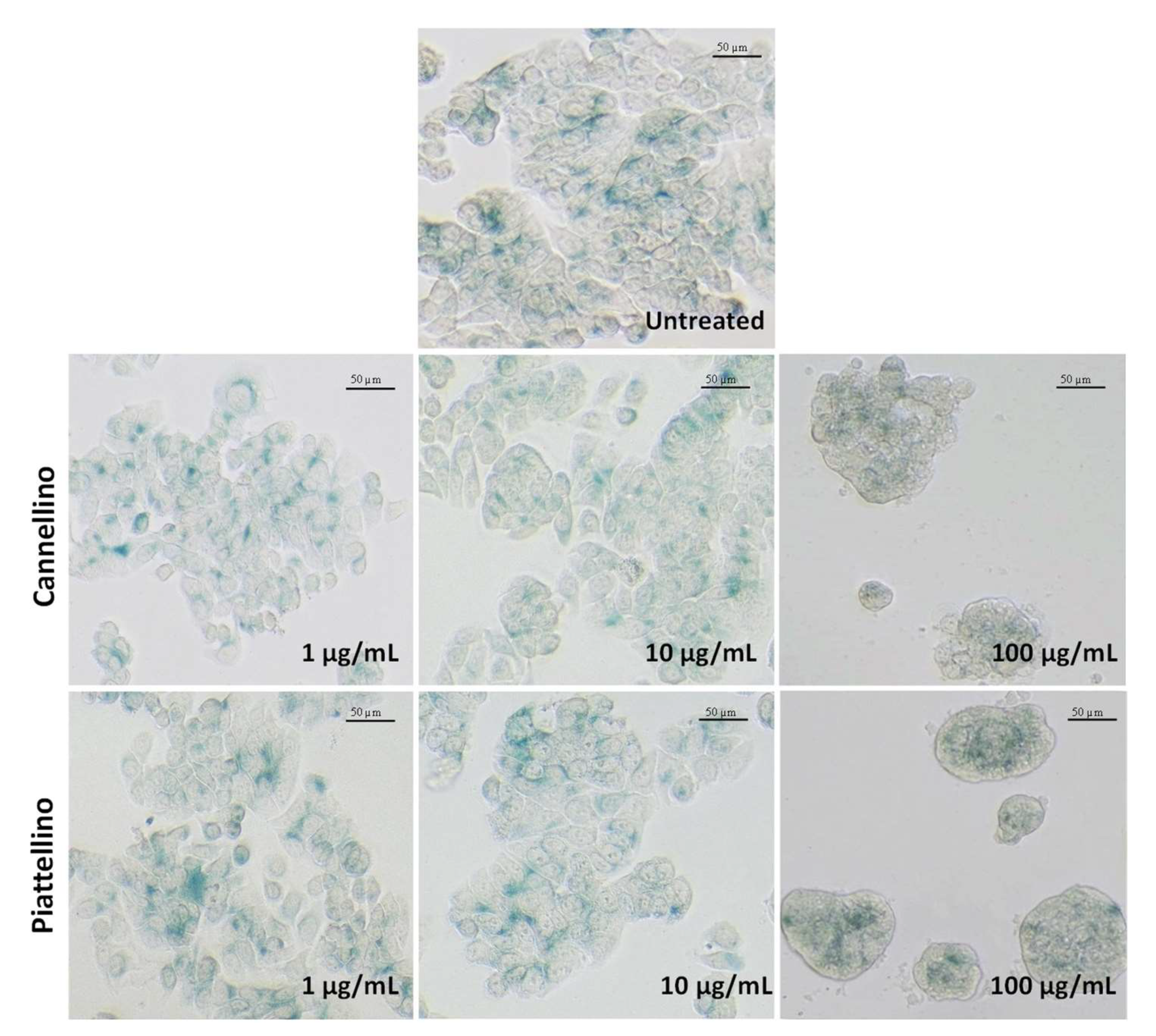
2.11. Senescence Assay
2.12. Statistical Analysis
3. Results
3.1. Chemical Analysis of P. Vulgaris Extracts
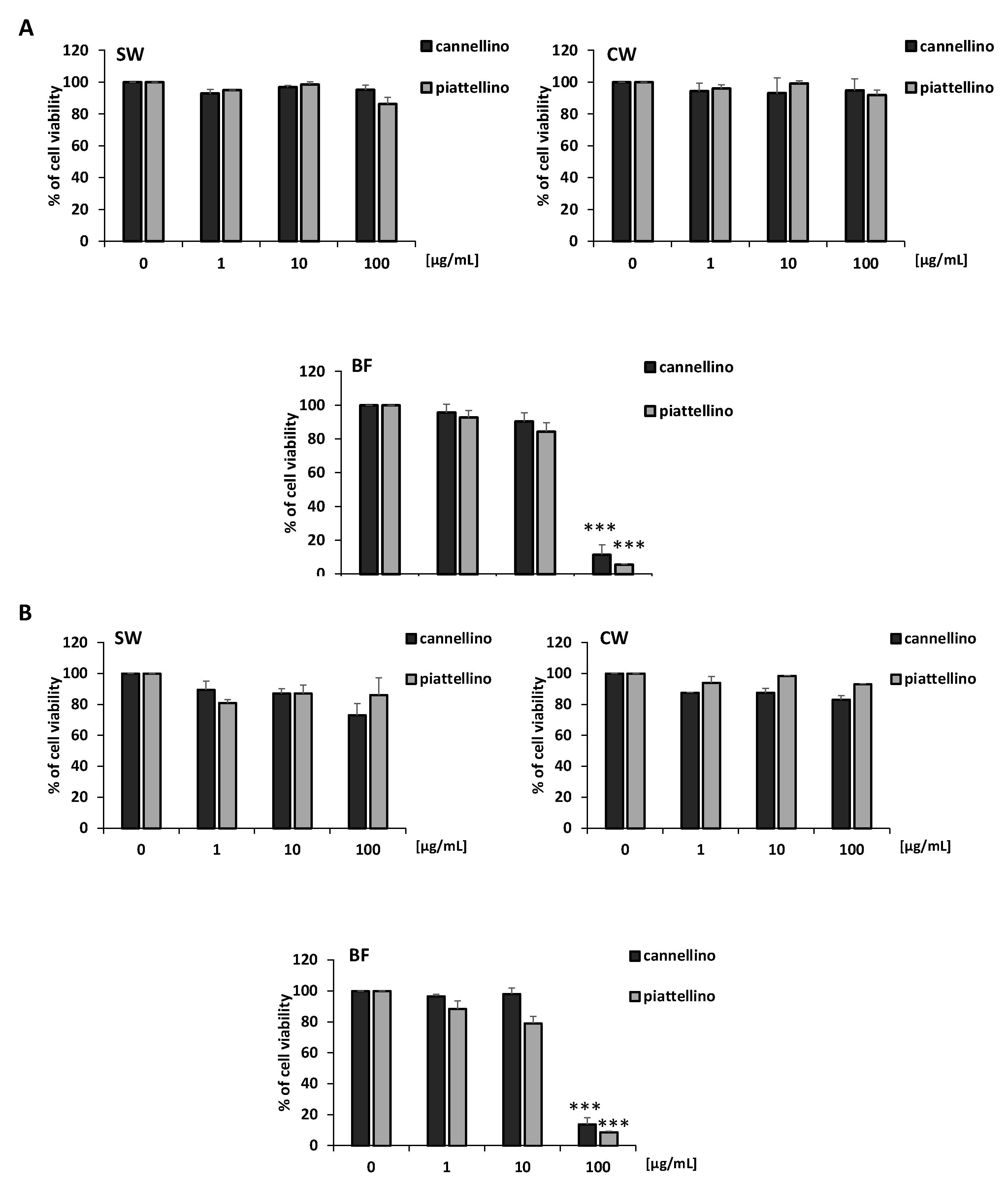
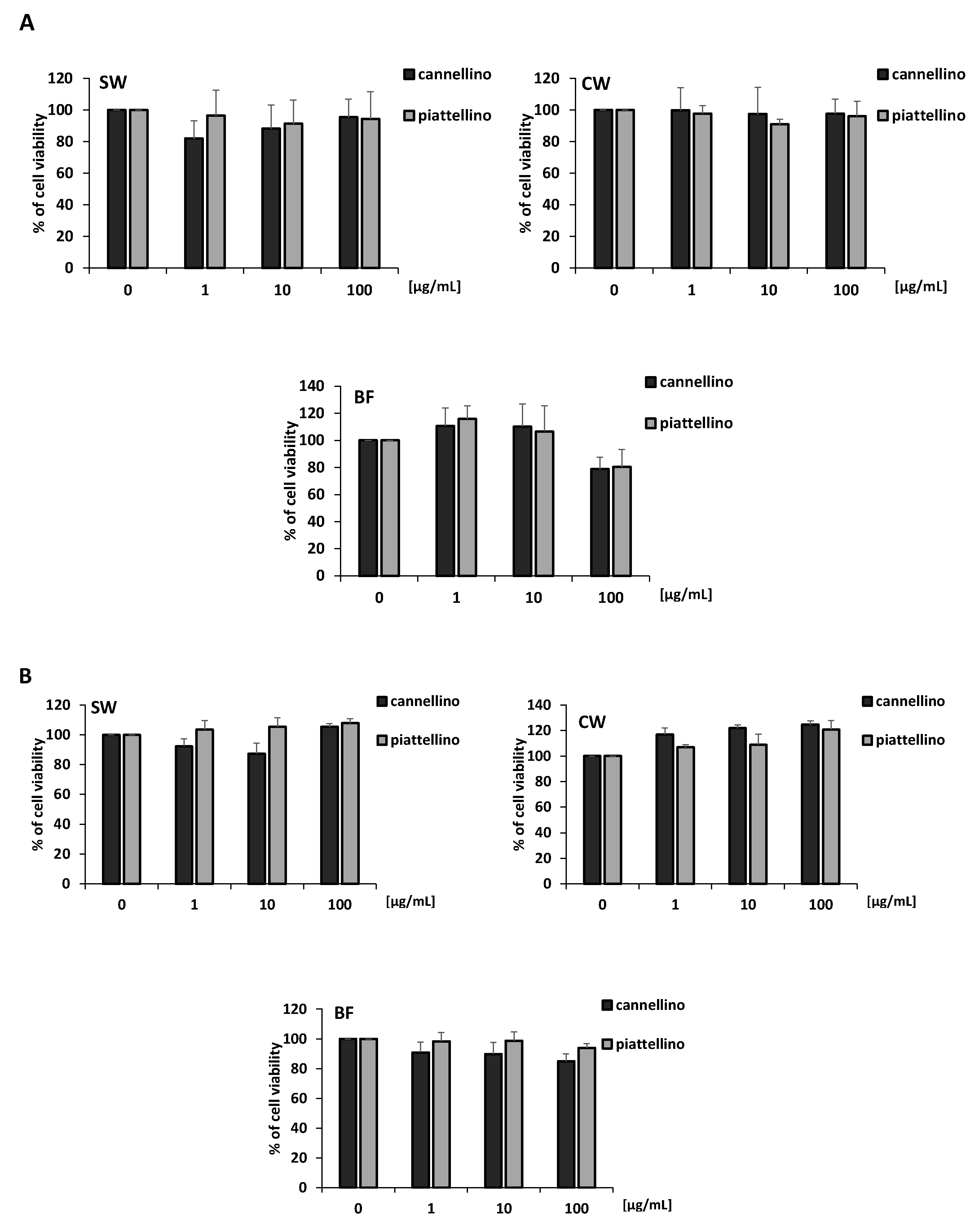
3.2. Effects of P. Vulgaris Extracts on Cell Vitality and Growth of Colon Cancer Cells
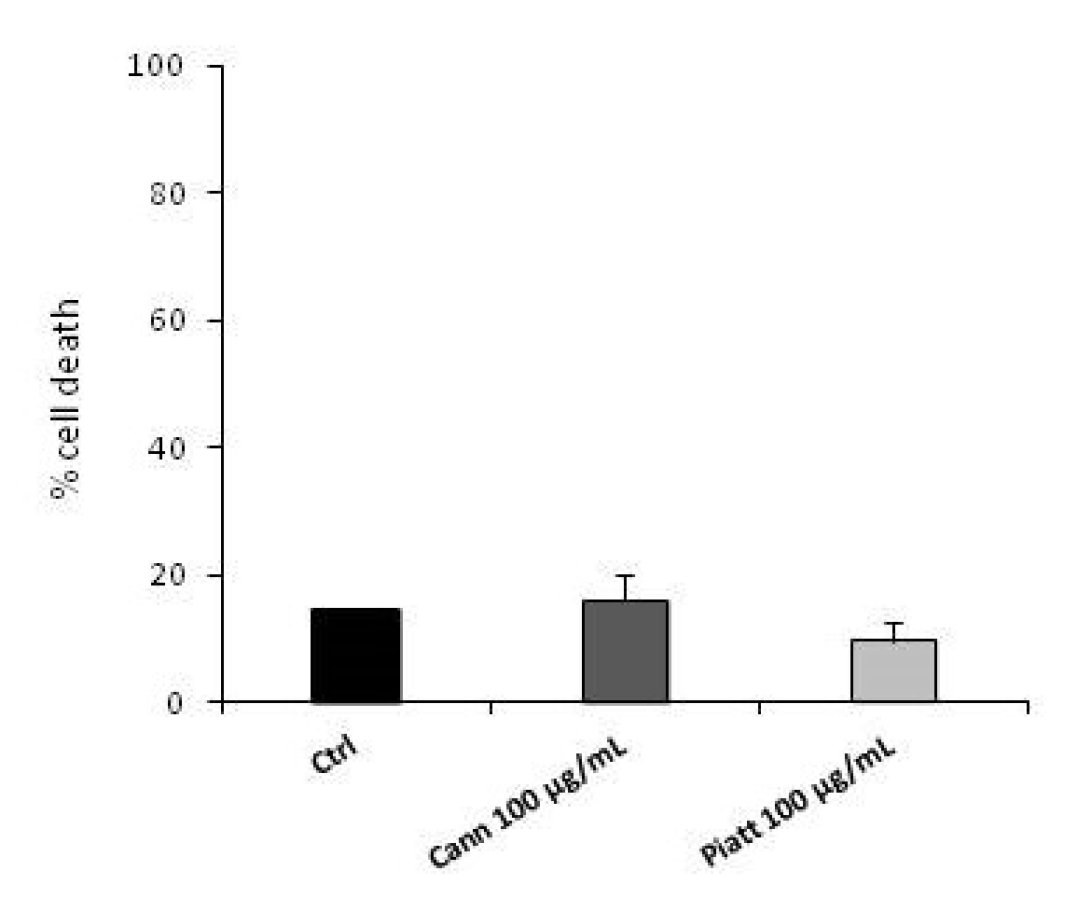
3.3. Bioaccessible Fraction of P. Vulgaris Beans Promotes Colon Cancer Cell Death through the Activation of Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suárez-Martínez, S.E.; Ferriz-Martínez, R.A.; Campos-Vega, R.; Elton-Puente, J.E.; de La Torre Carbot, K.; García-Gasca, T. Bean Seeds: Leading Nutraceutical Source for Human Health. CYTA J. Food 2016, 14, 131–137. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting Legumes Has Compromised Human Health and Sustainable Food Production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Thompson, H.J. Physiological Effects of Bean (Phaseolus vulgaris L.) Consumption on Cellular Signaling in Cancer. Cell Cycle 2012, 11, 835–836. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases-Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef]
- Tormo, M.A.; Gil-Exojo, I.; de Tejada, A.R.; Campillo, J.E. Hypoglycaemic and Anorexigenic Activities of an α-Amylase Inhibitor from White Kidney Beans (Phaseolus vulgaris) in Wistar Rats. Br. J. Nutr. 2004, 92, 785–790. [Google Scholar] [CrossRef]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T.; et al. White Kidney Bean (Phaseolus vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Althwab, S.A.; Qiu, H.; Zbasnik, R.; Urrea, C.; Carr, T.P.; Schlegel, V. Great Northern Beans (Phaseolus vulgaris L.) Lower Cholesterol in Hamsters Fed a High-Saturated-Fat Diet. J. Nutr. 2022, 152, 2080–2087. [Google Scholar] [CrossRef]
- Correa, P. Epidemiological Correlations Between Diet and Cancer Frequency. Cancer Res. 1981, 41, 3685–3689. [Google Scholar] [PubMed]
- Kolonel, L.N.; Hankin, J.H.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P.; Wilkens, L.R.; John, E.M.; Howe, G.R.; Dreon, D.M.; West, D.W.; et al. Vegetables, Fruits, Legumes and Prostate Cancer: A Multiethnic Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 795–804. [Google Scholar]
- Bogweh, N.E.; Ageyo, O.C. Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients 2021, 13, 3701. [Google Scholar] [CrossRef]
- Hughes, J.S.; Ganthavorn, C.; Wilson-Sanders, S. Dry Beans Inhibit Azoxymethane-Induced Colon Carcinogenesis in F344 Rats. J. Nutr. 1997, 127, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and Chemopreventive Effect of Polysaccharides from Common Beans (Phaseolus vulgaris L.) on Azoxymethane-Induced Colon Cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Castañeda, H.A.; Guevara-González, R.G.; Ramos-Gómez, M.; Reynoso-Camacho, R.; Guzmán-Maldonado, H.; Feregrino-Pérez, A.A.; Oomah, B.D.; Loarca-Piña, G. Non-Digestible Fraction of Cooked Bean (Phaseolus vulgaris L.) Cultivar Bayo Madero Suppresses Colonic Aberrant Crypt Foci in Azoxymethane-Induced Rats. Food Funct. 2010, 1, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Perez, A.A.; Piñol-Felis, C.; Gomez-Arbones, X.; Guevara-González, R.G.; Campos-Vega, R.; Acosta-Gallegos, J.; Loarca-Piña, G. A Non-Digestible Fraction of the Common Bean (Phaseolus vulgaris L.) Induces Cell Cycle Arrest and Apoptosis during Early Carcinogenesis. Plant Foods Hum. Nutr. 2014, 69, 248–254. [Google Scholar] [CrossRef]
- García-Cordero, J.M.; Martínez-Palma, N.Y.; Madrigal-Bujaidar, E.; Jiménez-Martínez, C.; Madrigal-Santillán, E.; Morales-González, J.A.; Paniagua-Pérez, R.; Álvarez-González, I. Phaseolin, a Protein from the Seed of Phaseolus vulgaris, Has Antioxidant, Antigenotoxic, and Chemopreventive Properties. Nutrients 2021, 13, 1750. [Google Scholar] [CrossRef]
- Thompson, M.D.; Mensack, M.M.; Jiang, W.; Zhu, Z.; Lewis, M.R.; McGinley, J.N.; Brick, M.A.; Thompson, H.J. Cell Signaling Pathways Associated with a Reduction in Mammary Cancer Burden by Dietary Common Bean (Phaseolus vulgaris L.). Carcinogenesis 2012, 33, 226–232. [Google Scholar] [CrossRef]
- Mensack, M.M.; McGinley, J.N.; Thompson, H.J. Metabolomic Analysis of the Effects of Edible Dry Beans (Phaseolus vulgaris L.) on Tissue Lipid Metabolism and Carcinogenesis in Rats. Br. J. Nutr. 2012, 108, S155–S165. [Google Scholar] [CrossRef]
- Ombra, M.N.; D’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxidative Med. Cell. Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Biagi, M.; Ercoli, J.; Macrì, G.; Miraldi, E.; Trabalzini, L. Phaseolus vulgaris L. var. Venanzio Grown in Tuscany: Chemical Composition and in vitro Investigation of Potential Effects on Colorectal Cancer. Antioxidants 2020, 9, 1181. [Google Scholar] [CrossRef]
- Nie, J.; Qin, X.; Li, Z. Revealing the Anti-Melanoma Mechanism of n-BuOH Fraction from the Red Kidney Bean Coat Extract Based on Network Pharmacology and Transcriptomic Approach. Food Res. Int. 2021, 140, 109880. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, M.R.; López-Barraza, R.; Cervantes-Cardoza, V.; Pérez-Ramírez, I.F.; Reyna-Rojas, J.A.; Gallegos-Infante, J.A.; Estrella, I.; Rojas-Contreras, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E. Mechanisms Associated to Apoptosis of Cancer Cells by Phenolic Extracts from Two Canned Common Beans Varieties (Phaseolus vulgaris L.). J. Food Biochem. 2019, 43, e12680. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in vitro Simulated Digestion on the Antioxidant Activity of Different Camellia Sinensis (L.) Kuntze Leaves Extracts. Eur. Food Res. Technol. 2022, 248, 119–128. [Google Scholar] [CrossRef]
- Trabalzini, L.; Ercoli, J.; Trezza, A.; Schiavo, I.; Macr, G.; Moglia, A.; Spiga, O.; Finetti, F. Pharmacological and In Silico Analysis of Oat Avenanthramides as EGFR Inhibitors: Effects on EGF-Induced Lung Cancer Cell Growth and Migration. Int. J. Mol. Sci. 2022, 23, 8534. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Singh, P.N.; Fraser, G.E. Dietary Risk Factors for Colon Cancer in a Low-Risk Population. Am. J. Epidemiol. 1998, 148, 761–774. [Google Scholar] [CrossRef]
- Haydé, V.C.; Ramón, G.G.; Lorenzo, G.O.; Dave, O.B.; Rosalía, R.C.; Paul, W.; Guadalupe, L.P. Non-Digestible Fraction of Beans (Phaseolus vulgaris L.) Modulates Signalling Pathway Genes at an Early Stage of Colon Cancer in Sprague-Dawley Rats. Br. J. Nutr. 2012, 108, S145–S154. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of Peptides from Common Bean Protein Isolates and Their Potential to Inhibit Markers of Type-2 Diabetes, Hypertension and Oxidative Stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Garcia, B.; de Barros, M.; de Souza Rocha, T. Bioactive Peptides from Beans with the Potential to Decrease the Risk of Developing Noncommunicable Chronic Diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 2003–2021. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; de Mejía, E.G. Characterization and Comparison of Protein and Peptide Profiles and Their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Optimization of Enzymatic Production of Anti-Diabetic Peptides from Black Bean (Phaseolus vulgaris L.) Proteins, Their Characterization and Biological Potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
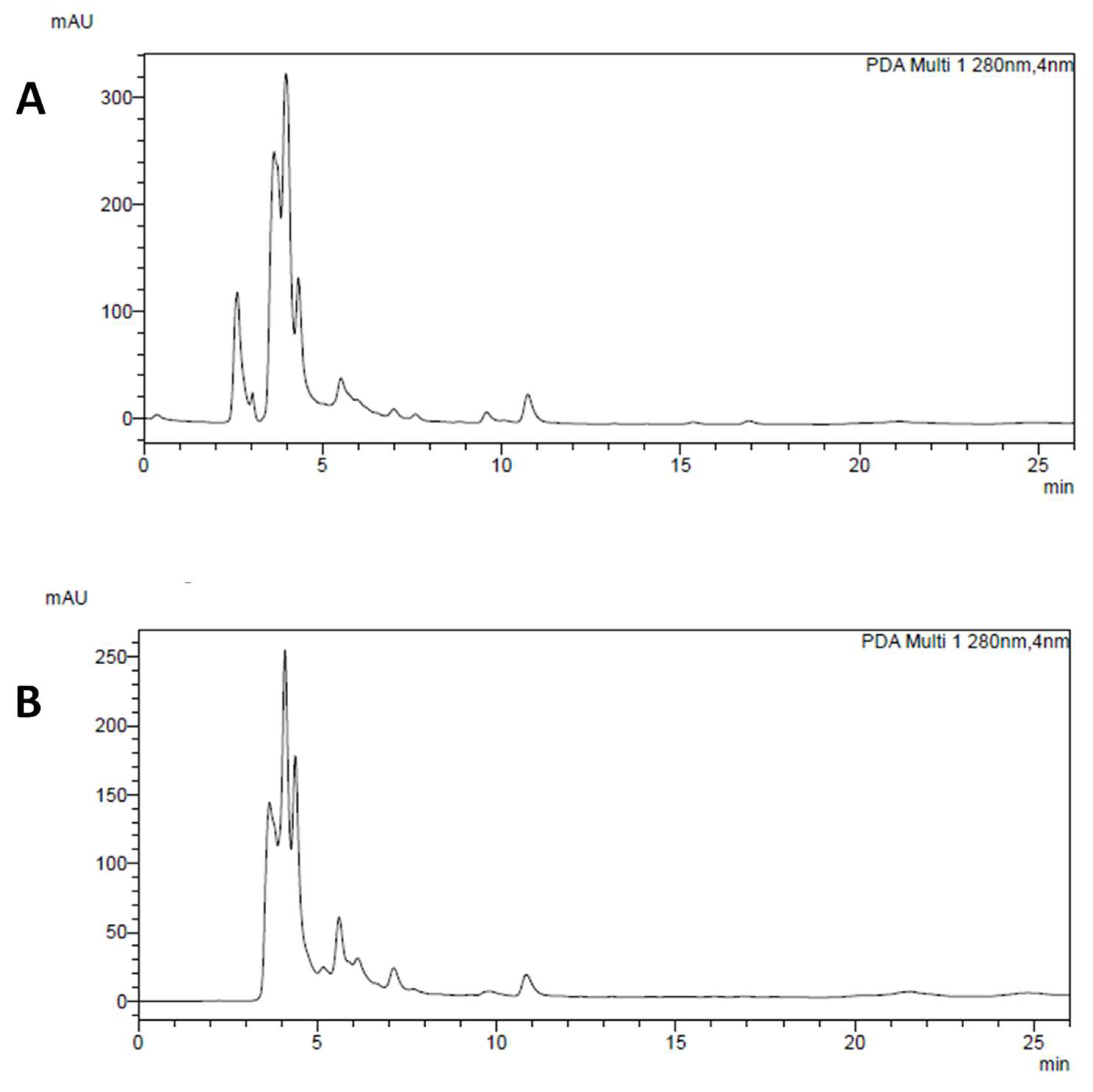


| Components | Quantification (mg/g) | |||
|---|---|---|---|---|
| Piattellino | Cannellino | |||
| CW | BF | CW | BF | |
| Total polyphenols | 0.75 ± 0.03 | n.d. | 0.75 ± 0.03 | n.d. |
| Total hydroxycinnamic derivatives | 0.21 ± 0.01 | 0.10 ± 0.01 | 0.27 ± 0.02 | 0.20 ± 0.04 |
| Gallic acid | 0.39 ± 0.03 | n.d. | 0.34 ± 0.03 | n.d. |
| Chlorogenic acid | 0.08 ± 0.01 | 0 | 0.12 ± 0.01 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, C.; Macrì, G.; Biagi, M.; Miraldi, E.; Finetti, F.; Trabalzini, L. In vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells. Foods 2023, 12, 839. https://doi.org/10.3390/foods12040839
Bernardi C, Macrì G, Biagi M, Miraldi E, Finetti F, Trabalzini L. In vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells. Foods. 2023; 12(4):839. https://doi.org/10.3390/foods12040839
Chicago/Turabian StyleBernardi, Clizia, Giulia Macrì, Marco Biagi, Elisabetta Miraldi, Federica Finetti, and Lorenza Trabalzini. 2023. "In vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells" Foods 12, no. 4: 839. https://doi.org/10.3390/foods12040839
APA StyleBernardi, C., Macrì, G., Biagi, M., Miraldi, E., Finetti, F., & Trabalzini, L. (2023). In vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells. Foods, 12(4), 839. https://doi.org/10.3390/foods12040839









