Process Modelling and Simulation of Key Volatile Compounds of Maillard Reaction Products Derived from Beef Tallow Residue Hydrolysate Based on Proxy Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Beef Tallow Residue Hydrolysates
2.3. Measurement of DH
2.4. Preparation of MRPs
2.5. Aroma Extract Dilution Analysis by GC-O-MS
2.6. Relative Quantification of Aromatic Compounds
2.7. Main Volatile Flavor Compounds Based on ROAV Analysis
2.8. Sensory Evaluation
2.9. Mathematical Modelling
- (1)
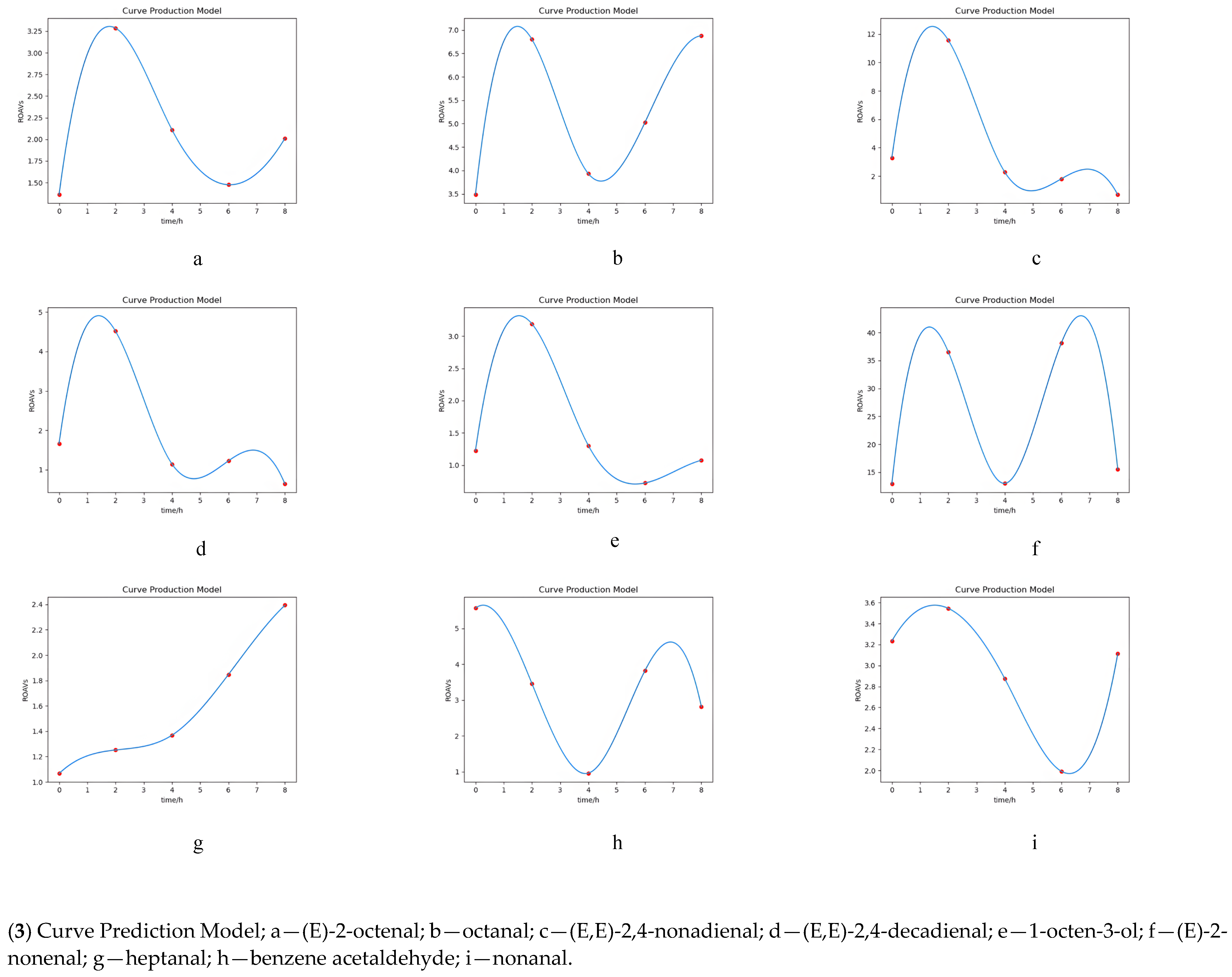
- CPM: Using initial values and 100 arithmetic progressions within the interval of independent variables, the predictive model fitted the curve. The independent variable predicted each dependent variable and was used for curve fitting.
- (2)
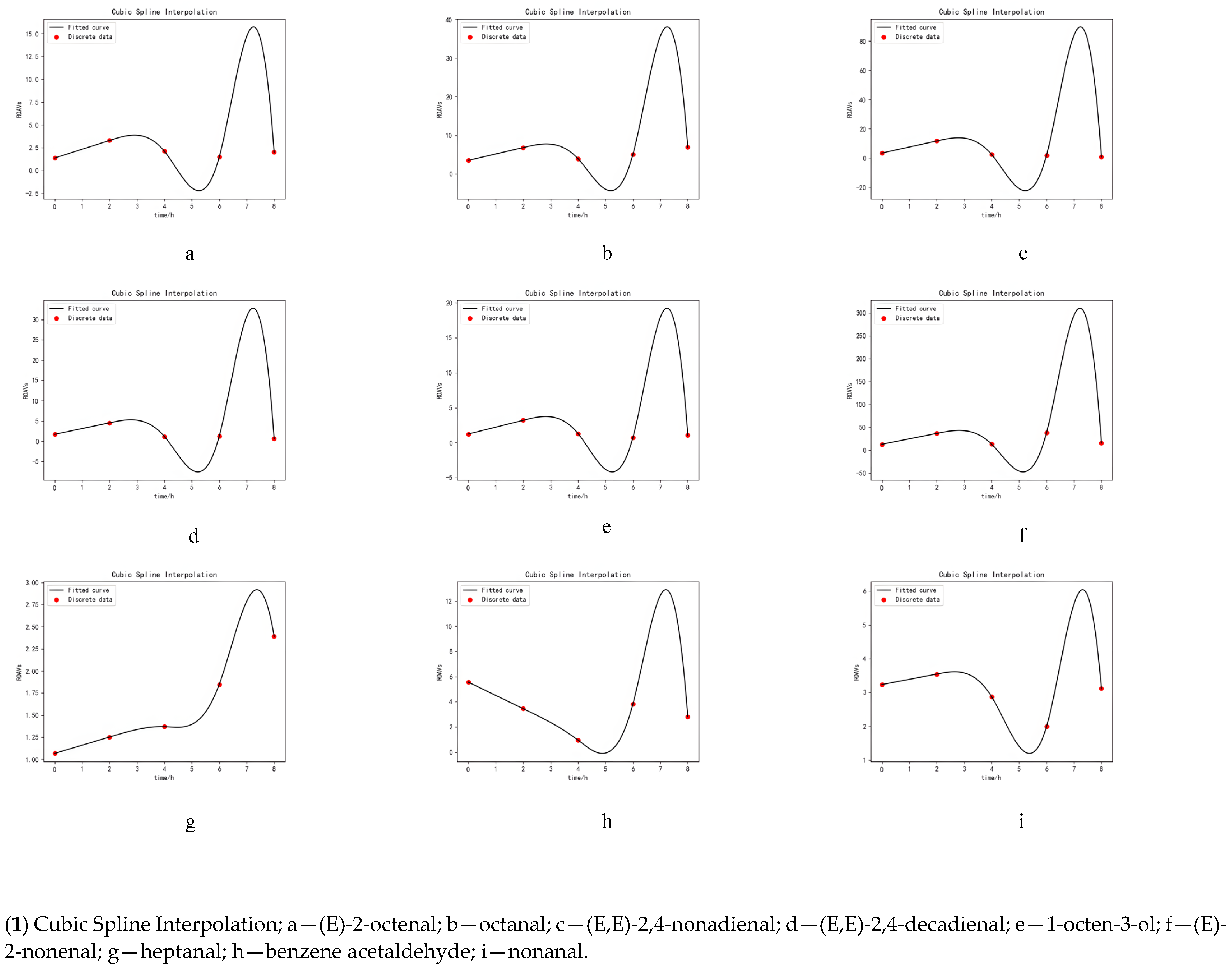
- CSI: To achieve the curve drawing, we used many function constraints, including:
- An internal node on the curve should have equal left and right values.
- The function’s first and last end points should appear in the corresponding equation.
- Each end of the node must have the exact derivative.
- Both ends of the node should have the same second derivative.
- The second derivative at the end point should be zero [18].
- (3)
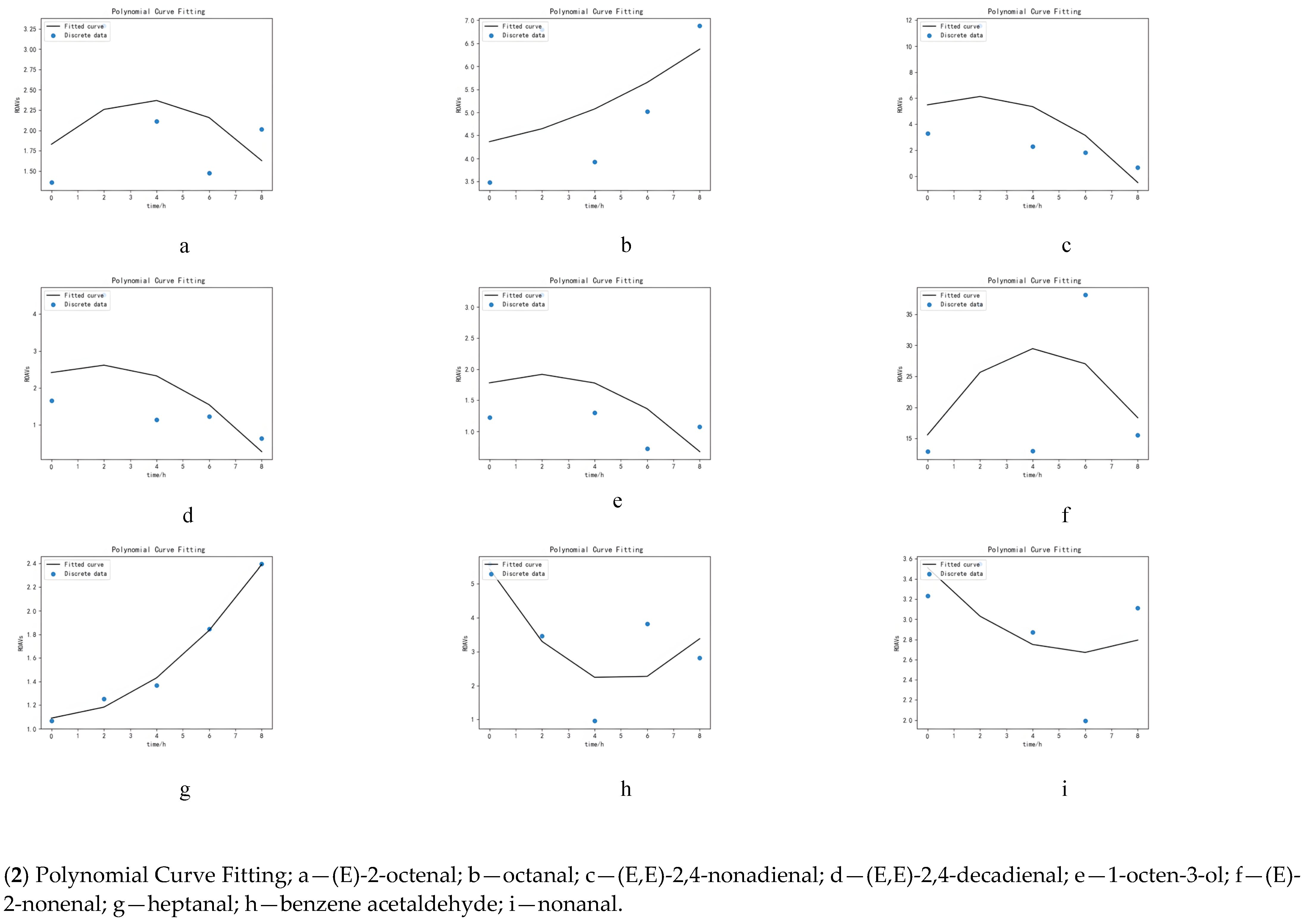
- PCF: Taking the partial derivative of the introduced coefficient and then making the partial derivative 0 allowed us to fit the curve by minimizing the square of the residuals between the dependent variables [19].
2.10. Verification Experiment
2.11. Statistical Analysis
3. Results
3.1. Analysis of DH in Hydrolysate
3.2. Overall Aroma Evaluation
3.3. Identification of Aroma-Active Compounds
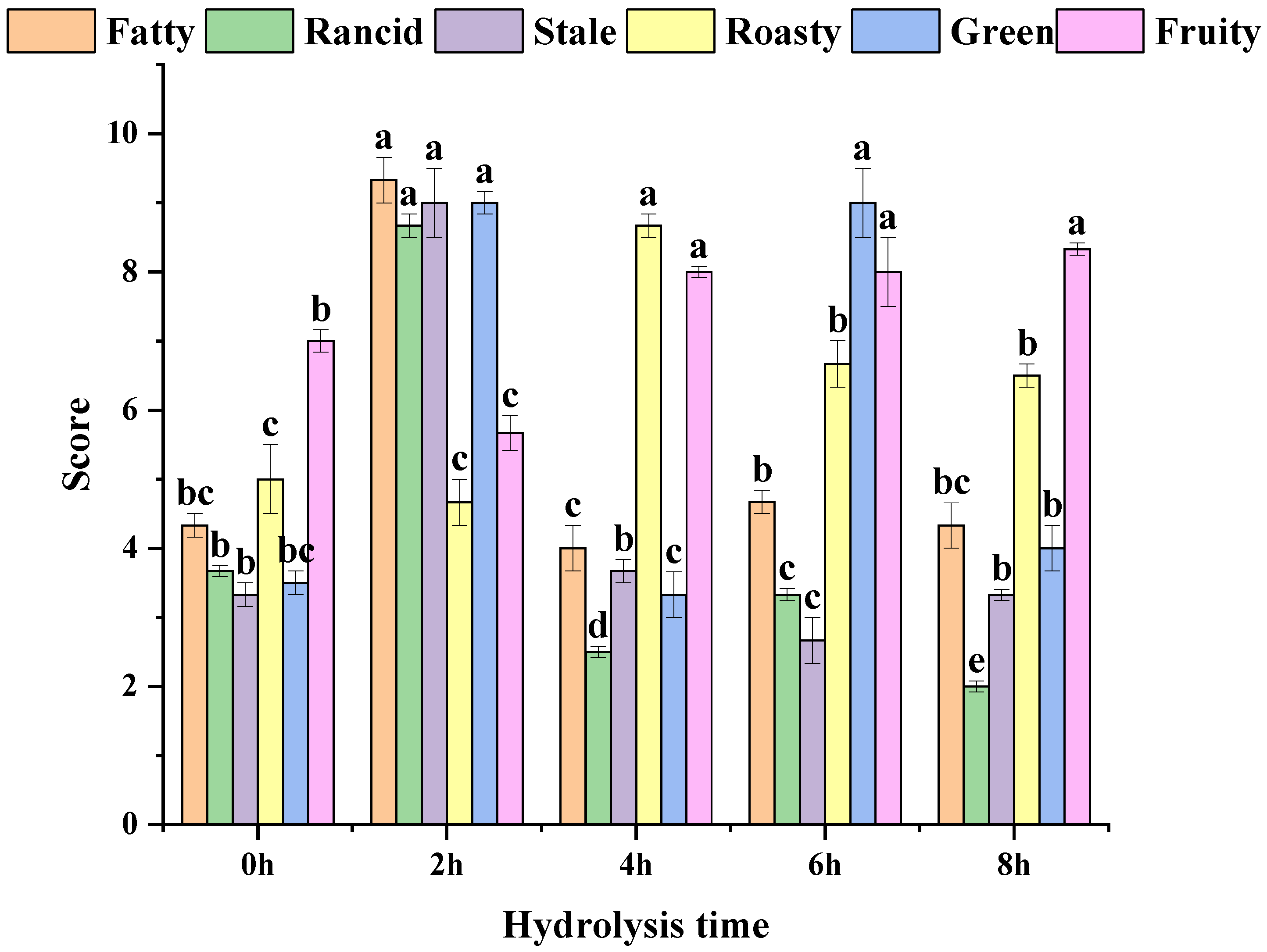
3.4. MRPs Sensory Analysis
3.5. Simulation of Key Volatile Components Curve
3.6. Validation Experiment of Volatile Components Curve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, A.; Wu, W.; Soladoye, O.P.; Aluko, R.E.; Bak, K.H.; Fu, Y.; Zhang, Y.H. Maillard reaction of food-derived peptides as a potential route to generate meat flavor compounds: A review. Food Res. Int. 2022, 151, 110823. [Google Scholar] [CrossRef]
- Guo, X.; Tian, S.; Small, D.M. Generation of meat-like flavourings from enzymatic hydrolysates of proteins from Brassica sp. Food Chem. 2010, 119, 167. [Google Scholar] [CrossRef]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPEM-GC-MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Xia, X.; Liu, Q.; Sun, F.; Kong, B. Flavour formation from hydrolysis of pork meat protein extract by the protease from Staphylococcus carnosus isolated from Harbin dry sausage. LWT-Food Sci. Technol. 2022, 163, 113525. [Google Scholar] [CrossRef]
- Xiao, C.-G.; Wu, J.-Y.; Meng, X.-H.; Tang, H.-G.; Chen, L.-H.; Wu, X.-F.; Shen, Y.-J. Study of enzymolysis technology and microwave Maillard preparation of Litopenaeus vannamei. Cyta-J. Food 2019, 17, 137. [Google Scholar] [CrossRef]
- Walter, J.; Greenberg, Y.; Sriramarao, P.; Ismail, B.P. Limited hydrolysis combined with controlled Maillard-induced glycation does not reduce immunoreactivity of soy protein for all sera tested. Food Chem. 2016, 213, 742. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Hayat, K.; Huang, M.; Liu, P.; Karangwa, E.; Gu, F.; Jia, C.; Xia, S.; Xiao, Z.; et al. Contribution of beef base to aroma characteristics of beeflike process flavour assessed by descriptive sensory analysis and gas chromatography olfactometry and partial least squares regression. J. Chromatogr. A 2021, 1217, 7788. [Google Scholar] [CrossRef]
- Kristoffersen, K.A.; Afseth, N.K.; Böcker, U.; Lindberg, D.; de Vogel-van den Bosch, H.; Ruud, M.L.; Wubshet, S.G. Average molecular weight, degree of hydrolysis and dry-film FTIR fingerprint of milk protein hydrolysates: Intercorrelation and application in process monitoring. Food Chem. 2020, 310, 125800. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, H.; Emrouznejad, A. Information representation of blockchain technology: Risk evaluation of investment by personalized quantifier with cubic spline interpolation. Inf. Processing Manag. 2021, 58, 102571. [Google Scholar] [CrossRef]
- Bucheli, J.; Dalhaus, T.; Finger, R. Temperature effects on crop yields in heat index insurance. Food Policy 2022, 107, 102214. [Google Scholar] [CrossRef]
- Deane, A.S.; Begun, D.R. Broken fingers: Retesting locomotor hypotheses for fossil hominoids using fragmentary proximal phalanges and high-resolution polynomial curve fitting (HR-PCF). J. Hum. Evol. 2008, 55, 691. [Google Scholar] [CrossRef] [PubMed]
- Nilsang, S.; Lertsiri, S.; Suphantharika, M.; Assavanig, A. Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J. Food Eng. 2005, 70, 571. [Google Scholar] [CrossRef]
- Song, N.; Tan, C.; Huang, M.G.; Liu, P.; Eric, K.; Zhang, X.M.; Xia, S.Q.; Jia, C.S. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chem. 2013, 136, 144. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.R.; Wu, G.C.; Ji, X.; Zhang, H.; Jin, Q.Z.; Wang, X.G. Influence of Prolonged Deep-Frying Using Various Oils on Volatile Compounds Formation of French Fries Using GC-MS, GC-O, and Sensory Evaluation. J. Am. Oil Chem. Soc. 2021, 98, 657. [Google Scholar] [CrossRef]
- Solah, V.A.; Meng, X.Q.; Wood, S.; Gahler, R.J.; Kerr, D.A.; James, A.P.; Pal, S.; Fenton, H.K.; Johnson, S.K. Effect of Training on the Reliability of Satiety Evaluation and Use of Trained Panellists to Determine the Satiety Effect of Dietary Fibre: A Randomised Controlled Trial. PLoS ONE 2015, 10, e0126202. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mei, X.; Chang, J.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Comparative characterization of key odorants of French fries and oils at the break-in, optimum, and degrading frying stages. Food Chem. 2022, 368, 130581. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, Z.; Zhang, C.; Du, L.; Xiao, D.; Xu, Y. Sensory and instrumental analysis-guided exploration of odor-active compounds recovery with oil during the water-boiling extraction of Pu-erh tea. Food Res. Int. 2020, 134, 109243. [Google Scholar] [CrossRef]
- Smith, R.J.; Gillies, M.H.; Newell, G.; Foley, J.P. A decision support model for travelling gun irrigation machines. Biosyst. Eng. 2008, 100, 126. [Google Scholar] [CrossRef]
- Richards, P.J.; Weatherhead, E.K. Prediction of raingun application patterns IN windy conditions. J. Agric. Eng. Res. 1993, 54, 281. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.-P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Sun, X.; Xu, J.; Xiong, Y.; Cao, Q.; Wang, Z.; Li, H.; Zhang, F.; Chen, Y.; Liu, Y. Characterization of the key aroma compounds in Chinese JingJiu by quantitative measurements, aroma recombination, and omission experiment. Food Chem. 2021, 352, 129450. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in stewed mutton (goat meat) added with thyme (Thymus vulgaris L.) based on the combination of instrumental analysis and sensory verification. Food Chem. 2022, 371, 131111. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. Odour Thresholds. Compilations of Odour Threshold Values in Air, Water and Other Media, Second Enlarged and Revised Ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Adams, A.; Kitryte, V.; Venskutonis, R.; de Kimpe, N. Formation and characterisation of melanoidin-like polycondensation products from amino acids and lipid oxidation products. Food Chem. 2009, 115, 904. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C. Retro-aldol related degradation of 2,4-decadienal in the development of staling flavors in fried foods. J. Food Sci. 1987, 52, 1186. [Google Scholar] [CrossRef]
- Choi, B.D.; Kang, S.J.; Lee, J.J.; Ho, C.T. Contribution of 2,4-decadienal and fish oil to volatile formation in the Maillard reaction of taurine with xylose. Food Sci. Biotechnol. 2000, 9, 133. [Google Scholar]
- Schirack, A.V.; Drake, M.A.; Sanders, T.H.; Sandeep, K.P. Characterization of aroma-active compounds in microwave blanched peanuts. J. Food Sci. 2006, 71, C513. [Google Scholar] [CrossRef]
- Zamora, R.; Alcon, E.; Hidalgo, F.J. Strecker-Type Degradation of Phenylalanine Initiated by 4-Oxo-2-alkenals in Comparison to That Initiated by 2,4-Alkadienals, 4,5-Epoxy-2-alkenals, or 4-Hydroxy-2-nonenal. J. Agric. Food Chem. 2013, 61, 10231. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1. [Google Scholar] [CrossRef]
- Ho, C.T.; Chen, Q.Y. Lipids in food flavors—An Overview. Lipids Food Flavors 1994, 2, 14. [Google Scholar]
- Cerny, C.; Grosch, W. Precursors of ethyldimethylpyrazine isomers and 2,3-diethyl-5-methylpyrazine formed in roasted beef. Z. Lebensm.-Unters.-Forsch. 1994, 198, 210. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhang, L.J.; Yu, X.W.; Xu, Y. The Biosynthesis Mechanism Involving 2,3-Pentanedione and Aminoacetone Describes the Production of 2-Ethyl-3,5-dimethylpyrazine and 2-Ethyl-3,6-dimethylpyrazine by Bacillus subtilis. J. Agric. Food Chem. 2020, 68, 3558. [Google Scholar] [CrossRef] [PubMed]
- Selke, E.; Rohwedder, W.K.; Dutton, H.J. Volatile components from triolein heated in air. J. Am. Oil Chem. Soc. 1977, 54, 62. [Google Scholar] [CrossRef]
- Assaf, S.; Hadar, Y.; Dosoretz, C.G. 1-octen-3-ol and 13-hydroperoxylinoleate are products of distinct pathways in the oxidative breakdown of linoleic acid by Pleurotus pulmonarius. Enzym. Microb. Technol. 1997, 21, 484. [Google Scholar] [CrossRef]
- Anese, M.; Suman, M. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res. Int. 2013, 51, 257. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Modifications induced by oxidation and suitable markers of oxidative status. J. Agric. Food Chem. 2003, 51, 6564. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. J. Am. Oil Chem. Soc. 2017, 94, 271. [Google Scholar] [CrossRef]
- ben Hammouda, I.; Freitas, F.; Ammar, S.; da Silva, M.D.R.G.; Bouaziz, M. Comparison and characterization of volatile compounds as markers of oils stability during frying by HS-SPME-GC/MS and Chemometric analysis. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2017, 1068, 322. [Google Scholar] [CrossRef]
- Zhong, A.; Chen, W.; Hu, L.; Wu, Z.; Xiao, Y.; Li, K.; Li, Z.; Wang, Y.; Wang, C. Characterisation of key volatile compounds in fermented sour meat after fungi growth inhibition. LWT 2022, 165, 113662. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, W.; Xu, F.; Huang, Y.J.; Zhang, N.N.; Li, K.; Hu, H.H.; Zhang, H. Comparative flavor analysis of eight varieties of Xinjiang flatbreads from the Xinjiang Region of China. Cereal Chem. 2019, 96, 1022. [Google Scholar] [CrossRef]
- Zhan, P.; Tian, H.; Zhang, X.; Wang, L. Contribution to aroma characteristics of mutton process flavor from the enzymatic hydrolysate of sheep bone protein assessed by descriptive sensory analysis and gas chromatography olfactometry. J. Chromatogr. B 2013, 921, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, X.; Nsor-Atindana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Optimization of key aroma compounds for dog food attractant. Anim. Feed Sci. Technol. 2017, 225, 173. [Google Scholar] [CrossRef]
- Ge, M.S.; Wu, P.T.; Zhu, D.L.; Zhang, L. Application of different curve interpolation and fitting methods in water distribution calculation of mobile sprinkler machine. Biosyst. Eng. 2018, 174, 316. [Google Scholar] [CrossRef]
- Fornberg, B.; Zuev, J. The Runge phenomenon and spatially variable shape parameters in RBF interpolation. Comput. Math. Appl. 2007, 54, 379. [Google Scholar] [CrossRef]
- Wu, C.F.; Yost, M.G.; Hashmonay, R.A.; Tsai, M.Y. Path concentration profile reconstruction of optical remote sensing measurements using polynomial curve fitting procedures. Atmos. Environ. 2003, 37, 1879. [Google Scholar] [CrossRef]

| No. | Aroma-Active Compounds | Flavor Dilution Factor a | Flavor b | ||||
|---|---|---|---|---|---|---|---|
| MRPs A | MRPs B | MRPs C | MRPs D | MRPs E | |||
| 1 | (E)-2-Octenal | 9 | 9 | 27 | 9 | 9 | fatty, plastic [16] |
| 2 | Octanal | 3 | 9 | 9 | 9 | 27 | fatty [16] |
| 3 | (E)-2-Decenal | 1 | 3 | 9 | 3 | 3 | fatty, green, waxy [16] |
| 4 | (E,E)-2,4-Nonadienal | 9 | 9 | 9 | 9 | 3 | fatty, green, waxy [16] |
| 5 | (E)-2-Undecenal | 1 | 3 | 3 | 3 | 3 | fatty, green, waxy [16] |
| 6 | 2-Pentyl- furan | 9 | 3 | 9 | 3 | 3 | fatty, fruity, green [16] |
| 7 | 1-Octanol | 1 | 3 | 3 | 3 | 3 | fatty, floral, green [16] |
| 8 | Decanal | 3 | 3 | 3 | 9 | 9 | fatty, floral, green [16] |
| 9 | (E)-2-Octen-1-ol | 3 | 9 | 27 | 9 | 3 | Sour, fatty, gravy [20] |
| 10 | 3-(Methylthio)propanal | 3 | 9 | 3 | 9 | 3 | boiled potato [16] |
| 11 | n-Decanoic acid | 3 | 3 | 3 | 3 | 3 | rubber, sour [21] |
| 12 | (E,E)-2,4-Decadienal | 9 | 27 | 9 | 9 | 9 | bedbug [16] |
| 13 | Butanoic acid | ND c | ND | 1 | 1 | 1 | rancid, moldy [16] |
| 14 | Octanoic acid | 9 | 3 | 9 | 9 | 9 | rancid, fermented [16] |
| 15 | (E,E)-3,5-Octadien-2-one | 3 | 9 | 9 | 9 | 9 | rancid [20] |
| 16 | Phenol | 9 | 9 | 9 | 9 | 9 | plastic, rancid [20] |
| 17 | 1-Octen-3-ol | 3 | 9 | 9 | 3 | 3 | moldy [21] |
| 18 | Trimethyl-pyrazine | ND | ND | 9 | 9 | 9 | moldy, plastic [22] |
| 19 | Nonanoic acid | 9 | 3 | 9 | 9 | 9 | moldy, rancid [16] |
| 20 | 2-Ethyl-6-methyl- pyrazine | 1 | 1 | 3 | 3 | 3 | peanut, roasted [16] |
| 21 | Ethyl myristate | 9 | 3 | 3 | 3 | 3 | peanut, rubber [20] |
| 22 | Hexanoic acid | 1 | 1 | 1 | 1 | 1 | rancid, bitter [16] |
| 23 | 2-Ethyl-3,5-dimethylpyrazine | 81 | 81 | 243 | 243 | 243 | peanut, roasted [16] |
| 24 | Hexanal | 1 | 1 | 1 | 1 | 1 | green [16] |
| 25 | p-Cresol | ND | ND | 1 | 9 | 3 | herbal medicine [23] |
| 26 | (E)-2-Nonenal | 27 | 27 | 27 | 27 | 27 | fatty, mushroom [16] |
| 27 | Heptanal | 9 | 9 | 27 | 27 | 27 | citrus-like [16] |
| 28 | Heptanoic acid | 1 | 1 | 1 | 1 | 1 | sweaty [16] |
| 29 | Benzene acetaldehyde | 27 | 27 | 27 | 27 | 27 | stale, floral [16] |
| 30 | Nonanal | 9 | 9 | 27 | 9 | 27 | citrus-like, fatty [16] |
| No. | Aroma-Active Compounds | Relative Content (%) * | Ions (m/z) | ||||
|---|---|---|---|---|---|---|---|
| MRPs A | MRPs B | MRPs C | MRPs D | MRPs E | |||
| 1 | (E)-2-Octenal | 2.16 d ± 0.42 | 4.47 b ± 0.24 | 6.38 a ± 0.05 | 3.45 c ± 0.15 | 4.64 b ± 0.62 | 41, 55, 70 |
| 2 | Octanal | 0.82 d ± 0.02 | 1.37 c ± 0.28 | 1.76 b ± 0.07 | 1.74 b ± 0.33 | 2.35 a ± 0.20 | 41, 57, 84 |
| 3 | (E)-2-Decenal | 0.72 c ± 0.04 | 1.58 a ± 0.03 | 1.62 a ± 0.19 | 1.49 b ± 0.05 | 1.48 b ± 0.02 | 41, 70, 55 |
| 4 | (E,E)-2,4-Nonadienal | 0.38 c ± 0.03 | 1.17 a ± 0.12 | 0.51 b ± 0.02 | 0.31 c ± 0.04 | 0.12 d ± 0.01 | 81, 41, 67 |
| 5 | (E)-2-Undecenal | 0.74 b ± 0.02 | 1.47 a ± 0.16 | 1.29 a ± 0.05 | 1.49 a ± 0.03 | 1.37 a ± 0.05 | 41, 70, 55 |
| 6 | 2-Pentyl- furan | 2.73 b ± 0.29 | 1.46 d ± 0.15 | 3.31 a ± 0.04 | 0.76 e ± 0.08 | 1.84 c ± 0.11 | 81, 53, 82 |
| 7 | 1-Octanol | 0.42 d ± 0.11 | 0.62 c ± 0.04 | 0.72 b ± 0.05 | 0.93 a ± 0.12 | 0.90 a ± 0.08 | 56, 41, 69 |
| 8 | Decanal | 0.53 c ± 0.07 | 0.61 c ± 0.02 | 0.36 d ± 0.01 | 0.79 b ± 0.08 | 0.91 a ± 0.04 | 41, 43, 57 |
| 9 | (E)-2-Octen-1-ol | 0.75 c ± 0.17 | 1.10 b ± 0.05 | 2.55 a ± 0.02 | 1.19 b ± 0.11 | 0.84 c ± 0.03 | 41, 55, 83 |
| 10 | 3-(Methylthio) propanal | 0.24 e ± 0.02 | 0.76 c ± 0.03 | 0.34 d ± 0.03 | 0.84 b ± 0.05 | 1.25 a ± 0.09 | 48, 104, 76 |
| 11 | n-Decanoic acid | 0.44 b ± 0.01 | 0.50 b ± 0.06 | 0.30 c ± 0.01 | 0.59 a ± 0.03 | 0.64 a ± 0.03 | 60, 73, 55 |
| 12 | (E,E)-2,4-Decadienal | 2.25 c ± 0.30 | 5.23 a ± 0.98 | 2.95 b ± 0.01 | 2.44 c ± 0.28 | 1.25 d ± 0.05 | 81, 41, 67 |
| 13 | Butanoic acid | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 0.08 b ± 0.00 | 0.80 a ± 0.13 | 0.05 c ± 0.01 | 60, 73, 39 |
| 14 | Octanoic acid | 0.23 b ± 0.03 | 0.15 c ± 0.01 | 0.37 a ± 0.02 | 0.40 a ± 0.04 | 0.40 a ± 0.10 | 60, 73, 43 |
| 15 | (E,E)-3,5-Octadien-2-one | 0.38 d ± 0.07 | 0.53 c ± 0.05 | 1.84 a ± 0.13 | 0.74 b ± 0.05 | 0.84 b ± 0.19 | 55, 43, 125 |
| 16 | Phenol | 0.24 a ± 0.08 | 0.18 a ± 0.03 | 0.11 b ± 0.01 | 0.16 a ± 0.01 | 0.17 a ± 0.02 | 68, 40, 55 |
| 17 | 1-Octen-3-ol | 1.94 b ± 0.53 | 4.34 a ± 0.65 | 3.94 a ± 0.09 | 1.69 c ± 0.35 | 2.48 b ± 0.20 | 56, 41, 59 |
| 18 | Trimethyl-pyrazine | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 0.48 b ± 0.03 | 0.48 b ± 0.07 | 0.72 a ± 0.07 | 121, 67, 80 |
| 19 | Nonanoic acid | 0.32 b ± 0.03 | 0.17 d ± 0.01 | 0.40 b ± 0.07 | 0.40 b ± 0.03 | 0.52 a ± 0.03 | 60, 73, 41 |
| 20 | 2-Ethyl-6-methyl- pyrazine | 0.16 c ± 0.01 | 0.16 c ± 0.02 | 0.94 b ± 0.09 | 1.00 b ± 0.12 | 1.28 a ± 0.20 | 42, 108, 39 |
| 21 | Ethyl myristate | 2.20 a ± 0.36 | 1.30 b ± 0.33 | 0.98 b ± 0.02 | 1.19 b ± 0.15 | 1.13 b ± 0.13 | 77, 106, 51 |
| 22 | Hexanoic acid | 0.46 e ± 0.17 | 2.56 b ± 0.05 | 2.24 c ± 0.07 | 2.89 a ± 0.17 | 1.56 d ± 0.17 | 60, 73, 41 |
| 23 | 2-Ethyl-3,5-dimethylpyrazine | 0.59 c ± 0.03 | 0.50 d ± 0.03 | 1.12 a ± 0.03 | 0.87 b ± 0.32 | 0.85 b ± 0.07 | 135, 56, 39 |
| 24 | Hexanal | 0.45 a ± 0.09 | 0.36 b ± 0.01 | 0.38 b ± 0.02 | 0.31 c ± 0.01 | 0.51 a ± 0.03 | 44, 56, 41 |
| 25 | p-Cresol | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 0.00 c ± 0.00 | 0.67 a ± 0.03 | 0.28 b ± 0.02 | 43, 57, 128 |
| 26 | (E)-2-Nonenal | 0.68 d ± 0.10 | 1.66 b ± 0.22 | 1.31 b ± 0.15 | 2.97 a ± 0.32 | 1.19 c ± 0.03 | 41, 55, 70 |
| 27 | Heptanal | 0.57 c ± 0.08 | 0.57 c ± 0.07 | 1.38 b ± 0.04 | 1.44 b ± 0.45 | 1.84 a ± 0.17 | 70, 55, 44 |
| 28 | Heptanoic acid | 0.06 b ± 0.00 | 0.06 b ± 0.00 | 0.07 b ± 0.02 | 0.07 b ± 0.02 | 0.11 a ± 0.01 | 60, 73, 87 |
| 29 | Benzene acetaldehyde | 5.57 a ± 0.87 | 2.97 b ± 0.83 | 1.83 c ± 0.07 | 5.62 a ± 1.33 | 4.09 a ± 0.87 | 91, 120, 65 |
| 30 | Nonanal | 4.95 c ± 0.93 | 4.65 c ± 0.75 | 8.37 a ± 0.24 | 4.49 c ± 0.21 | 6.90 b ± 0.69 | 41, 57, 70 |
| No. | Aroma-Active Compounds | RI a | Odor Threshold in Water (µg/kg) b | ROAV | ||||
|---|---|---|---|---|---|---|---|---|
| MRPs A | MRPs B | MRPs C | MRPs D | MRPs E | ||||
| 1 | (E,E)-3,5-Octadien-2-one | 1068 | 0.1 | 0.007 | 0.010 | 0.016 | 0.009 | 0.010 |
| 2 | Hexanal | 1087 | 0.0011 | 0.687 | 0.644 | 0.309 | 0.32 | 0.548 |
| 3 | Heptanal | 1182 | 0.0009 | 1.066 | 1.252 | 1.369 | 1.848 | 2.394 |
| 4 | 2-Pentyl- furan | 1235 | 0.019 | 0.244 | 0.153 | 0.156 | 0.046 | 0.113 |
| 5 | p-Cresol | 1251 | 0.0084 | 0 | 0 | 0 | 0.092 | 0.039 |
| 6 | Octanal | 1291 | 0.0004 | 3.48 | 6.801 | 3.929 | 5.023 | 6.876 |
| 7 | Ethyl myristate | 1322 | 0.18 | 0.021 | 0.014 | 0.005 | 0.008 | 0.007 |
| 8 | 2-Ethyl-3,5-dimethylpyrazine | 1346 | 0.00001 | 100 | 100 | 100 | 100 | 100 |
| 9 | 2-Ethyl-6-methyl-pyrazine | 1371 | 0.04 | 0.007 | 0.008 | 0.021 | 0.029 | 0.037 |
| 10 | Nonanal | 1395 | 0.0026 | 3.234 | 3.545 | 2.874 | 1.992 | 3.113 |
| 11 | Trimethyl-pyrazine | 1402 | 0.033 | 0 | 0 | 0.013 | 0.017 | 0.026 |
| 12 | (E)-2-Octenal | 1426 | 0.0027 | 1.359 | 3.286 | 2.11 | 1.475 | 2.013 |
| 13 | 1-Octen-3-ol | 1438 | 0.0027 | 1.222 | 3.191 | 1.303 | 0.723 | 1.076 |
| 14 | 3-(Methylthio)propanal | 1454 | 0.0014 | 0.298 | 1.083 | 0.214 | 0.69 | 1.047 |
| 15 | Decanal | 1497 | 0.0026 | 0.352 | 0.466 | 0.124 | 0.35 | 0.409 |
| 16 | (E)-2-Nonenal | 1531 | 0.00009 | 12.884 | 36.53 | 12.996 | 38.106 | 15.54 |
| 17 | (E)-2-Octen-1-ol | 1544 | 0.04 | 0.032 | 0.055 | 0.057 | 0.034 | 0.025 |
| 18 | 1-Octanol | 1559 | 0.022 | 0.032 | 0.056 | 0.029 | 0.049 | 0.048 |
| 19 | Butanoic acid | 1631 | 0.004 | 0 | 0 | 0.017 | 0.23 | 0.015 |
| 20 | Benzene acetaldehyde | 1643 | 0.0017 | 5.564 | 3.462 | 0.96 | 3.818 | 2.818 |
| 21 | (E)-2-Decenal | 1654 | 0.0027 | 0.457 | 1.162 | 0.536 | 0.636 | 0.644 |
| 22 | (E,E)-2,4-Nonadienal | 1778 | 0.0002 | 3.285 | 11.567 | 2.277 | 1.807 | 0.686 |
| 23 | (E)-2-Undecenal | 1861 | 0.044 | 0.029 | 0.066 | 0.026 | 0.039 | 0.036 |
| 24 | (E,E)-2,4-Decadienal | 2001 | 0.0023 | 1.665 | 4.514 | 1.145 | 1.227 | 0.638 |
| 25 | Phenol | 2020 | 0.046 | 0.009 | 0.008 | 0.002 | 0.004 | 0.004 |
| 26 | Hexanoic acid | 2050 | 0.04 | 0.02 | 0.127 | 0.05 | 0.083 | 0.046 |
| 27 | Heptanoic acid | 2130 | 0.022 | 0.005 | 0.006 | 0.003 | 0.004 | 0.006 |
| 28 | Octanoic acid | 2264 | 0.0051 | 0.077 | 0.058 | 0.065 | 0.091 | 0.092 |
| 29 | n-Decanoic acid | 2276 | 0.05 | 0.015 | 0.02 | 0.005 | 0.014 | 0.015 |
| 30 | Nonanoic acid | 2370 | 0.02 | 0.027 | 0.017 | 0.018 | 0.023 | 0.031 |
| Aroma-Active Compounds | Relative Content | Error | |||||
|---|---|---|---|---|---|---|---|
| Actual Measured | PCF | CSI | CPM | PCF | CSI | CPM | |
| (E)-2-Octenal | 1.682 | 2.263 | 1.895 | 1.638 | 0.581 | 0.213 | 0.044 |
| Octanal | 3.972 | 5.361 | 3.971 | 3.972 | 1.389 | 0.001 | 0 |
| (E,E)-2,4-Nonadienal | 1.032 | 4.265 | −21.173 | 0.971 | 3.233 | 22.205 | 0.061 |
| (E,E)-2,4-Decadienal | 0.843 | 1.966 | −6.910 | 0.803 | 1.123 | 7.753 | 0.040 |
| 1-Octen-3-ol | 0.826 | 1.568 | −3.742 | 0.791 | 0.742 | 4.568 | 0.035 |
| (E)-2-Nonenal | 20.535 | 28.317 | −45.821 | 22.492 | 7.782 | 66.356 | 1.957 |
| Heptanal | 1.576 | 1.646 | 1.394 | 1.575 | 0.070 | 0.182 | 0.001 |
| Benzene acetaldehyde | 1.994 | 2.254 | 0.042 | 2.061 | 0.260 | 1.952 | 0.067 |
| Nonanal | 2.295 | 2.722 | 1.421 | 2.344 | 0.427 | 0.874 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Wang, Y.; Zhang, H.; Li, J.; Wang, Q.; Yang, L.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Process Modelling and Simulation of Key Volatile Compounds of Maillard Reaction Products Derived from Beef Tallow Residue Hydrolysate Based on Proxy Models. Foods 2022, 11, 2962. https://doi.org/10.3390/foods11192962
Cui J, Wang Y, Zhang H, Li J, Wang Q, Yang L, Zhang H, Jin Q, Wu G, Wang X. Process Modelling and Simulation of Key Volatile Compounds of Maillard Reaction Products Derived from Beef Tallow Residue Hydrolysate Based on Proxy Models. Foods. 2022; 11(19):2962. https://doi.org/10.3390/foods11192962
Chicago/Turabian StyleCui, Jingwei, Yinhan Wang, Huihuang Zhang, Jiulin Li, Qiaojun Wang, Lixue Yang, Hui Zhang, Qingzhe Jin, Gangcheng Wu, and Xingguo Wang. 2022. "Process Modelling and Simulation of Key Volatile Compounds of Maillard Reaction Products Derived from Beef Tallow Residue Hydrolysate Based on Proxy Models" Foods 11, no. 19: 2962. https://doi.org/10.3390/foods11192962
APA StyleCui, J., Wang, Y., Zhang, H., Li, J., Wang, Q., Yang, L., Zhang, H., Jin, Q., Wu, G., & Wang, X. (2022). Process Modelling and Simulation of Key Volatile Compounds of Maillard Reaction Products Derived from Beef Tallow Residue Hydrolysate Based on Proxy Models. Foods, 11(19), 2962. https://doi.org/10.3390/foods11192962







