Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders
Abstract
1. Introduction
2. Relationship between Dietary Polysaccharides and Gut Microbiota
2.1. Dietary Polysaccharide
2.2. Metabolism of Dietary Polysaccharides by Gut Microbiota
2.3. The Regulatory Effect of Dietary Polysaccharides on the Structure of Intestinal Flora
3. The Role of Gut Microbiota in Metabolic Disorders
3.1. Metabolic Disorders and Metabolic Diseases
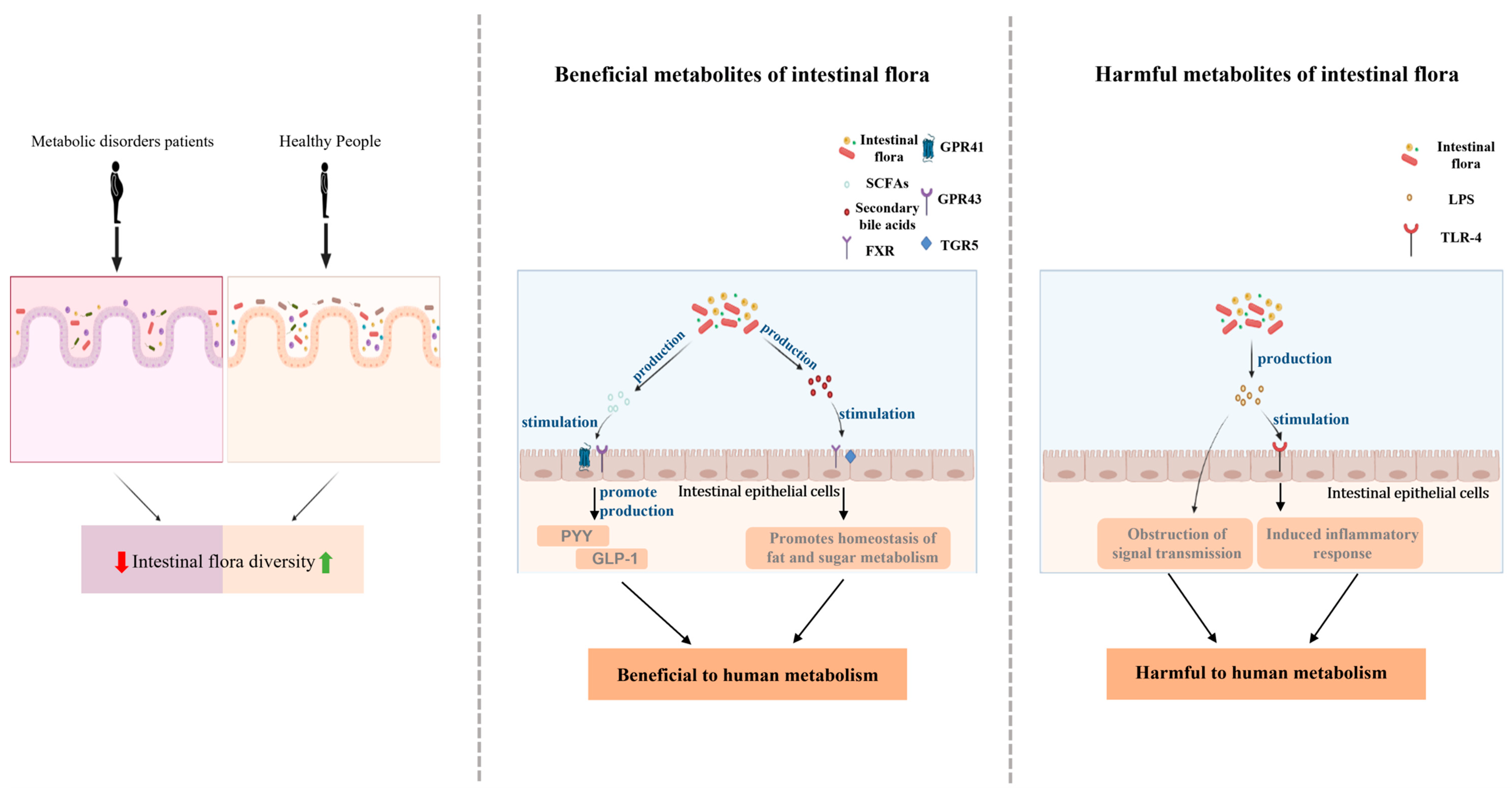
3.2. The Strong Link between Intestinal Flora and Metabolic Disorders
4. Dietary Intervention Modulates Gut Microbiota to Prevent and Treat Metabolic Diseases
4.1. Treatment and Prevention of Type 2 Diabetes
4.2. Treatment and Prevention of Hyperlipidemia
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.F.A.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, 2179. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut microbiota markers in obese adolescent and adult patients: Age-dependent differential patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex differences in gut microbiota. World J. Mens Health 2019, 38, 48–60. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Y.; Wang, R.; An, Y.; Gao, W.; Bai, L.; Li, Y.; Zhao, S.; Fan, J.; Liu, E. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis. 2018, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of mediterranean diet on human gut microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espin, J.C.; Margolles, A.; Azpiroz, F. Differential effects of western and mediterranean-type diets on gut microbiota: A metagenomics and metabolomics approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Shi, Z. Gut microbiota: An important link between western diet and chronic diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Li, Z.R.; Jia, R.B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (ziziphus jujuba mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, B.; Zheng, N.; Wu, G.; Ma, J.; Tao, X.; Chen, L.; Zhong, J.; Sheng, L.; Li, H. Integrated metagenomic and metabolomic analyses of the effect of Astragalus polysaccharides on alleviating high-fat diet-induced metabolic disorders. Front. Pharmacol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes. Control Ther. 2017, 4. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Beverly, J.K.; Budoff, M.J. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 2020, 12, 102–104. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Isabella, V.M.; Ha, B.N.; Castillo, M.J.; Lubkowicz, D.J.; Rowe, S.E.; Millet, Y.A.; Anderson, C.L.; Li, N.; Fisher, A.B.; West, K.A.; et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 2018, 36, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahluwalia, T.S. Editorial: The role of genetic and lifestyle factors in metabolic diseases. Front. Endocrinol. 2019, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2016, 15, 43. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef]
- Cui, S.W. Structural analysis of polysaccharides. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005; p. 432. [Google Scholar]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.L.; Kang, C.G.; Park, K.H.; Shim, J.Y.; Kim, Y.R. Development of reduced-fat mayonnaise using 4αGTase-modified rice starch and xanthan gum. Int. J. Biol. Macromol. 2009, 44, 400–407. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef]
- Gudi, R.; Suber, J.; Brown, R.; Johnson, B.M.; Vasu, C. Pretreatment with yeast-derived complex dietary polysaccharides suppresses gut inflammation, alters the microbiota composition, and increases immune regulatory short-chain fatty acid production in C57BL/6 mice. J. Nutr. 2020, 150, 1291–1302. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. Modeling of in vitro digestogram by consecutive reaction kinetics model reveals the nature of starch digestive characteristics. Food Hydrocoll. 2022, 124, 107203. [Google Scholar] [CrossRef]
- Hernandez-Maldonado, L.M.; Blancas-Benitez, F.J.; Zamora-Gasga, V.M.; Cardenas-Castro, A.P.; Tovar, J.; Sayago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of high dietary fiber and antioxidant-rich mango (Mangifera indica L.) “Ataulfo”-based fruit bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef] [PubMed]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Pietilä, S.; Toivonen, R.; Rokka, A.; Mokkala, K.; Laitinen, K.; Elo, L.; Hänninen, A. A carbohydrate-active enzyme (CAZy) profile links successful metabolic specialization of prevotella to its abundance in gut microbiota. Sci. Rep. 2020, 10, 12411. [Google Scholar] [CrossRef] [PubMed]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Bolognini, D.; Tobin, A.B.; Milligan, G.; Moss, C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol. Pharmacol. 2016, 89, 388–398. [Google Scholar] [CrossRef]
- Lee, J.; d’Aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.; Putluri, N.; et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 2020, 127, 453–465. [Google Scholar] [CrossRef]
- Zhan, K.; Jiang, M.; Gong, X.; Zhao, G. Effect of short-chain fatty acids on the expression of genes involved in short-chain fatty acid transporters and inflammatory response in goat jejunum epithelial cells. In Vitro Cell Dev. Biol. Anim. 2018, 54, 311–320. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Maningi, N.E.; Fourie, P.B. Mycobacterium tuberculosis, antimicrobials, immunity, and lung–gut microbiota crosstalk: Current updates and emerging advances. Ann. N. Y. Acad. Sci. 2020, 1467, 21–47. [Google Scholar] [CrossRef]
- Yao, Y.; Yan, L.; Chen, H.; Wu, N.; Wang, W.; Wang, D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Zhao, F.; Cao, J.; Shen, X.; Li, C. Holothuria leucospilota polysaccharides ameliorate hyperlipidemia in high-fat diet-induced rats via short-chain fatty acids production and lipid metabolism regulation. Int. J. Mol. Sci. 2019, 20, 4738. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Huang, G.; Li, X.A.; Liao, W.; Leong, W.K.; Xia, W.; Bian, X.; Wu, J.; Hsiao, W.L.W. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in ApcMin/+ mice. Pharmacol. Res. 2019, 148, 104448. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-obesity effect of Dictyophora indusiata mushroom polysaccharide (DIP) in high fat diet-induced obesity via regulating inflammatory cascades and intestinal microbiome. Front. Endocrinol. 2020, 11, 558874. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Yin, H.M.; Wang, S.N.; Nie, S.P.; Xie, M.Y. Coix polysaccharides: Gut microbiota regulation and immunomodulatory. Bioact. Carbohydr. Diet. Fibre 2018, 16, 53–61. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2018, 9, 937–950. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan brick tea polysaccharides attenuate metabolic syndrome in high-fat diet induced mice in association with modulation in the gut microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Kharroubi, A.T. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2019, 125, 128–135. [Google Scholar] [CrossRef]
- Balakumar, P.; Babbar, L. Preconditioning the hyperlipidemic myocardium: Fact or fantasy? Cell. Signal. 2012, 24, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Fischer, M.; Strack, C.; Schmitz, G.; Loew, T.; Lahmann, C.; Baessler, A. Successful weight reduction improves left ventricular diastolic function and physical performance in severe obesity. Int. Heart J. 2015, 56, 196–202. [Google Scholar] [CrossRef][Green Version]
- De Miguel-Yanes, J.M.; Shrader, P.; Pencina, M.J.; Fox, C.S.; Manning, A.K.; Grant, R.W.; Dupuis, J.; Florez, J.C.; D’Agostino, R.B., Sr.; Cupples, L.A.; et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care 2011, 34, 121–125. [Google Scholar] [CrossRef]
- Zhu, W. Exercise is medicine for type 2 diabetes: An interview with Dr. Sheri R. Colberg. J. Sport Health Sci. 2022, 11, 179–183. [Google Scholar] [CrossRef]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Method. 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of propolis on diet-induced hyperlipidemia and atherogenic indices in mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Wen, J.; Su, M. A randomized trial of Tai Chi on preventing hypertension and hyperlipidemia in middle-aged and elderly patients. Int. J. Environ. Res. Pub. Health 2021, 18, 5480. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.M.; Li, P.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.; et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, H.B.; Lee, E.; Park, H.Y. The effects of gelatinized wheat starch and high salt diet on gut microbiota and metabolic disorder. Nutrients 2020, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, G.; Li, Q.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitin oligosaccharide modulates gut microbiota and attenuates high-fat-diet-induced metabolic syndrome in mice. Mar. Drugs 2018, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.H.; Smith, D.M.; Arch, J.R.S. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Backhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, 453–463. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, Q.M.; Ni, W.W.; Zhang, X.; Li, Y.; Li, A.L.; Du, P.; Li, C.; Yu, S.S. Modulatory effect of lactobacillus acidophilus KLDS 1.0738 on intestinal short-chain fatty acids metabolism and GPR41/43 expression in beta-lactoglobulin-sensitized mice. Microbiol. Immunol. 2019, 63, 303–315. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Preston, T.; Brignardello, J.; Garcia-Perez, I.; Holmes, E.; Frost, G.S.; Morrison, D.J. The effect of L-rhamnose on intestinal transit time, short chain fatty acids and appetite regulation: A pilot human study using combined 13CO2/H2 breath tests. J. Breath Res. 2018, 12, 046006. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.-K.; Yun, B.-S.; Matsuzaki, K.; Furukawa, M.; Min, H.-K.; Bajaj, J.S.; et al. Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit clostridium difficile: Role of secondary bile acids. Cell Chemical. Biology. 2019, 26, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki-Anzai, S.; Masuda, M.; Levi, M.; Keenan, A.L.; Miyazaki, M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE 2014, 9, e108270. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry extract improves obesity through regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. IScience 2019, 19, 676–690. [Google Scholar] [CrossRef]
- Tveter, K.M.; Villa-Rodriguez, J.A.; Cabales, A.J.; Zhang, L.; Bawagan, F.G.; Duran, R.M.; Roopchand, D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care 2020, 8, e001386. [Google Scholar] [CrossRef]
- Tian, F.; Huang, S.; Xu, W.; Chen, L.; Su, J.; Ni, H.; Feng, X.; Chen, J.; Wang, X.; Huang, Q. Compound K attenuates hyperglycemia by enhancing glucagon-like peptide-1 secretion through activating TGR5 via the remodeling of gut microbiota and bile acid metabolism. J. Ginseng Res. 2022, in press. [CrossRef]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.-H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Doganyigit, Z.; Okan, A.; Kaymak, E.; Pandir, D.; Silici, S. Investigation of protective effects of apilarnil against lipopolysaccharide induced liver injury in rats via TLR 4/ HMGB-1/ NF-kappaB pathway. Biomed. Pharmacother. 2020, 125, 109967. [Google Scholar] [CrossRef]
- Gonzalez, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.B. Thoughts on the progression of type 2 diabetes drug discovery. Expert Opin. Drug Discov. 2015, 10, 107–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapilevich, L.V.; Zakharova, A.N.; Dyakova, E.Y.; Kironenko, T.A.; Milovanova, K.G.; Kalinnikova, J.G.; Chibalin, A.V. Mice experimental model of diabetes mellitus type ii based on high fat diet. Byulleten Sibirskoy Meditsiny 2019, 18, 53–61. [Google Scholar] [CrossRef]
- Yu, S.C.; Zhang, G.Q.; Jin, L.H. A high-sugar diet affects cellular and humoral immune responses in drosophila. Exp. Cell Res. 2018, 368, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Q.; Cao, J.; Xu, Y.; Pei, Z.; Fan, H.; Yuan, Y.; Shen, X.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Liao, B.; Sun, B.; Lin, L.; Zhao, M. Mitigation mechanisms of Hizikia fusifarme polysaccharide consumption on type 2 diabetes in rats. Int. J. Biol. Macromol. 2020, 164, 2659–2670. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Gao, H.; Fan, L.; Chen, H.; Nie, S. Polysaccharide from plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocoll. 2019, 86, 34–42. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Davidson, M.A.; Mattison, D.R.; Azoulay, L.; Krewski, D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: Past, present and future. Crit. Rev. Toxicol. 2018, 48, 52–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, Y.; Tao, Y.; Meng, E.; Tang, J.; Liu, Y.; Li, F. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2020, 159, 107990. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.P.; Joshi, S.G.; Rane, B.N. Development of an experimental diet model in rats to study hyperlipidemia and insulin resistance, markers for coronary heart disease. Indian J. Pharmacol. 2014, 46, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Karam, I.; Yang, M.; Li, Y.J. Induce hyperlipidemia in rats using high fat diet investigating blood lipid and histopathology. J. Hematol. Blood Disord. 2018, 4, 104. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F. Dietary fiber: An opportunity for a global control of hyperlipidemia. Oxid. Med. Cell Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; An, J.; Song, Y.; Lee, C.K.; Kim, K.; Kong, H. Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front. Microbiol. 2019, 10, 1947. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from whole-grain oat alleviated high-fat diet-induced hyperlipidemia via regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.; Qin, R.; Lv, S.; Wang, S. Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef]
- Tong, A.J.; Hu, R.K.; Wu, L.X.; Lv, X.C.; Li, X.; Zhao, L.N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from rosa roxburghii tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Chen, Z.; Cai, Y.; Xiao, C.; Zhou, F.; Sun, B.; Zhao, M. In vitro digestion and fermentation of three polysaccharide fractions from laminaria japonica and their impact on lipid metabolism-associated human gut microbiota. J. Agric. Food Chem. 2019, 67, 7496–7505. [Google Scholar] [CrossRef] [PubMed]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, Y.; Huang, S.; Li, J.; Zhao, K.; Li, X.; Wen, X.; Li, X.A. Fecal microbiota transplantation for ulcerative colitis: A prospective clinical study. BMC Gastroenterol. 2019, 19, 116. [Google Scholar] [CrossRef]
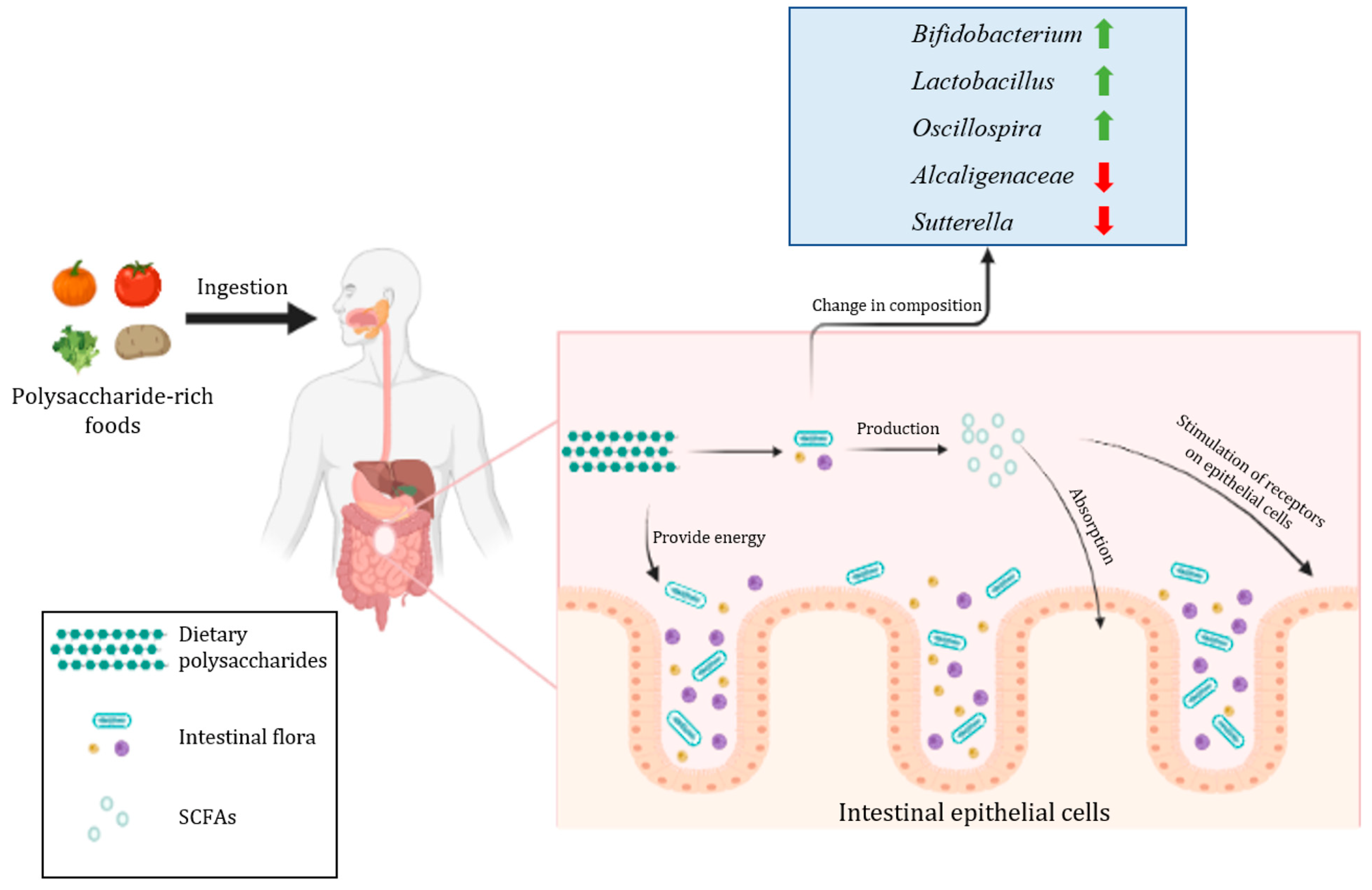
| Polysaccharide Sources | Experimental Model | Affected Intestinal Flora | Experimental Results | Reference |
|---|---|---|---|---|
| Pumpkin | Rats with type 2 diabetes mellitus | The relative abundance of Bacteroidetes, Deltaproteobacteri, and Veillonellaceae increased | Diabetes symptoms have subsided | [106] |
| Tea | The relative abundance of Lachnospira, Victivallis, Roseburia, and Fluviicola was recovered | [107] | ||
| Sea cucumber | The relative abundance of beneficial bacteria increased and that of harmful bacteria decreased | [108] | ||
| Plantago asiatica | The relative abundance of Bacteroides vulgatus, Prevotella loescheii, and Bacteroides vulgates increased | [110] | ||
| Ganoderma lucidum | The relative abundance of harmful bacteria Aerococcus, Ruminococcus decreased and the beneficial bacteria Parabacteroides and Bacteroides increased | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, X.; Zhang, X. Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders. Foods 2022, 11, 2961. https://doi.org/10.3390/foods11192961
Zhang L, Wang X, Zhang X. Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders. Foods. 2022; 11(19):2961. https://doi.org/10.3390/foods11192961
Chicago/Turabian StyleZhang, Li, Xinzhou Wang, and Xin Zhang. 2022. "Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders" Foods 11, no. 19: 2961. https://doi.org/10.3390/foods11192961
APA StyleZhang, L., Wang, X., & Zhang, X. (2022). Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders. Foods, 11(19), 2961. https://doi.org/10.3390/foods11192961







