Analysis of Water Distribution and Muscle Quality of Silver Carp (Hypophthalmichthys molitrix) Chunks Based on Electron-Beam Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Processing
2.3. Determination of Water Distribution
2.4. Determination of Moisture Content and Expressible Moisture Content (EMC)
2.5. Determination of Whiteness and pH Values
2.6. Determination of AVs, Peroxide Values (POVs), and TBA Values
2.7. Determination of Total Protein Content, Myofibrillar Protein Content, and Actomyosin Content
2.8. Data Analysis
3. Results and Discussion
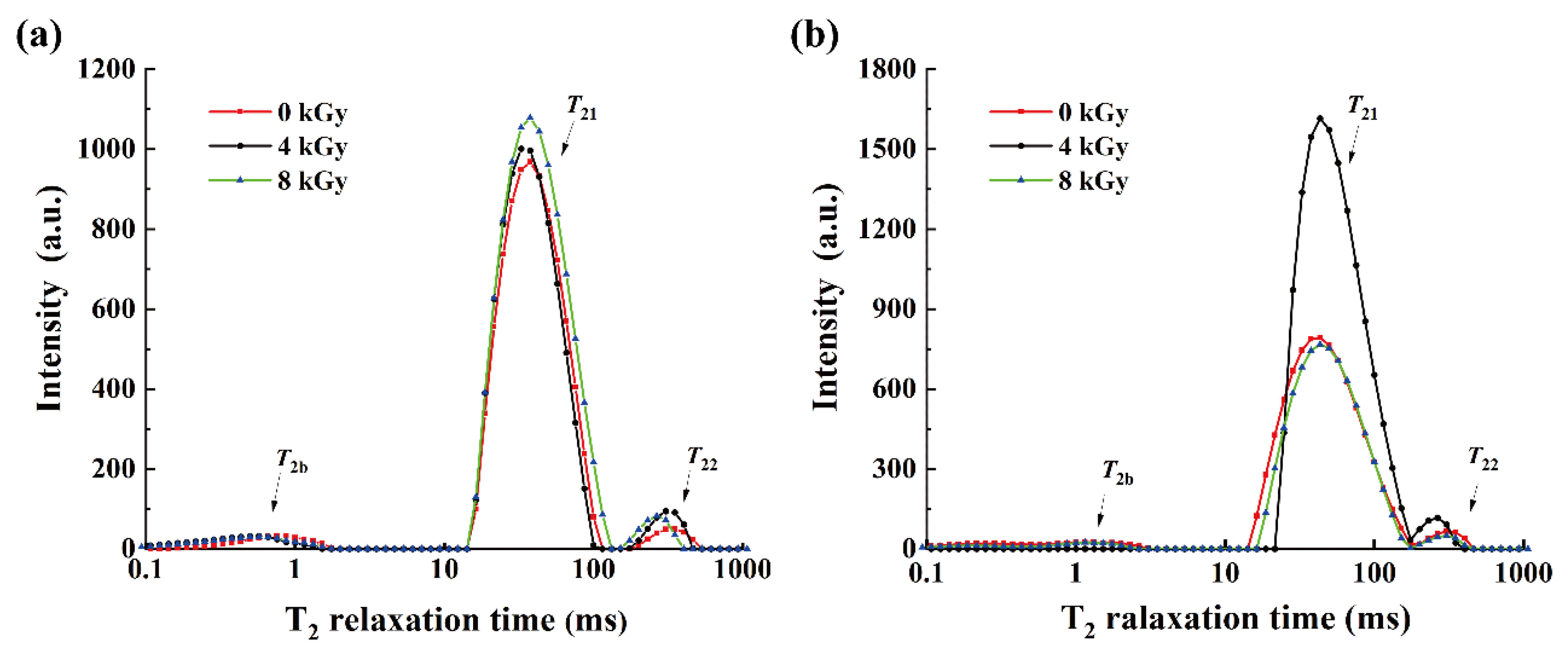
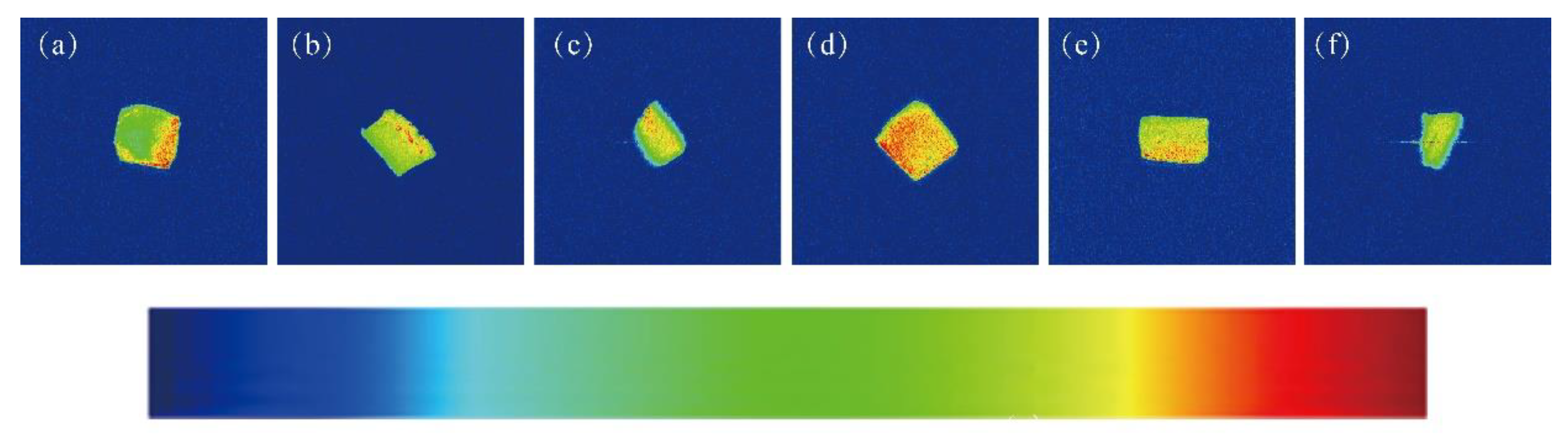
3.1. Effects of Different EBI Doses on the Water Distribution in SCCs
3.2. Effects of Different EBI Doses on the Moisture Content and EMC Loss of SCCs
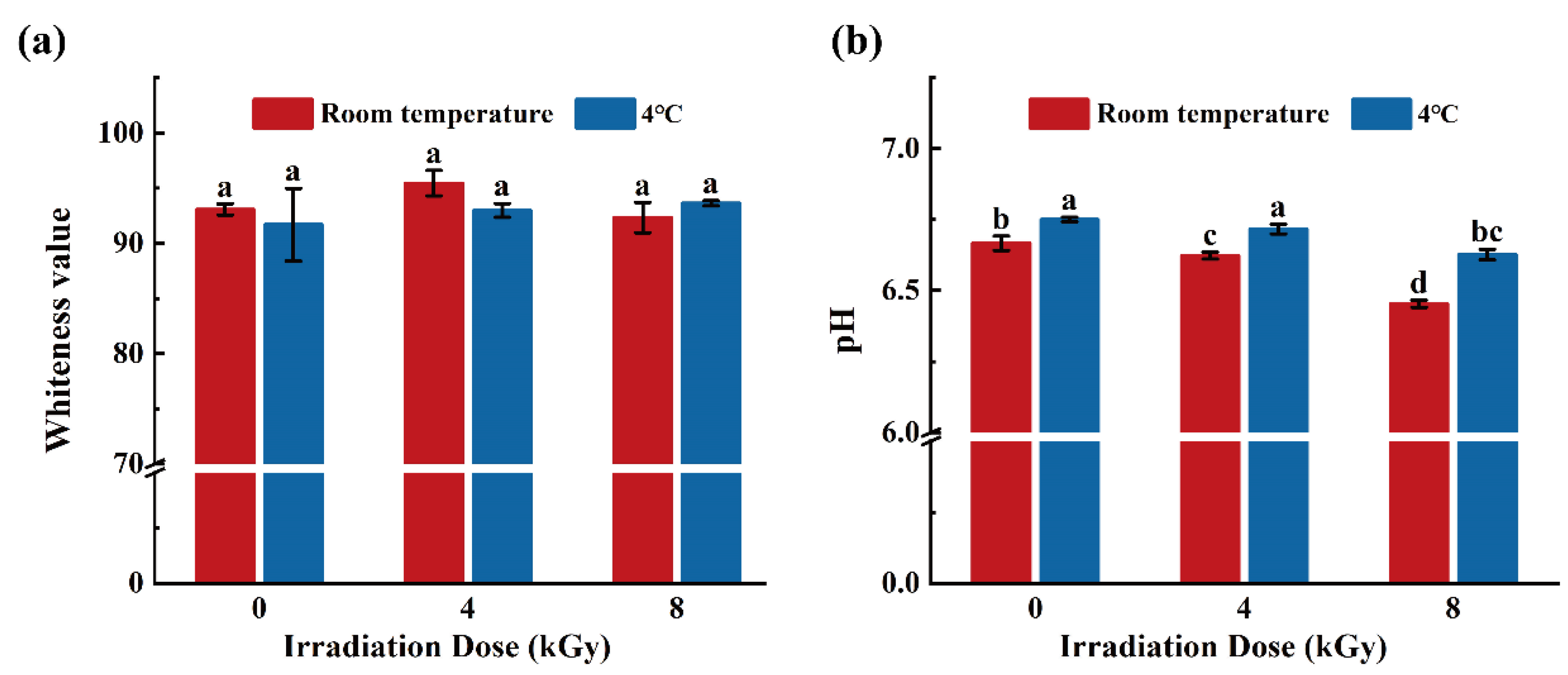
3.3. Effects of Different EBI Doses on the Whiteness and pH Values of SCCs
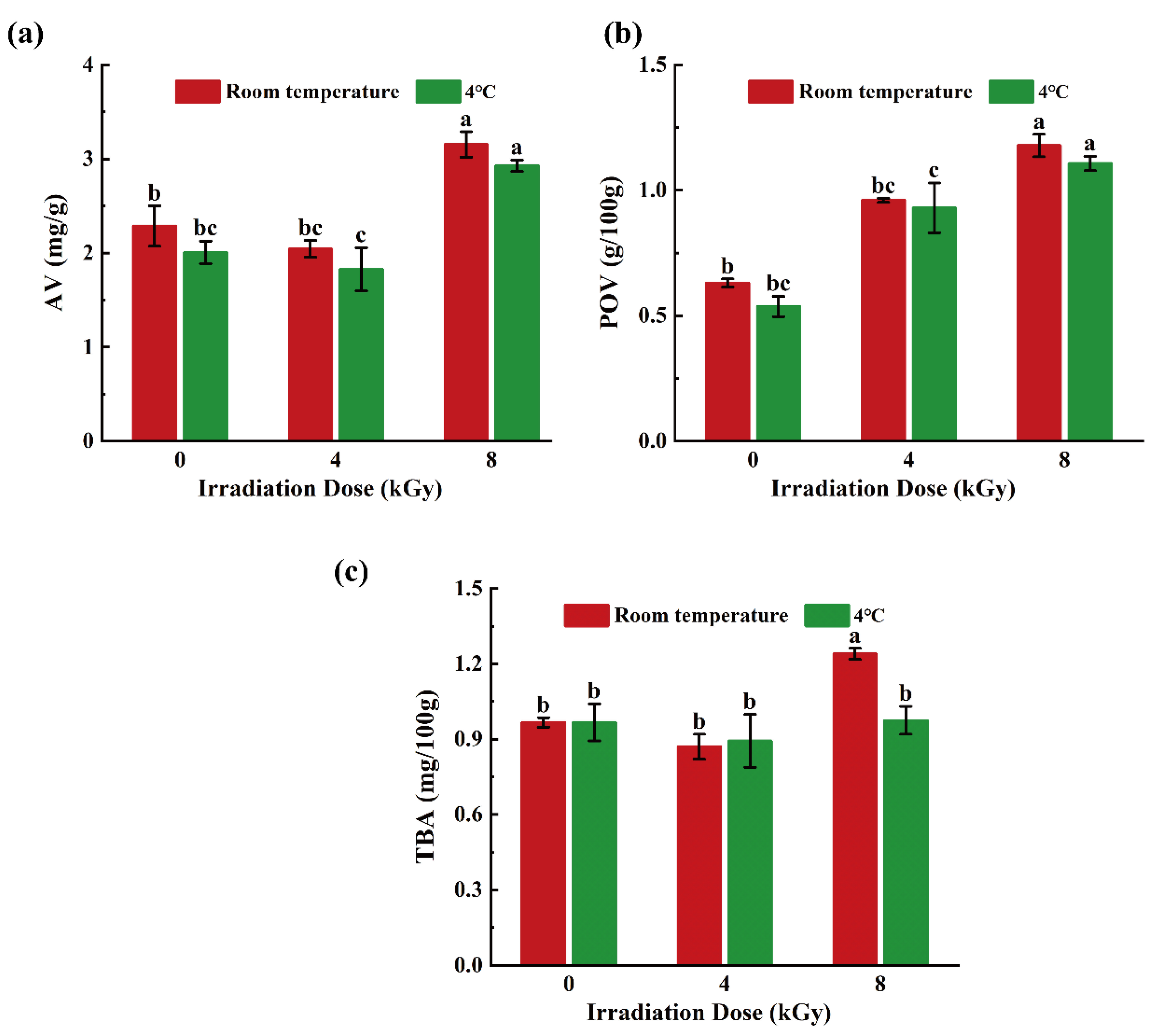
3.4. Effects of Different EBI Doses on AVs, POVs, and TBA Values of SCCs
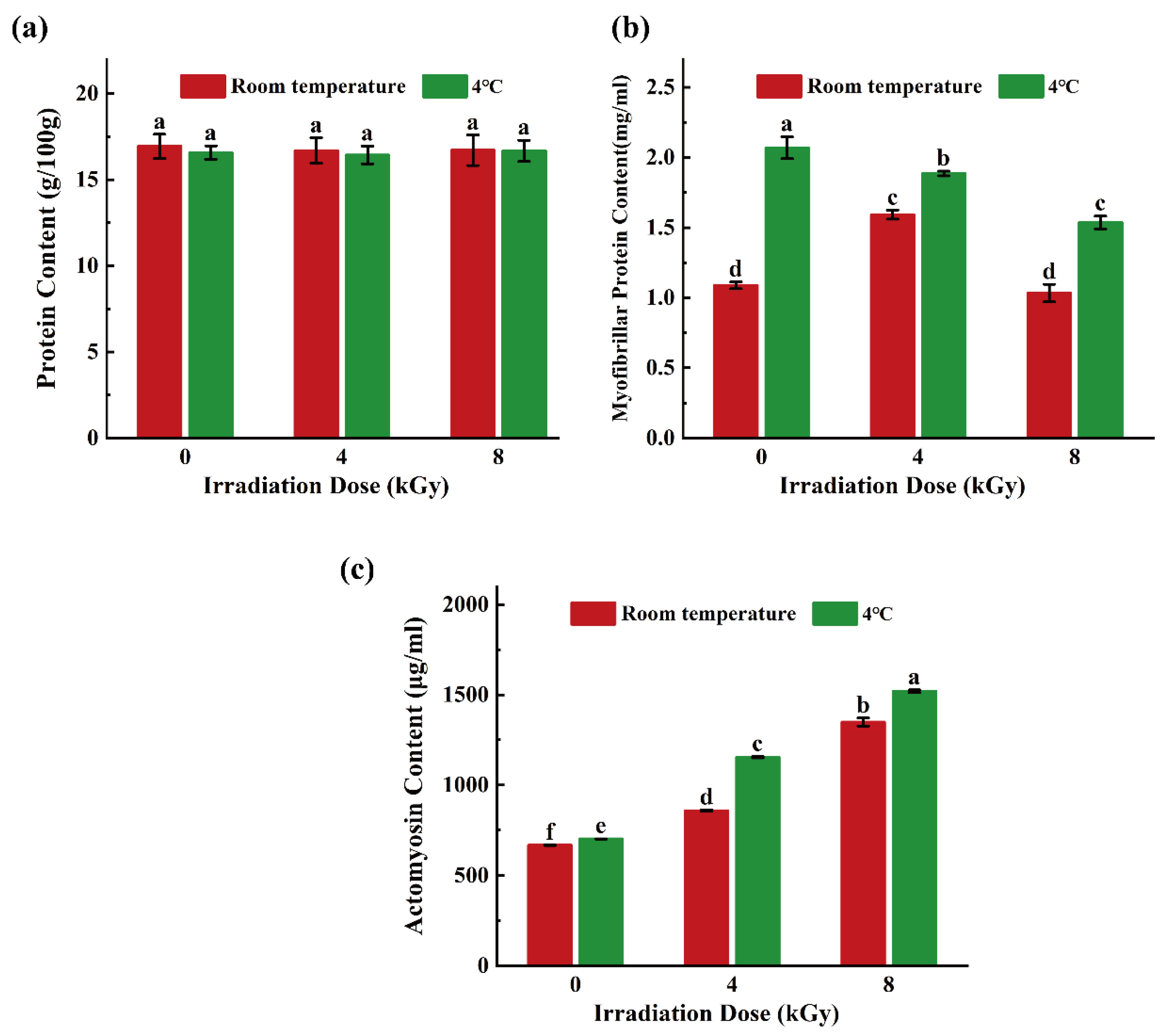
3.5. Effects of Different EBI Doses on Total Protein Content, Myofibrillar Protein Content, and Actomyosin Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Majdoubi, F.-Z.; Ouizgane, A.; Farid, S.; Mossetti, L.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Fry Survival Rate as a Predictive Marker of Optimal Production of Silver Carp (Hypophthalmichthys molitrix, Valenciennes 1844): A Biostatistical Study in Deroua Fish Farm, Morocco. Proc. Zool. Soc. 2021, 75, 152–160. [Google Scholar] [CrossRef]
- Li, D.P.; Li, Q.; Zhang, Y.M.; Liu, X.C.; Hong, H.; Luo, Y.K. Quality changes and microbiological spoilage analysis of air-packed and vacuum-packed silver carp (Hypophthalmichthys molitrix) fillets during chilled storage. J. Food Process. Preserv. 2018, 42, e13389. [Google Scholar] [CrossRef]
- DeWitt, C.A.; Oliveira, A.C. Modified Atmosphere Systems and Shelf Life Extension of Fish and Fishery Products. Foods 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Xia, W.; Jiang, Q. Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. Eur. Food Res. Technol. 2014, 238, 753–761. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, Q.; Yu, D.; Xu, Y.; Gao, P.; Xia, W. Quality of giant freshwater prawn (Macrobrachium rosenbergii) during the storage at −18 °C as affected by different methods of freezing. Int. J. Food Prop. 2018, 21, 2100–2109. [Google Scholar] [CrossRef]
- Annamalai, J.; Sivam, V.; Unnikrishnan, P.; Kuppa Sivasankara, S.; Kaushlesh Pansingh, R.; Shaik Abdul, K.; Lakshmi, N.M.; Chandragiri Nagarajarao, R. Effect of electron beam irradiation on the biochemical, microbiological and sensory quality of Litopenaeus vannamei during chilled storage. J. Food Sci. Technol. 2020, 57, 2150–2158. [Google Scholar] [CrossRef]
- Fernandes, A.; Antonio, A.L.; Oliveira, M.B.; Martins, A.; Ferreira, I.C. Effect of gamma and electron beam irradiation on the physico-chemical and nutritional properties of mushrooms: A review. Food Chem. 2012, 135, 641–650. [Google Scholar] [CrossRef]
- Dong, S.; Guo, J.; Yu, J.; Bai, J.; Xu, H.; Li, M. Effects of electron-beam generated X-ray irradiation on the postharvest storage quality of Agaricus bisporus. Innov. Food Sci. Emerg. Technol. 2022, 80, 103079. [Google Scholar] [CrossRef]
- Aguirre, J.; Rodríguez, M.R.; González, R.; García de Fernando, G. E-beam irradiation affects the maximum specific growth rate ofBacillus cereus. Int. J. Food Sci. Technol. 2013, 48, 382–386. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Wholesomeness and safety aspects of irradiated foods. Food Chem. 2019, 285, 363–368. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, J.; Li, H.; Zhao, Y.; Xia, S.; Qiu, Y.; Zhu, J. Effects of E-beam irradiation on the physicochemical properties of Atlantic cod (Gadus morhua). Food Biosci. 2022, 101803. [Google Scholar] [CrossRef]
- Guo, H.; Feng, T.; Qi, W.; Kong, Q.; Yue, L.; Wang, H. Effects of electron-beam irradiation on volatile flavor compounds of salmon fillets by the molecular sensory science technique. J. Food Sci. 2021, 86, 184–193. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, H.; Qian, C.; Shao, H.; Han, J.; Li, Y.; Lou, Y. Determination of the optimal electron beam irradiation dose for treating shrimp (Solenocera melantho) by means of physical and chemical properties and bacterial communities. Lwt 2022, 153, 112539. [Google Scholar] [CrossRef]
- Luo, J.; Li, M.; Zhang, Y.; Zheng, M.; Ming Ling, C. The low-field NMR studies the change in cellular water in tilapia fillet tissue during different drying conditions. Food Sci. Nutr. 2021, 9, 2644–2657. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, T.; Yao, L.; Wang, X.; Song, Y.; Wang, H.; Wang, H.; Tan, M. Use of low-field-NMR and MRI to characterize water mobility and distribution in pacific oyster (Crassostrea gigas) during drying process. Dry. Technol. 2017, 36, 630–636. [Google Scholar] [CrossRef]
- Sanchez-Alonso, I.; Martinez, I.; Sanchez-Valencia, J.; Careche, M. Estimation of freezing storage time and quality changes in hake (Merluccius merluccius, L.) by low field NMR. Food Chem. 2012, 135, 1626–1634. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Qing, C.; Chen, L.; Gao, P.; Huang, M. Impacts of electron-beam-irradiation on microstructure and physical properties of yam (Dioscorea opposita Thunb.) flour. LWT 2022, 163, 113531. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Ma, J.; Yang, K.; Feng, X.; You, X.; Wang, S.; Zhang, Y.; Xiong, G.; Wang, L.; et al. Effects of radio frequency heating on water distribution and structural properties of grass carp myofibrillar protein gel. Food Chem. 2021, 343, 128557. [Google Scholar] [CrossRef]
- Al-Habsi, N.A.; Al-Hadhrami, S.; Al-Kasbi, H.; Rahman, M.S. Molecular mobility of fish flesh measured by low-field nuclear magnetic resonance (LF-NMR) relaxation: Effects of freeze–thaw cycles. Fish. Sci. 2017, 83, 845–851. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Yang, C. Effect of low-temperature vacuum frying assisted by microwave on the property of fish fillets (Aristichthys nobilis). J. Food Process Eng. 2019, 42, e13050. [Google Scholar] [CrossRef]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, F.; Wei, L.; Lin, H.; Jiang, J.; Hui, G. Jumbo squid (Dosidicus gigas) quality enhancement using complex bio-preservative during cold storage. J. Food Meas. Charact. 2017, 12, 78–86. [Google Scholar] [CrossRef]
- Gulcan, U.; Candal Uslu, C.; Mutlu, C.; Arslan-Tontul, S.; Erbas, M. Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Pourashouri, P.; Salaun, F.; Quek, S.Y. Shelf-life and quality of chicken nuggets fortified with encapsulated fish oil and garlic essential oil during refrigerated storage. J. Food Sci. Technol. 2021, 58, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Mahmoud, A.A.; Ramadan, M.F.; Roby, M.H.H. Biochemical and microbiological characteristics of some Mediterranean salted fish products. Rend. Lincei Sci. Fis. E Nat. 2021, 32, 343–355. [Google Scholar] [CrossRef]
- Wei, D.; Li, L.; He, S.; Yu, J.; Tian, X.; Wu, Z. Improving the lipid oxidation in pork fat processing for Chi-aroma Baijiu through pretreatments and segmented soaking with liquor. LWT-Food Sci. Technol. 2020, 130, 109624. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Chen, X.; Liu, Y.; Wang, J.; Wang, X.; Wang, C.; Song, H. Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 2020, 162, 838–844. [Google Scholar] [CrossRef]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, L.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Comparison of silver carp fin gelatins extracted by three types of methods: Molecular characteristics, structure, function, and pickering emulsion stabilization. Food Chem. 2022, 368, 130818. [Google Scholar] [CrossRef]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Zhou, A.; Lin, L.; Liang, Y.; Benjakul, S.; Shi, X.; Liu, X. Physicochemical properties of natural actomyosin from threadfin bream (Nemipterus spp.) induced by high hydrostatic pressure. Food Chem. 2014, 156, 402–407. [Google Scholar] [CrossRef]
- Zhai, Y.; Pan, L.; Luo, X.; Zhang, Y.; Wang, R.; Chen, Z. Effect of electron beam irradiation on storage, moisture and eating properties of high-moisture rice during storage. J. Cereal Sci. 2022, 103, 103407. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Wang, W.; Qi, W.; Yue, L.; Ye, Q. Effect of 10 MeV E-beam irradiation combined with vacuum-packaging on the shelf life of Atlantic salmon fillets during storage at 4 degrees C. Food Chem. 2014, 145, 535–541. [Google Scholar] [CrossRef]
- Zheng, M.J.; Liu, X.; Chuai, P.J.; Jiang, Z.D.; Zhu, Y.B.; Zhang, B.; Ni, H.; Li, Q.B. Effects of crude fucoidan on physicochemical properties, antioxidation and bacteriostasis of surimi products. Food Control 2021, 122, 107806. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, W.; Zhang, S.F.; Wang, H.Y.; Ye, Q.F. Influence of 10-MeV E-Beam Irradiation and Vacuum Packaging on the Shelf-Life of Grass Carp Surimi. Food Bioprocess Technol. 2016, 9, 830–838. [Google Scholar] [CrossRef]
- Ucar, Y.; Özogul, Y.; Özogul, F.; Durmuş, M.; Köşker, A.R. Effect of nisin on the shelf life of sea bass (Dicentrarchus labrax L.) fillets stored at chilled temperature (4 ± 2 °C). Aquac. Int. 2020, 28, 851–863. [Google Scholar] [CrossRef]
- Ham, Y.-K.; Kim, H.-W.; Hwang, K.-E.; Song, D.-H.; Kim, Y.-J.; Choi, Y.-S.; Song, B.-S.; Park, J.-H.; Kim, C.-J. Effects of irradiation source and dose level on quality characteristics of processed meat products. Radiat. Phys. Chem. 2017, 130, 259–264. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xie, J.; Chen, X.J. Differences in lipid composition of Bigeye tuna (Thunnus obesus) during storage at 0 °C and 4 °C. Food Res. Int. 2021, 143, 110233. [Google Scholar] [CrossRef]
- An, K.A.; Arshad, M.S.; Jo, Y.; Chung, N.; Kwon, J.H. E-Beam Irradiation for Improving the Microbiological Quality of Smoked Duck Meat with Minimum Effects on Physicochemical Properties During Storage. J. Food Sci. 2017, 82, 865–872. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Tajik, H.; Rohani, S.M.R.; Moradi, M.; Hashemi, M.; Aliakbarlu, J. Effect of functional chitosan coating and gamma irradiation on the shelf-life of chicken meat during refrigerated storage. Radiat. Phys. Chem. 2017, 141, 103–109. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, M.; Yong, H.I.; Bae, Y.S.; Jung, S.; Jo, C. Synergistic Effects of Electron-beam Irradiation and Leek Extract on the Quality of Pork Jerky during Ambient Storage. Asian-Australas. J. Anim. Sci. 2013, 26, 596–602. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kwon, J.H.; Ahmad, R.S.; Ameer, K.; Ahmad, S.; Jo, Y. Influence of E-beam irradiation on microbiological and physicochemical properties and fatty acid profile of frozen duck meat. Food Sci. Nutr. 2020, 8, 1020–1029. [Google Scholar] [CrossRef]
- Oyekunle, J.A.O.; Ore, O.T.; Durodola, S.S.; Oyinloye, J.A.; Oyebode, B.A.; Ajanaku, O.L. Heavy metal levels and changes in trimethylamine content of smoked fish and meat under different storage conditions. Sn Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Fallah, A.A.; Tajik, H.; Farshid, A.A. Chemical Quality, Sensory Attributes and Ultrastructural Changes of Gamma-Irradiated Camel Meat. J. Muscle Foods 2010, 21, 597–613. [Google Scholar] [CrossRef]
- Zhao, N.N.; Yang, X.Q.; Li, Y.J.; Wu, H.H.; Chen, Y.P.; Gao, R.C.; Xiao, F.; Bai, F.; Wang, J.L.; Liu, Z.Y.; et al. Effects of protein oxidation, cathepsins, and various freezing temperatures on the quality of superchilled sturgeon fillets. Mar. Life Sci. Technol. 2022, 4, 117–126. [Google Scholar] [CrossRef]
- Fan, X.; Konno, K.; Lin, X.; Yu, X.; Liu, Y.; Dong, X. The effect of fish freshness on myosin denaturation in flounder Paralichthys olivaceus muscle during frozen storage. Fish. Sci. 2020, 86, 1111–1120. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-L.; Li, M.-J.; Zhao, Q.; Huang, J.-J.; Zu, X.-Y. Analysis of Water Distribution and Muscle Quality of Silver Carp (Hypophthalmichthys molitrix) Chunks Based on Electron-Beam Irradiation. Foods 2022, 11, 2963. https://doi.org/10.3390/foods11192963
Li H-L, Li M-J, Zhao Q, Huang J-J, Zu X-Y. Analysis of Water Distribution and Muscle Quality of Silver Carp (Hypophthalmichthys molitrix) Chunks Based on Electron-Beam Irradiation. Foods. 2022; 11(19):2963. https://doi.org/10.3390/foods11192963
Chicago/Turabian StyleLi, Hai-Lan, Mei-Jin Li, Qing Zhao, Jia-Jun Huang, and Xiao-Yan Zu. 2022. "Analysis of Water Distribution and Muscle Quality of Silver Carp (Hypophthalmichthys molitrix) Chunks Based on Electron-Beam Irradiation" Foods 11, no. 19: 2963. https://doi.org/10.3390/foods11192963
APA StyleLi, H.-L., Li, M.-J., Zhao, Q., Huang, J.-J., & Zu, X.-Y. (2022). Analysis of Water Distribution and Muscle Quality of Silver Carp (Hypophthalmichthys molitrix) Chunks Based on Electron-Beam Irradiation. Foods, 11(19), 2963. https://doi.org/10.3390/foods11192963





