Characterization of Volatile Compounds by HS-GC-IMS and Chemical Composition Analysis of Colored Highland Barley Roasted at Different Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Roasting Treatment of CHB at Different Temperatures
2.3. Color Parameters Analysis of the RCHB
2.4. Volatile Profiles Analysis of the RCHB
2.4.1. Electronic Nose (E-Nose) Analysis
2.4.2. Headspace-Gas-Chromatography-Ion-Mobility Spectroscopy (HS-GC-IMS) Analysis
2.5. Chemical Composition Analysis of CHB Roasted at Different Temperatures
2.5.1. Total Starch, β-Glucan, and Protein Analysis
2.5.2. Total Polyphenols Analysis
2.5.3. Total Flavonoids Analysis
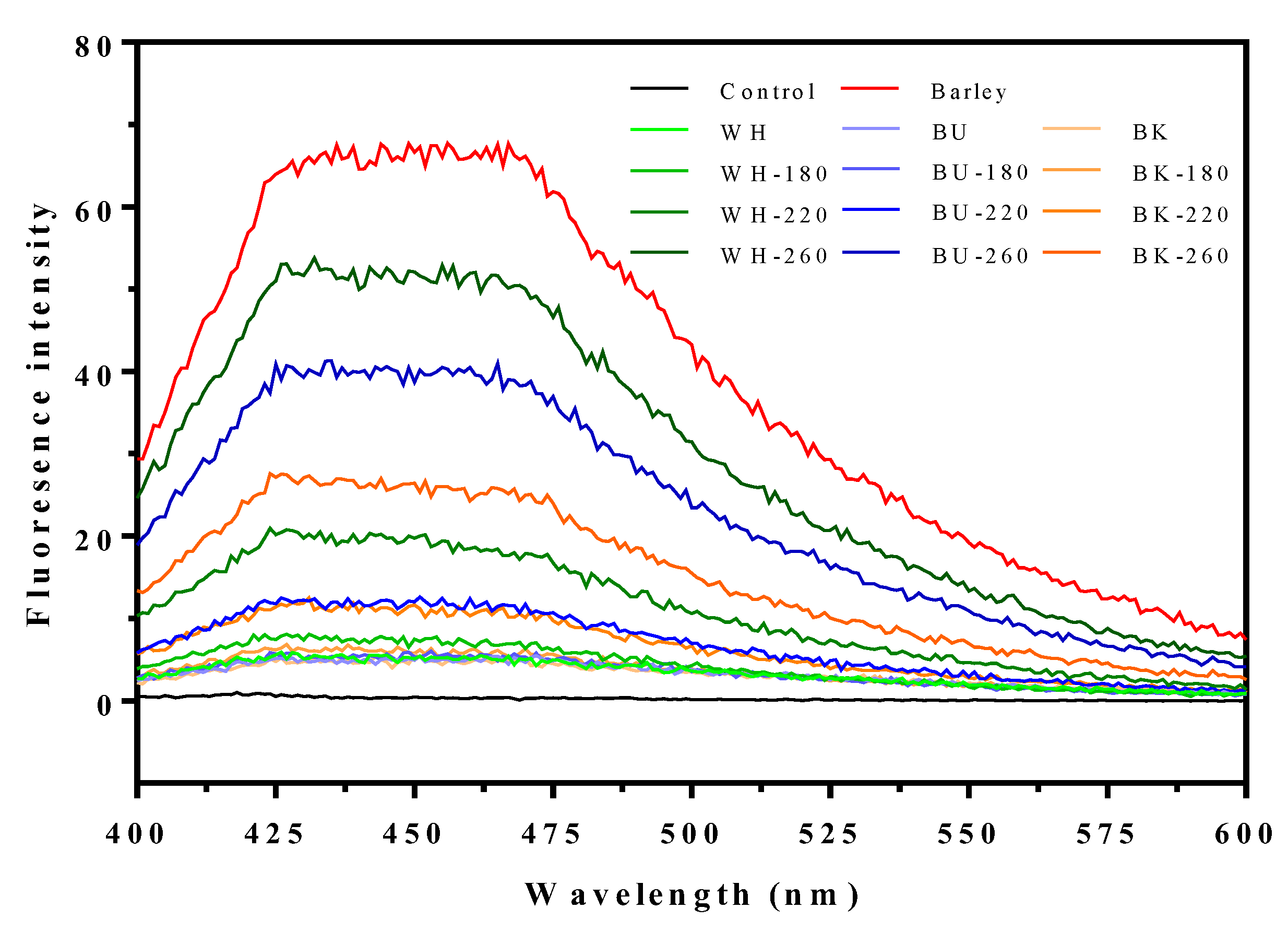
2.5.4. Free Fluorescence Intermediary Compounds Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Color Parameters of CHB Roasted at Different Temperatures
3.2. Volatile Compounds Analysis of CHB Roasted at Different Temperatures
3.2.1. Volatile Aromas Analyzed by E-Nose
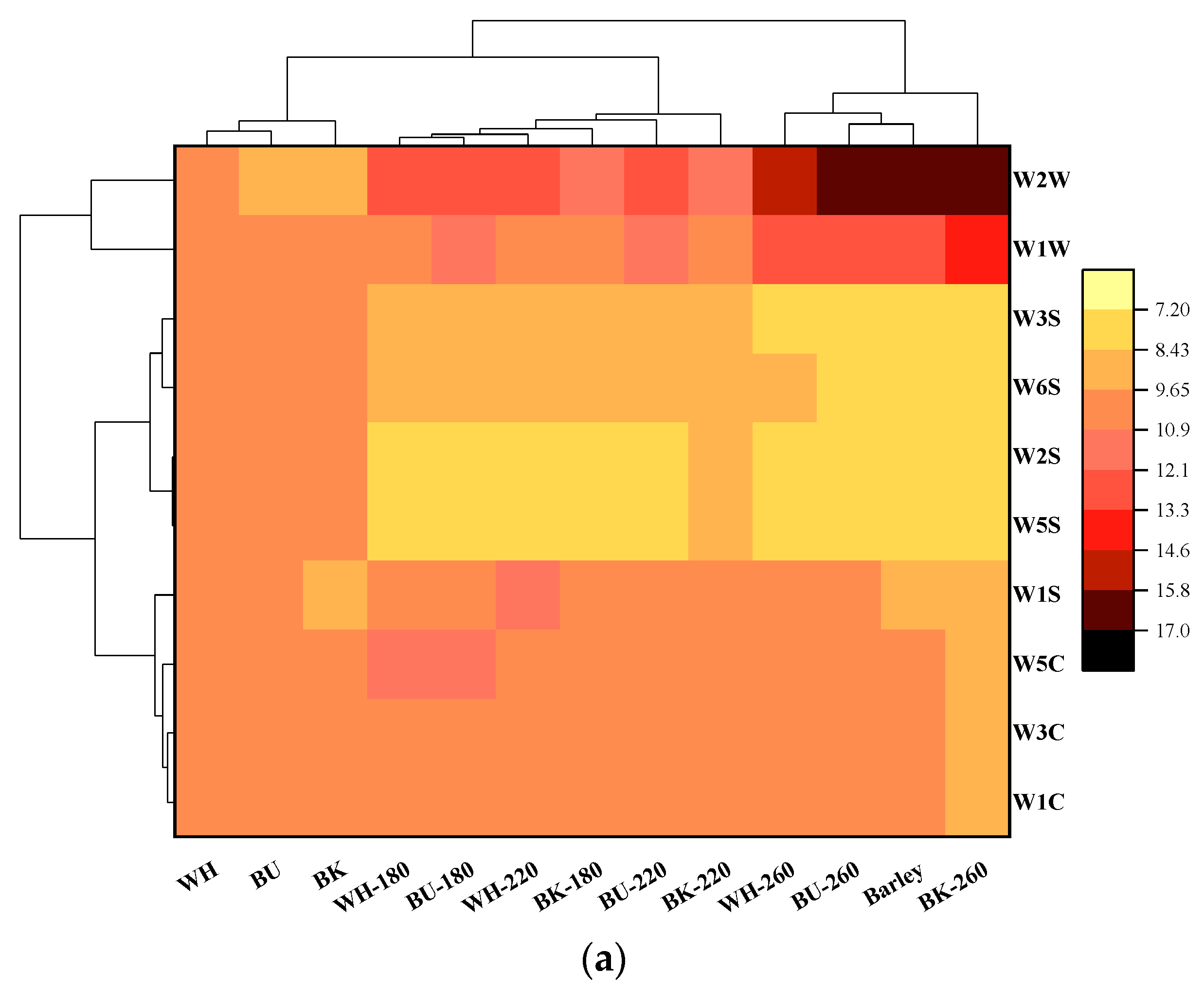
3.2.2. Volatiles Fingerprints Established by HS-GC-IMS
3.3. Chemical Composition of CHB Roasted at Different Temperatures
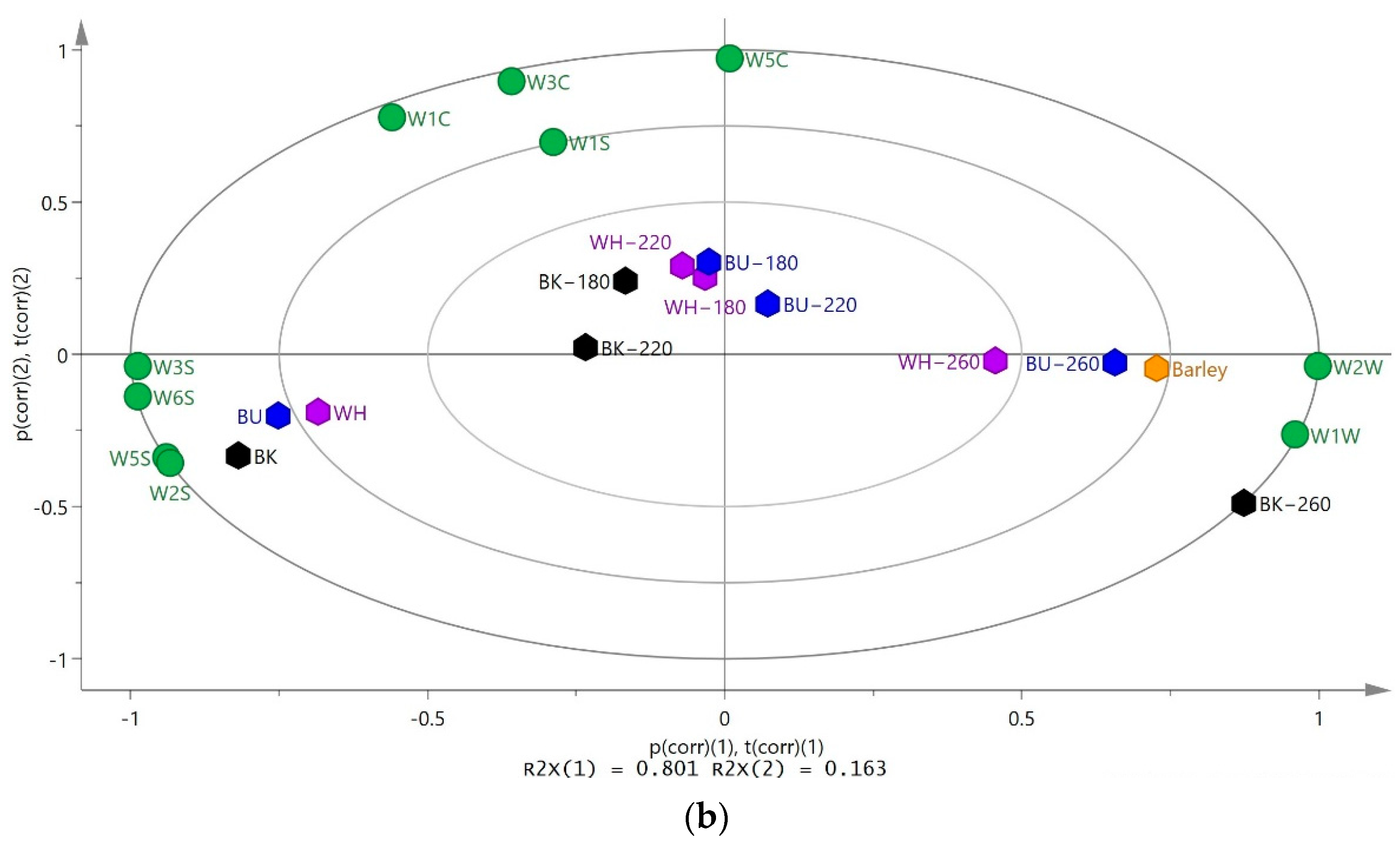
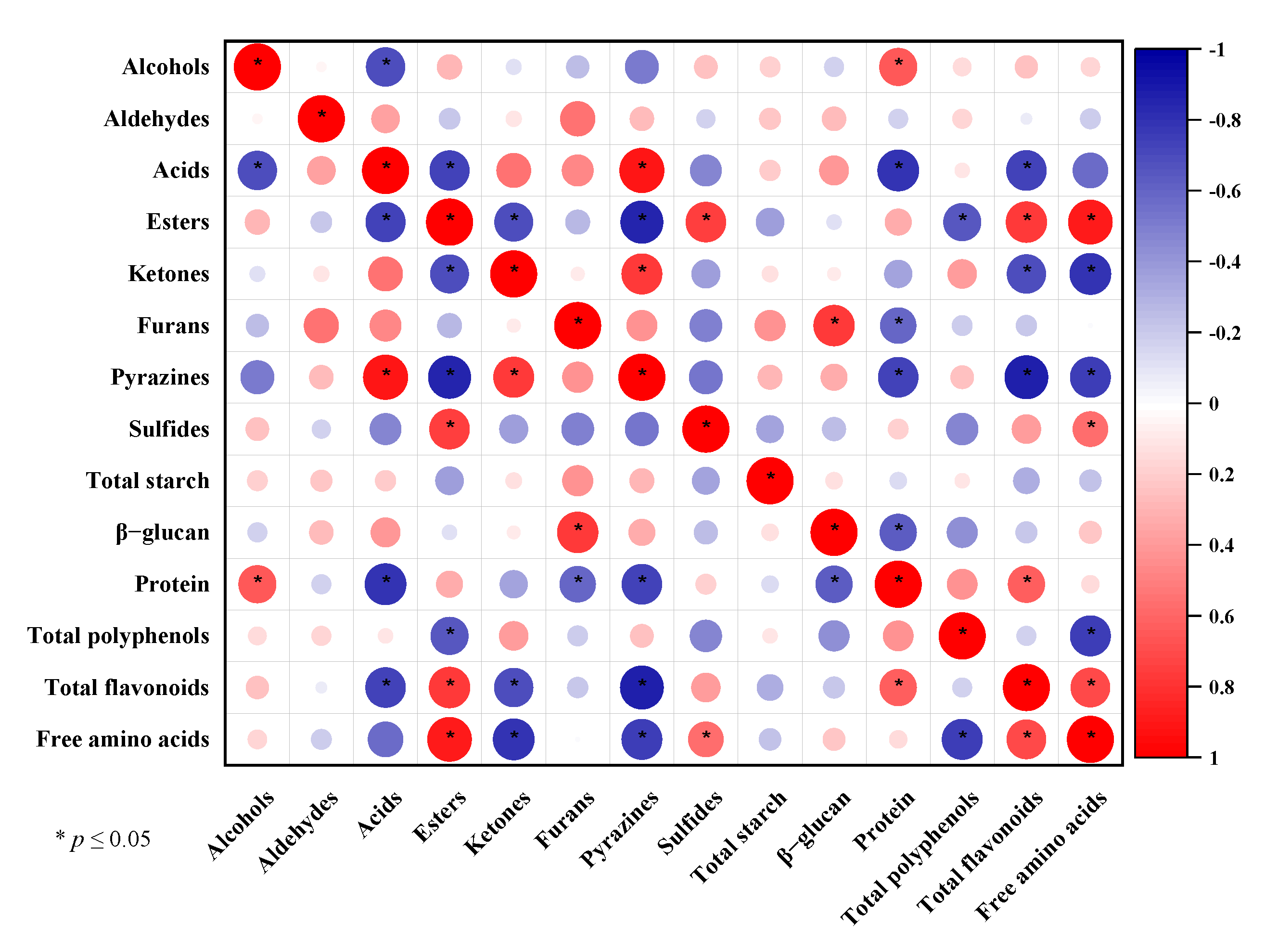
3.4. Correlation between the Chemical Composition and Volatile Substances of CHB Roasted at Different Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Ming, J.; Zhao, G.; Lei, L. Color, starch digestibility, and in vitro fermentation of roasted highland barley flour with different fractions. Foods 2022, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, X.; Meng, M.; Han, L.; Li, C.; Hou, L.; Qi, W.; Wang, C. Inhibitory effect on HT-29 colon cancer cells of a water-soluble polysaccharide obtained from highland barley. Int. J. Biol. Macromol. 2016, 92, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shang, J.; Liu, L.; Tong, L.T.; Zhou, X.; Wang, S.; Zhang, Y.; Wang, L.; Zhou, S. Effect of roasting process on enzymes inactivation and starch properties of highland barley. Int. J. Biol. Macromol. 2020, 165, 675–682. [Google Scholar] [CrossRef]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Liu, R.H. Bioactive compounds of highland barley and their health benefits. J. Cereal Sci. 2022, 103, 103366. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Guo, H.; Lin, S.; Lu, M.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Lin, D.R.; Qin, W.; Wu, D.T. Characterization, in vitro binding properties, and inhibitory activity on pancreatic lipase of β-glucans from different Qingke (Tibetan hulless barley) cultivars. Int. J. Biol. Macromol. 2018, 120, 2517–2522. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res. Int. 2011, 44, 235–240. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food processing and maillard reaction products: Effect on human health and nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Zhang, W.G.; Zhang, Y.; Yang, X.J.; Dang, B.; Zhang, J.; Du, Y.; Chen, D.S. GC-MS analysis of volatile flavor substances in different varieties of roasted hulless barley. Food Sci. 2019, 40, 192–201. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhang, W.G.; Dang, B.; Yang, X.J.; Chen, D.S.; Hao, J. Effects of stir-frying time and method on volatile flavor compounds in highland barley. Food Sci. 2020, 41, 271–277. (In Chinese) [Google Scholar]
- Schlörmann, W.; Zetzmann, S.; Wiege, B.; Haase, N.U.; Greiling, A.; Lorkowski, S.; Dawczynski, C.; Glei, M. Impact of different roasting conditions on chemical composition, sensory quality and physicochemical properties of waxy-barley products. Food Funct. 2019, 10, 5436–5445. [Google Scholar] [CrossRef] [PubMed]
- Wronkowska, M.; Piskuła, M.K.; Zieliński, H. Effect of roasting time of buckwheat groats on the formation of Maillard reaction products and antioxidant capacity. Food Chem. 2016, 196, 355–358. [Google Scholar]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, C.; Gao, L.; Zhuang, H.; Feng, T.; Xu, G. Analysis of volatile flavor compounds of green wheat under different treatments by GC-MS and GC-IMS. J. Food Biochem. 2022, 46, 13875. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Zhang, C.; Feng, T.; Zhuang, H. Analysis of volatile flavor compounds of corn under different treatments by GC-MS and GC-IMS. Front. Chem. 2022, 10, 725208. [Google Scholar] [CrossRef]
- Cai, J.S.; Zhu, Y.Y.; Ma, R.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Effects of roasting level on physicochemical, sensory, and volatile profiles of soybeans using electronic nose and HS-SPME-GC–MS. Food Chem. 2021, 340, 127880. [Google Scholar] [CrossRef]
- Schmitt, M.R.; Wise, M.L. Barley and oat β-glucan content measured by calcofluor fluorescence in a microplate assay. Cereal Chem. 2009, 86, 187–190. [Google Scholar] [CrossRef]
- Rosen, H. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Yuan, G.; Pan, Y.; Li, W.; Wang, C.; Chen, H. Effect of extrusion on physicochemical properties, functional properties and antioxidant activities of shrimp shell wastes protein. Int. J. Biol. Macromol. 2019, 136, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Mao, X.; Huang, H.; Wang, T.; Qu, Z.; Miao, J.; Gao, W. Effects of drying processes on starch-related physicochemical properties, bioactive components and antioxidant properties of yam flours. Food Chem. 2017, 224, 224–232. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Matiacevich, S.B.; Pilar Buera, M. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006, 95, 423–430. [Google Scholar] [CrossRef]
- Bagheri, H.; Kashaninejad, M.; Ziaiifar, A.M.; Aalami, M. Textural, color and sensory attributes of peanut kernels as affected by infrared roasting method. Inf. Process. Agric. 2019, 6, 255–264. [Google Scholar] [CrossRef]
- Suh, C.S.; Chun, J.K. Relationships among the roasting conditions, colors and extractable solid content of roastad barley. Korean J. Food Sci. Technol. 1981, 13, 334–339. [Google Scholar]
- Carvalho, D.O.; Øgendal, L.H.; Andersen, M.L.; Guido, L.F. High molecular weight compounds generated by roasting barley malt are pro-oxidants in metal-catalyzed oxidations. Eur. Food Res. Technol. 2016, 242, 1545–1553. [Google Scholar] [CrossRef]
- Mendes, L.C.; De Menezes, H.C.; Aparecida, M.; Da Silva, A.P. Optimization of the roasting of robusta coffee (C. canephora conillon) using acceptability tests and rsm. Food Qual. Prefer. 2001, 12, 153–162. [Google Scholar] [CrossRef]
- Özdemir, M.; Devres, O. Analysis of color development during roasting of hazelnuts using response surface methodology. J. Food Eng. 2000, 45, 17–24. [Google Scholar] [CrossRef]
- Mizukami, Y.; Yoshida, M.; Ono, H. Acrylamide elution from roasted barley grains into mugicha and its formation during roasting. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.V.; Ravi, R.; Bhat, K.K.; Raghavarao, K.S.M.S. Studies on roasting of wheat using fluidized bed roaster. J. Food Eng. 2008, 89, 336–342. [Google Scholar] [CrossRef]
- Kahyaoglu, T.; Kaya, S. Modeling of moisture, color and texture changes in sesame seeds during the conventional roasting. J. Food Eng. 2006, 75, 167–177. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Kara, Ş.; Ercȩlebi, E.A. Thermal degradation kinetics of anthocyanins and visual colour of Urmu mulberry (Morus nigra L.). J. Food Eng. 2013, 116, 541–547. [Google Scholar] [CrossRef]
- Parr, H.; Bolat, I.; Cook, D. Identification and categorization of volatile sulfur flavor compounds in roasted malts and barley. J. Am. Soc. Brew. Chem. 2021, 1–12. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-compound fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a benchtop alternative to 1H NMR profiling for assessment of the authenticity of honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products-An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Gaca, A.; Jeleń, H.H. Changes in volatile compound profiles of cold-pressed berry seed oils induced by roasting. LWT 2021, 148, 111718. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Fang, Y.; Wang, H.; Tao, H.; Pei, F.; Li, P.; Xu, B.; Hu, Q. A comprehensive analysis of aroma compounds and microstructure changes in brown rice during roasting process. LWT 2018, 98, 613–621. [Google Scholar] [CrossRef]
- Cui, D.D.; Liu, Y.; Chen, Y.P.; Feng, X.; Lu, Y.; Yu, B. Application of SPME-GC-TOFMS, E-nose, and sensory evaluation to investigate the flavor characteristics of Chinese Yunnan coffee at three different conditions (beans, ground powder, and brewed coffee). Flavour Fragr. J. 2020, 35, 541–560. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, M.; Huang, F.; Zheng, C.; Deng, Q. Effect of pretreatment with dehulling and microwaving on the flavor characteristics of cold-pressed rapeseed oil by GC-MS-PCA and electronic nose discrimination. J. Food Sci. 2013, 78, C961–C970. [Google Scholar] [CrossRef]
- Wang, K.; Ma, B.; Feng, T.; Chen, D.; Yao, L.; Lu, J.; Sun, M. Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities. Open Chem. 2021, 19, 518–529. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar Garlapati, V.; Banerjee, R.; Nag, A. Enzymatic synthesis of fruit flavor esters by immobilized lipase from Rhizopus oligosporus optimized with response surface methodology. J. Mol. Catal. B Enzym. 2009, 60, 57–63. [Google Scholar] [CrossRef]
- Davídek, T.; Devaud, S.; Robert, F.; Blank, I. Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic β-dicarbonyl cleavage mechanism. J. Agric. Food Chem. 2006, 54, 6667–6676. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.J.; Her, J.Y.; Kim, Y.G.; Kim, M.Y.; Jeong, S.Y.; Kim, M.K.; Lee, J.Y.; Kim, C.I.; Yoon, H.J.; Lee, K.G. Furan in thermally processed foods—A review. Toxicol. Res. 2015, 31, 241–253. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, M.; Liu, H.; Fan, Z.; Zhang, Y.; Qin, X.; Liu, X. Effects of β-glucan and various thermal processing methods on the in vitro digestion of hulless barley starch. Food Chem. 2021, 360, 129952. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akindahunsi, A.A. The effect of roasting on the nutritional and antioxidant properties of yellow and white maize varieties. Int. J. Food Sci. Technol. 2010, 45, 1236–1242. [Google Scholar] [CrossRef]
- Liaquat, M.; Pasha, I.; Ahsin, M.; Salik, A. Roasted fox nuts (Euryale Ferox L.) contain higher concentration of phenolics, flavonoids, minerals and antioxidants, and exhibit lower Glycemic Index (GI) in human subjects. Food Prod. Process. Nutr. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Hong, Q.; Chen, G.; Wang, Z.; Chen, X.; Kan, J. Effects of different thermal processing methods on bioactive components, phenolic compounds, and antioxidant activities of Qingke (Highland hull-less barley). Food Sci. Hum. Wellness 2023, 12, 119–129. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Liu, C.; Li, W.; Huang, F. Influence of microwaves treatment of rapeseed on phenolic compounds and canolol content. J. Agric. Food Chem. 2014, 62, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Guillot, F.L.; Malnoë, A.; Stadler, R.H. Antioxidant properties of novel tetraoxygenated phenylindan isomers formed during thermal decomposition of caffeic acid. J. Agric. Food Chem. 1996, 44, 2503–2510. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ni, Z.; Liu, X.; Xia, B.; Hu, L.; Thakur, K.; Wei, Z. Effects of sugars on the flavor and antioxidant properties of the Maillard reaction products of camellia seed meals. Food Chem. X 2021, 11, 100127. [Google Scholar] [CrossRef]
- Zielinski, H.; Michalska, A.; Amigo-Benavent, M.; Del Castillo, M.D.; Piskula, M.K. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J. Agric. Food Chem. 2009, 57, 4771–4776. [Google Scholar] [CrossRef]
- Cheng, K.W.; Zeng, X.; Yun, S.T.; Wu, J.J.; Liu, Z.; Sze, K.H.; Chu, I.K.; Chen, F.; Wang, M. Inhibitory mechanism of naringenin against carcinogenic acrylamide formation and nonenzymatic browning in maillard model reactions. Chem. Res. Toxicol. 2009, 22, 1483–1489. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Piechowska, P.; Waśkiewicz, A.; Zawirska-Wojtasiak, R. Effects of polyphenols on volatile profile and acrylamide formation in a model wheat bread system. Food Chem. 2019, 297, 125008. [Google Scholar] [CrossRef]
- Misnawi; Jinap, S.; Jamilah, B.; Nazamid, S. Changes in polyphenol ability to produce astringency during roasting of cocoa liquor. J. Sci. Food Agric. 2005, 85, 917–924. [Google Scholar] [CrossRef]


| Samples | L* | a* | b* | BI |
|---|---|---|---|---|
| WH | 56.79 ± 1.44 a | 7.85 ± 0.68 cd | 26.53 ± 1.16 ab | 69.58 ± 0.77 ef |
| WH-180 | 53.00 ± 2.60 ab | 9.79 ± 0.92 ab | 26.62 ± 1.26 ab | 81.37 ± 5.06 bc |
| WH-220 | 47.86 ± 2.44 bc | 9.73 ± 0.20 ab | 24.85 ± 1.50 bc | 85.88 ± 3.49 b |
| WH-260 | 46.07 ± 1.32 c | 7.32 ± 1.32 de | 28.11 ± 1.28 a | 100.78 ± 2.62 a |
| BU | 52.08 ± 2.13 ab | 4.37 ± 0.57 f | 21.14 ± 1.23 d | 57.11 ± 4.38 g |
| BU-180 | 53.51 ± 5.14 a | 7.12 ± 0.42 de | 23.94 ± 2.12 bcd | 67.74 ± 1.46 ef |
| BU-220 | 52.83 ± 1.39 ab | 10.16 ± 0.10 a | 25.50 ± 0.20 abc | 78.33 ± 2.10 cd |
| BU-260 | 45.80 ± 0.39 c | 6.50 ± 0.27 e | 22.86 ± 0.41 cd | 77.49 ± 2.88 cd |
| BK | 35.81 ± 1.52 d | 10.53 ± 0.51 a | 14.54 ± 1.09 ef | 72.56 ± 2.25 de |
| BK-180 | 35.56 ± 5.50 d | 10.29 ± 0.81 a | 15.89 ± 3.95 e | 78.58 ± 6.05 cd |
| BK-220 | 36.58 ± 4.55 d | 10.14 ± 0.98 a | 16.91 ± 2.58 e | 81.07 ± 2.05 bc |
| BK-260 | 34.43 ± 1.60 d | 8.67 ± 0.47 bc | 16.63 ± 1.45 e | 82.63 ± 3.67 bc |
| NO. | Compound | RI | Dt [RIPrel] | Relative Content (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WH | WH-180 | WH-220 | WH-260 | BU | BU-180 | BU-220 | BU-260 | BK | BK-180 | BK-220 | BK-260 | Barley | ||||
| Aldehydes | ||||||||||||||||
| 1 | Nonanal | 1096 | 1.4755 | 8.5 | 3.1 | 2.9 | 2.4 | 8.4 | 3.9 | 3.4 | 2.2 | 5.9 | 2.6 | 1.6 | 1.2 | 0.54 |
| 2 | Heptanal | 902.5 | 1.3295 | 1.9 | 0.71 | 0.55 | 0.35 | 2.1 | 0.96 | 0.62 | 0.35 | 1.3 | 0.75 | 0.48 | 0.31 | 0.2 |
| 3 | Hexanal | 799.8 | 1.2591 | 3.4 | 1.6 | 1.6 | 1.1 | 3.2 | 1.9 | 1.8 | 1.1 | 2.4 | 1.6 | 1.4 | 1.1 | 0.54 |
| 4 | 3-Methylbutanal | 651.4 | 1.4028 | 9.4 | 10.0 | 7.1 | 3 | 11.0 | 12.0 | 7.9 | 3.1 | 7.0 | 11.0 | 6.5 | 2.2 | 1.5 |
| 5 | Propanal | 491.7 | 1.0481 | 9.1 | 3.1 | 3.2 | 2.5 | 8.1 | 4.1 | 4.0 | 2.6 | 7.0 | 3.1 | 3.2 | 2.5 | 1.7 |
| 6 | (E)-hept-2-enal | 965.3 | 1.2613 | 2.1 | 0.67 | 0.91 | 0.47 | 1.2 | 0.56 | 0.74 | 0.54 | 2.0 | 1.2 | 1.2 | 0.75 | 0.2 |
| 7 | 5-Methylfurfural | 970.8 | 1.1241 | nd | 2.3 | 2.4 | 4 | nd | 1.8 | 2.7 | 4.2 | nd | 1.9 | 2.2 | 3.1 | 2.2 |
| 8 | 3-Methylthiopropanal | 911.3 | 1.395 | nd | 4.3 | 4.5 | 5 | nd | 2.5 | 4.5 | 4.4 | nd | 2.6 | 2.9 | 3.3 | 6.2 |
| 9 | Furfural | 827.2 | 1.3386 | nd | 8.2 | 9.6 | 13 | nd | 5.7 | 8.1 | 13.0 | nd | 9.1 | 10.0 | 12.0 | 12.3 |
| 10 | 2-Methylpropanal | 558.1 | 1.2883 | 1.3 | 6.2 | 4.3 | 2 | 2.4 | 6.2 | 4.7 | 2.4 | nd | 7.1 | 3.7 | 1.4 | 0.5 |
| 11 | Benzaldehyde | 963.5 | 1.1501 | nd | 0.72 | 0.72 | 0.68 | nd | 0.61 | 0.65 | 0.61 | nd | 0.96 | 1.0 | 1.1 | 0.73 |
| 12 | Butanal | 595.5 | 1.2897 | 0.53 | 0.54 | 0.57 | 0.53 | 0.52 | 0.54 | 0.53 | 0.42 | 0.4 | 0.55 | 0.57 | 0.63 | 1.5 |
| 13 | Benzene acetaldehyde | 1040.7 | 1.2566 | 2.9 | 2.2 | 1.0 | nd | 3.0 | 2.7 | 1.2 | nd | 2.1 | 2.1 | 0.78 | nd | 0.15 |
| 14 | (E)-2-octenal | 1056.4 | 1.3389 | 1.2 | 0.53 | 0.79 | 0.43 | 1.1 | 0.49 | 0.72 | 0.63 | 1.2 | 0.8 | 1.1 | 0.49 | 0.24 |
| 15 | Heptanal (DIMER) | 900.9 | 1.701 | 1.2 | 0.32 | 0.18 | nd | 2.3 | 0.46 | 0.17 | 0.1 | 0.81 | 0.41 | 0.2 | 0.12 | 0.083 |
| 16 | 5-Methylfurfural (DIMER) | 968.1 | 1.4766 | nd | nd | nd | 9.6 | nd | nd | nd | 9.6 | nd | nd | 2.1 | 5.2 | 12.5 |
| 17 | (E)-hept-2-enal (DIMER) | 962.2 | 1.6661 | 0.86 | 0.3 | 0.47 | 0.42 | 0.39 | 0.2 | 0.3 | 0.45 | 1.2 | 1.1 | 1.2 | 1.1 | 0.18 |
| Alcohols | ||||||||||||||||
| 18 | Pentan-1-ol | 760.4 | 1.2556 | 2.1 | 0.8 | 0.75 | 0.49 | 2.0 | 1.0 | 0.8 | 0.48 | 1.6 | 0.94 | 0.77 | 0.47 | 0.54 |
| 19 | 2-Methyl-1-propanol | 619.9 | 1.1731 | 1.4 | 0.61 | 0.47 | 0.45 | 1.9 | 0.77 | 0.51 | 0.4 | 0.53 | 0.74 | 0.7 | 0.43 | 0.33 |
| 20 | 1-Propanol | 567.6 | 1.1142 | 3.8 | 1.6 | 1.8 | 1.3 | 3.9 | 2.3 | 2.1 | 1.3 | 2.1 | 1.4 | 1.4 | 1.0 | 1.0 |
| 21 | (Z)-3-hexen-1-ol | 847.2 | 1.5176 | 3.0 | 1.1 | 0.55 | nd | 4.2 | 2.0 | 0.71 | nd | 2.2 | 0.92 | 0.55 | nd | 0.37 |
| 22 | 4-Methylpentanol | 854.3 | 1.3099 | 3.1 | 2.8 | 3.2 | 6.4 | 3.7 | 1.8 | 2.8 | 7.0 | 2.0 | nd | 5.0 | 8.0 | 4.8 |
| 23 | (Z)-3-nonen-1-ol | 1153.9 | 1.8295 | 8.0 | 2.3 | 2.5 | 2.9 | nd | 4.7 | 3.7 | 2.3 | 3.0 | nd | nd | nd | 6.3 |
| 24 | 3-Octanol | 994.8 | 1.4076 | 0.41 | 0.29 | 0.23 | 0.15 | 0.56 | 0.3 | 0.22 | 0.14 | 0.3 | 0.32 | 0.22 | 0.17 | 0.39 |
| 25 | 2-Octanol | 993.8 | 1.4381 | 0.15 | 0.23 | 0.27 | 0.16 | 0.24 | 0.22 | 0.17 | 0.12 | 0.14 | 0.33 | 0.38 | 0.34 | 0.32 |
| 26 | 1-Heptanol | 971.7 | 1.3899 | nd | 1.7 | 1.4 | 6.0 | nd | 1.6 | 1.3 | 5.6 | nd | nd | 1.5 | 3.8 | 7.7 |
| 27 | 2,3-Butanediol | 792.4 | 1.3617 | 0.6 | 0.46 | 0.47 | 0.36 | 0.53 | 0.46 | 0.47 | 0.35 | 0.5 | 0.49 | 0.46 | 0.5 | nd |
| 28 | Pentan-1-ol (DIMER) | 757.1 | 1.5174 | 0.77 | 0.26 | 0.19 | 0.1 | 0.75 | 0.31 | 0.22 | 0.12 | 0.61 | 0.39 | 0.33 | 0.12 | 0.095 |
| Ketones | ||||||||||||||||
| 29 | Methyl-5-hepten-2-one | 683 | 1.4291 | 3.9 | 3.9 | 4.3 | 3.6 | 4 | 3.7 | 3.9 | 3.4 | 3.1 | 4.4 | 5.3 | 5.5 | 4.3 |
| 30 | 2-Heptanone | 888.5 | 1.2642 | 0.58 | 0.47 | 0.45 | 0.29 | 0.57 | 0.55 | 0.38 | 0.26 | 0.55 | 0.49 | 0.35 | 0.34 | 0.097 |
| 31 | 2-Butanone | 592.1 | 1.2444 | nd | 3.5 | 3.9 | 3.3 | 1.8 | 3.0 | 3.7 | 2.8 | nd | 3.0 | 3.3 | 3.9 | 2.8 |
| 32 | 1-Hydroxy-2-propanone | 675.4 | 1.2253 | nd | 1.2 | 1.9 | 2.4 | nd | 0.76 | 1.4 | 2.2 | nd | 1.3 | 1.7 | 2.9 | 2.7 |
| 33 | Acetone | 499.6 | 1.1201 | 6.0 | 3.8 | 6.3 | 5.4 | 5.5 | 3.0 | 6.1 | 6.8 | 3.6 | 2.5 | 4.1 | 6.3 | 4.1 |
| 34 | Dihydro-2(3h)-furanone | 914.7 | 1.3031 | 0.42 | 0.94 | 0.93 | 0.55 | 0.82 | 0.49 | 0.85 | 0.64 | 0.4 | 0.71 | 1.3 | 1.0 | 0.22 |
| 35 | 2-Hexanone | 791.2 | 1.5027 | 0.15 | 0.28 | 0.24 | 0.17 | 0.2 | 0.34 | 0.26 | 0.18 | 0.16 | 0.28 | 0.23 | 0.14 | 0.13 |
| 36 | 1-Octen-3-one | 988.5 | 1.274 | 0.55 | 0.21 | 0.27 | 0.16 | 0.37 | 0.12 | 0.18 | 0.16 | 0.66 | 0.41 | 0.35 | 0.32 | 0.17 |
| 37 | 2-Heptanone (DIMER) | 889.5 | 1.6298 | 0.23 | 0.37 | 0.32 | 0.33 | 0.3 | 0.3 | 0.18 | 0.2 | 0.21 | 0.39 | 0.26 | 0.36 | 0.26 |
| Esters | ||||||||||||||||
| 38 | Methyl 2-methylpropanoate | 683 | 1.4291 | 1.5 | 1.0 | 0.76 | 0.35 | 2.1 | 2.0 | 0.87 | 0.45 | 1.5 | 1.4 | 0.86 | 0.51 | 0.16 |
| 39 | Ethyl butyrate | 794.2 | 1.5588 | 9.1 | 4.0 | 4.4 | 2.2 | 8.6 | 4.7 | 4.4 | 2.9 | 7.6 | 4.6 | 5.0 | 2.9 | 1.2 |
| 40 | Acetic acid, hexyl ester | 1012.2 | 1.4061 | 2.3 | 0.83 | 0.65 | 0.51 | 2.3 | 0.93 | 0.74 | 0.51 | 1.3 | 0.6 | 0.52 | 0.4 | 0.23 |
| 41 | Ethyl acetate | 606.7 | 1.0971 | 0.78 | 0.37 | 0.5 | 0.36 | 0.54 | 0.44 | 0.52 | 0.36 | 0.79 | 0.41 | 0.42 | 0.43 | 0.43 |
| 42 | n-Propyl acetate | 710.9 | 1.1573 | nd | 1.3 | 1.6 | 1.6 | nd | 1.2 | 1.4 | 1.7 | nd | 1.6 | 1.9 | 1.8 | 1.2 |
| 43 | Ethyl acetate (DIMER) | 605.6 | 1.3403 | 0.66 | 0.36 | 0.34 | 0.31 | 0.68 | 0.4 | 0.31 | 0.24 | 0.79 | 0.44 | 0.39 | 0.37 | 4.3 |
| Acids | ||||||||||||||||
| 44 | Acetic acid | 600.1 | 1.1547 | nd | 1.5 | 1.5 | 1.7 | nd | 1.1 | 1.0 | 1.4 | nd | 1.7 | 1.9 | 2.1 | 1.2 |
| 45 | Butanoic acid | 816.2 | 1.3903 | nd | 1.9 | 1.7 | 1.7 | nd | 1.2 | 1.5 | 1.5 | nd | 0.46 | 1.6 | 1.5 | 2.0 |
| 46 | 2-Methylbutanoic acid | 848.4 | 1.4718 | nd | 0.7 | 0.48 | 0.21 | nd | 0.54 | 0.41 | 0.19 | nd | 1.7 | 0.33 | 0.2 | 0.21 |
| 47 | Hexanoic acid | 997.3 | 1.3012 | 0.78 | 2.0 | 1.5 | 0.66 | 1.2 | 1.8 | 1.2 | 0.59 | 1.2 | 2.4 | 1.5 | 1.0 | 0.85 |
| Pyrazines | ||||||||||||||||
| 48 | Methylpyrazine | 828.8 | 1.0823 | nd | 3.5 | 3.7 | 2.7 | nd | 3.4 | 3.7 | 2.9 | nd | 3.5 | 3.3 | 2.7 | 1.4 |
| 49 | Trimethylpyrazine | 1008.5 | 1.162 | nd | 1.8 | 2.4 | 2.3 | nd | 1.6 | 2.8 | 2.3 | nd | 1.6 | nd | 2.8 | 1.8 |
| 50 | 2-Acetylpyrazine | 1023.9 | 1.1468 | nd | 3.0 | 2.9 | 2.4 | nd | 2.3 | 2.7 | 2.1 | nd | 3.2 | 2.3 | 4.7 | 3.0 |
| 51 | 2-Ethyl-6-methylpyrazine | 1005.1 | 1.2003 | 0.32 | 0.3 | 0.38 | 0.44 | 0.32 | 0.34 | 0.53 | 0.43 | 0.31 | 0.53 | 3.9 | 0.34 | 0.23 |
| Furans | ||||||||||||||||
| 52 | 2-Pentyl furan | 1001.4 | 1.2561 | 0.74 | 0.47 | 0.35 | 0.21 | 0.91 | 0.67 | 0.29 | 0.23 | 0.95 | 0.63 | 0.51 | 0.16 | 0.097 |
| 53 | 2-Acetylfuran | 910.3 | 1.1174 | 0.72 | 1.9 | 2.1 | 1.6 | 0.56 | 2.4 | 2.6 | 1.6 | nd | 1.8 | 0.31 | 1.2 | 0.85 |
| Sulfides | ||||||||||||||||
| 54 | Dipropenyldisulfide (diallyl disulfide) | 1083.2 | 1.2123 | 1.4 | 0.59 | 0.62 | 0.47 | 1.1 | 0.7 | 0.66 | 0.4 | 0.84 | 0.56 | 1.47 | 0.37 | 0.3 |
| 55 | 1,4-Dioxane | 702.5 | 1.3301 | nd | 2.5 | 2.6 | 1.5 | nd | 2.6 | 2.9 | 1.4 | nd | 2.6 | 0.52 | 2.4 | nd |
| Samples | Content (%) | |||||
|---|---|---|---|---|---|---|
| Total Starch | β-Glucan | Protein | Total Polyphenols | Total Flavonoids | Free Amino Acids | |
| WH | 50.38 ± 1.79 c | 4.31 ± 0.099 de | 8.82 ± 0.96 a | 0.063 ± 0.001 e | 0.59 ± 0.007 bc | 1.65 ± 0.04 a |
| WH-180 | 54.53 ± 1.47 bc | 5.18 ± 0.40 a | 5.11 ± 0.32 bc | 0.064 ± 0.002 de | 0.54 ± 0.003 def | 1.27 ± 0.06 c |
| WH-220 | 53.11 ± 2.45 bc | 4.88 ± 0.063 b | 4.92 ± 0.42 bc | 0.069 ± 0.005 cde | 0.54 ± 0.010 def | 0.98 ± 0.11 d |
| WH-260 | 61.44 ± 2.52 a | 3.55 ± 0.14 g | 8.58 ± 0.24 a | 0.095 ± 0.004 a | 0.55 ± 0.012 de | 0.52 ± 0.01 f |
| BU | 54.62 ± 2.69 bc | 3.36 ± 0.059 gh | 10.11 ± 1.09 a | 0.064 ± 0.002 d | 0.64 ± 0.020 a | 1.49 ± 0.11 b |
| BU-180 | 64.13 ± 3.71 a | 4.65 ± 0.18 bc | 3.99 ± 0.77 c | 0.056 ± 0.001 f | 0.52 ± 0.018 ef | 1.30 ± 0.05 c |
| BU-220 | 56.02 ± 4.02 b | 4.58 ± 0.086 cd | 4.86 ± 0.42 bc | 0.069 ± 0.001 cde | 0.55 ± 0.033 de | 1.02 ± 0.02 d |
| BU-260 | 52.81 ± 0.51 bc | 3.52 ± 0.14 gh | 9.06 ± 1.53 a | 0.086 ± 0.005 b | 0.53 ± 0.010 def | 0.52 ± 0.02 f |
| BK | 52.03 ± 2.83 bc | 3.41 ± 0.39 gh | 9.90 ± 0.48 a | 0.068 ± 0.001 cd | 0.61 ± 0.023 ab | 1.54 ± 0.06 b |
| BK-180 | 50.68 ± 1.50 c | 4.11 ± 0.20 ef | 4.06 ± 0.71 c | 0.070 ± 0.002 cd | 0.56 ± 0.007 cd | 0.97 ± 0.02 d |
| BK-220 | 52.74 ± 1.96 bc | 3.23 ± 0.13 h | 5.37 ± 0.31 b | 0.068 ± 0.002 cde | 0.51 ± 0.003 fg | 0.84 ± 0.02 e |
| BK-260 | 56.28 ± 1.49 b | 3.85 ± 0.067 f | 5.52 ± 0.30 bc | 0.073 ± 0.005 c | 0.48 ± 0.016 g | 0.52 ± 0.01 f |
| Samples | FIC Values (FU) | |||
|---|---|---|---|---|
| 0.0 °C | 180.0 °C | 220.0 °C | 260.0 °C | |
| White cultivar | 0.11 ± 0.002 j | 0.16 ± 0.006 g | 0.44 ± 0.005 d | 1.15 ± 0.007 a |
| Blue cultivar | 0.11 ± 0.004 j | 0.13 ± 0.004 i | 0.27 ± 0.009 e | 0.92 ± 0.003 b |
| Black cultivar | 0.11 ± 0.002 j | 0.14 ± 0.004 h | 0.26 ± 0.013 f | 0.59 ± 0.001 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, Z.; Zhang, X.; Tian, X.; Chen, K.; Zeng, X. Characterization of Volatile Compounds by HS-GC-IMS and Chemical Composition Analysis of Colored Highland Barley Roasted at Different Temperatures. Foods 2022, 11, 2921. https://doi.org/10.3390/foods11182921
Wang C, Zhang Z, Zhang X, Tian X, Chen K, Zeng X. Characterization of Volatile Compounds by HS-GC-IMS and Chemical Composition Analysis of Colored Highland Barley Roasted at Different Temperatures. Foods. 2022; 11(18):2921. https://doi.org/10.3390/foods11182921
Chicago/Turabian StyleWang, Cong, Zhiming Zhang, Xiayin Zhang, Xinyi Tian, Kai Chen, and Xiaoxiong Zeng. 2022. "Characterization of Volatile Compounds by HS-GC-IMS and Chemical Composition Analysis of Colored Highland Barley Roasted at Different Temperatures" Foods 11, no. 18: 2921. https://doi.org/10.3390/foods11182921
APA StyleWang, C., Zhang, Z., Zhang, X., Tian, X., Chen, K., & Zeng, X. (2022). Characterization of Volatile Compounds by HS-GC-IMS and Chemical Composition Analysis of Colored Highland Barley Roasted at Different Temperatures. Foods, 11(18), 2921. https://doi.org/10.3390/foods11182921