Changes in Fusarium and Aspergillus Mycotoxin Content and Fatty Acid Composition after the Application of Ozone in Different Maize Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial
2.2. Harvest, Assessment, and Sampling
2.3. Sample Preparation
2.4. Chemicals
2.5. Ozone Generation and Maize Decontamination Procedure
2.6. Mycotoxin Determination
2.7. Seed Oil Determination by Soxhlet Extraction Method
2.8. Fatty Acid Composition Analysis
2.9. Statistical Analysis
2.9.1. Ear Root Assessment
2.9.2. Fatty Acid Data
3. Results and Discussion
3.1. Ear Rot Assessment
3.2. Effectiveness of Ozonation on Mycotoxin Levels
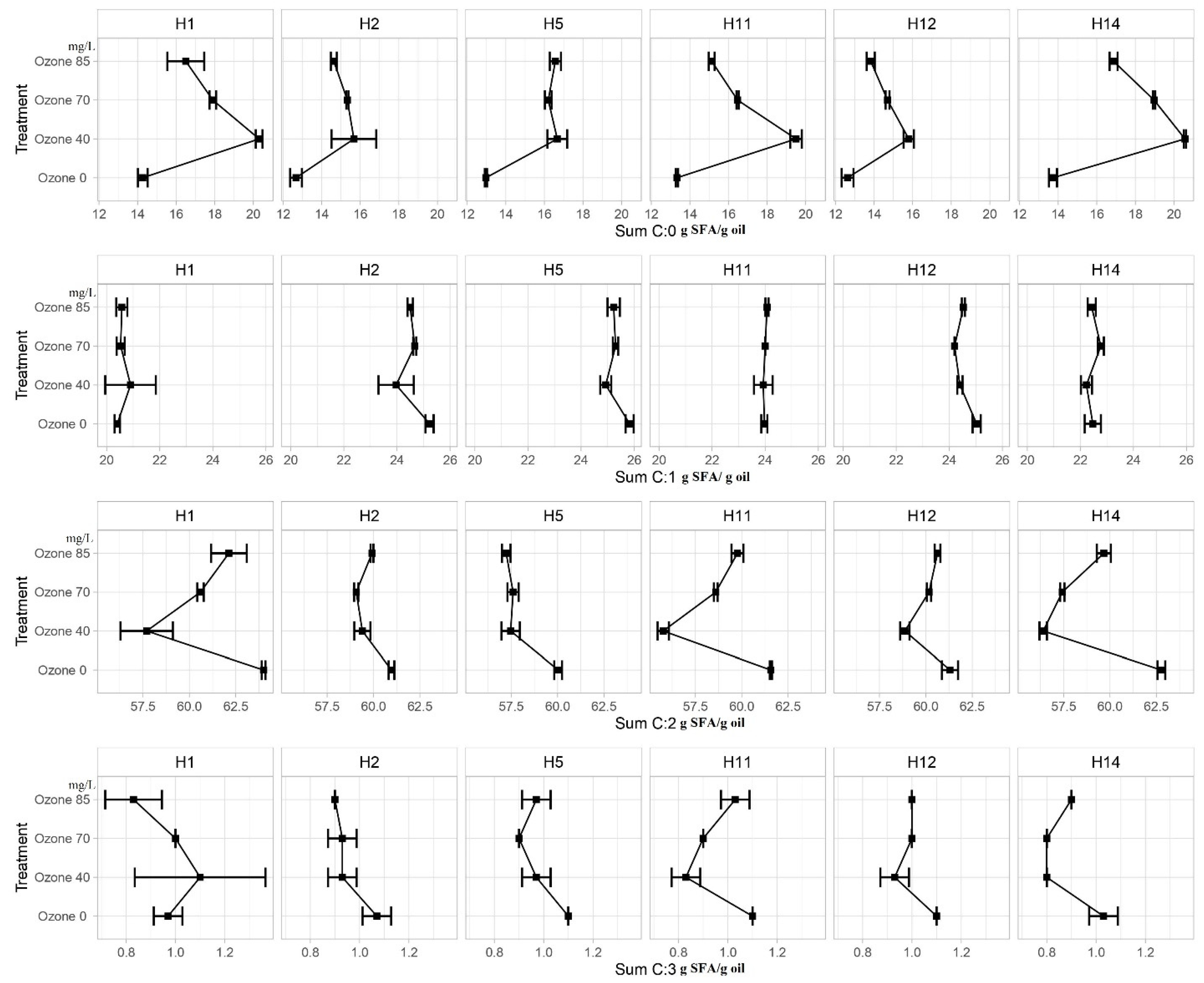
3.3. Fatty Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 April 2022).
- Dissemination Database Search. Available online: https://data.stat.gov.rs/Home/Result/130102?languageCode=en-US (accessed on 28 April 2022).
- Krnjaja, V.; Lukic, M.; Delic, N.; Tomic, Z.; Mandic, V.; Bijelic, Z.; Gogic, M. Mycobiota and Mycotoxins in Freshly Harvested and Stored Maize. Biotechnol. Anim. Husb. 2015, 31, 291–302. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin Production in Major Crops as Influenced by Growing, Harvesting, Storage and Processing, with Emphasis on the Achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited “FAO Estimate” of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Kocic-Tanackov, S.; Dimic, G. Fungi and Mycotoxins: Food Contaminants. Chem. Ind. 2013, 67, 639–653. [Google Scholar] [CrossRef]
- Obradovic, A.; Krnjaja, V.; Nikolic, M.; Delibasic, G.; Filipovic, M.; Stankovic, G.; Stankovic, S. Impacts of Climatic Conditions on Aflatoxin B1 and Fumonisins Contamination of Maize Kernels and Their Co-Occurrence. Biotechnol. Anim. Husb. 2018, 34, 469–480. [Google Scholar] [CrossRef]
- Turner, P. Deoxynivalenol and Nivalenol Occurrence and Exposure Assessment. World Mycotoxin J. 2010, 3, 315–321. [Google Scholar] [CrossRef]
- Pitt, J.I. Biocontrol of aflatoxinsin peanuts. In Meeting the Mycotoxin Menace; Barug, D., van Egmond, H., LopezGarcia, R., van Osenbruggen, T., Visconti, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004; pp. 141–152. 3p, Available online: https://vdoc.pub/documents/meeting-the-mycotoxin-menace-3psmpfk6m0vg (accessed on 31 May 2022).
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the Mycotoxins Incidence in Serbia in the Period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Stanković, S.; Obradović, A.; Vasić, T.; Lukić, M.; Bijelić, Z. Influence of Plant Density on Toxigenic Fungal and Mycotoxin Contamination of Maize Grains. Crop Prot. 2019, 116, 126–131. [Google Scholar] [CrossRef]
- Nikolic, M.; Srdic, J.; Savic, I.; Zilic, S.; Stevanovic, M.; Kandic, V.; Stankovic, S. The Occurrence of Mycotoxins in Sweet Maize Hybrids. Genetika 2021, 53, 1311–1320. [Google Scholar] [CrossRef]
- Kos, J.; Janić Hajnal, E.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in Maize Harvested in Republic of Serbia in the Period 2012–2015. Part 1: Regulated Mycotoxins and Its Derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef]
- Payne, G.A.; Widstrom, N.W. Aflatoxin in Maize. Crit. Rev. Plant Sci. 1992, 10, 423–440. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of Two Mycotoxins Deoxynivalenol and Fumonisin on Pig Intestinal Health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Squire, R.A. Ranking Animal Carcinogens: A Proposed Regulatory Approach. Science 1981, 214, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, H.M.; Laishes, B.A. Toxicity of aflatoxin B1 in rat and mouse hepatocytes in vivo and in vitro. Toxicology 1984, 30, 185–193. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Chemical Agents and Related Occupations; IARC Monographs on the Evaluation of Carcinogenic Risk to Humans: Lyon, France, 2012; ISBN 9789283201380. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Chemical-Agents-And-Related-Occupations-2012 (accessed on 31 May 2022).
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, R.; Vanara, F.; Reyneri, A. Impact of Sowing Time, Hybrid and Environmental Conditions on the Contamination of Maize by Emerging Mycotoxins and Fungal Metabolites. Ital. J. Agron. 2017, 12, 215–224. [Google Scholar] [CrossRef]
- Ghiasian, S.A.; Shephard, G.S.; Yazdanpanah, H. Natural Occurrence of Aflatoxins from Maize in Iran. Mycopathologia 2011, 172, 153–160. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Krulj, J.; Krstović, S.; Jajić, I.; Pezo, L.; Šarić, B.; Nedeljković, N. Aflatoxins Contamination of Maize in Serbia: The Impact of Weather Conditions in 2015. Food Addit. Contam. Part A 2017, 34, 1999–2010. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Lanubile, A.; Dall’Asta, C.; Pietri, A.; Battilani, P. The Impact of Seasonal Weather Variation on Mycotoxins: Maize Crop in 2014 in Northern Italy as a Case Study. World Mycotoxin J. 2020, 13, 25–36. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural Mycotoxin Contamination of Maize (Zea Mays L.) in the South Region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of Maize with Deoxynivalenol and Zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Li, Y.; Li, T.; He, Q.; Tang, Y.; Liu, H.; Su, Y.; Yin, Y.; Liao, P. Aflatoxin B1, Zearalenone and Deoxynivalenol in Feed Ingredients and Complete Feed from Different Province in China. J. Anim. Sci. Biotechnol. 2016, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B. The Fate of Mycotoxins during Thermal Food Processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Krstović, S.; Krulj, J.; Jakšić, S.; Bočarov-Stančić, A.; Jajić, I. Ozone as Decontaminating Agent for Ground Corn Containing Deoxynivalenol, Zearalenone, and Ochratoxin A. Cereal Chem. 2020, 98, 135–143. [Google Scholar] [CrossRef]
- Hoigné, J. Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes. In Quality and Treatment of Drinking Water II; Hrubec, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 83–141. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Smith, S.A.; Scott, D.T.; Taylor, D.P. Ozone Application in Aquaculture; Virginia Cooperative Extension, Virginia Tech, Virginia State University: Petersburg, VA, USA, 2017; Available online: https://www.rashkiyan.com/File/News/70/file/1.pdf (accessed on 29 August 2022).
- Kottapalli, B.; Wolf-Hall, C.E.; Schwarz, P. Evaluation of Gaseous Ozone and Hydrogen Peroxide Treatments for Reducing Fusarium Survival in Malting Barley. J. Food Prot. 2005, 68, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Santos Alexandre, A.P.; Vela-Paredes, R.S.; Santos, A.S.; Costa, N.S.; Canniatti-Brazaca, S.G.; Calori-Domingues, M.A.; Augusto, P.E.D. Ozone Treatment to Reduce Deoxynivalenol (DON) and Zearalenone (ZEN) Contamination in Wheat Bran and Its Impact on Nutritional Quality. Food Addit. Contam. Part A 2018, 35, 1189–1199. [Google Scholar] [CrossRef]
- Sun, C.; Ji, J.; Wu, S.; Sun, C.; Pi, F.; Zhang, Y.; Tang, L.; Sun, X. Saturated Aqueous Ozone Degradation of Deoxynivalenol and Its Application in Contaminated Grains. Food Control 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Shao, H.; Chen, Z. Effect of Deoxynivalenol Detoxification by Ozone Treatment in Wheat Grains. Food Control 2016, 66, 137–144. [Google Scholar] [CrossRef]
- Wang, L.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Chen, Z. Effect of Ozone Treatment on Deoxynivalenol and Wheat Quality. PLoS ONE 2016, 11, e0147613. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.S.; Sarr, A.B.; Mayura, K.; Bailey, R.H.; Miller, D.R.; Rogers, T.D.; Norred, W.P.; Voss, K.A.; Plattner, R.D.; Kubena, L.F.; et al. Oxidative Degradation and Detoxification of Mycotoxins Using a Novel Source of Ozone. Food Chem. Toxicol. 1997, 35, 807–820. [Google Scholar] [CrossRef]
- Qi, L.; Li, Y.; Luo, X.; Wang, R.; Zheng, R.; Wang, L.; Li, Y.; Yang, D.; Fang, W.; Chen, Z. Detoxification of Zearalenone and Ochratoxin a by Ozone and Quality Evaluation of Ozonised Corn. Food Addit. Contam. Part A 2016, 33, 1700–1710. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Castanha, N.; Costa, N.S.; Santos, A.S.; Badiale-Furlong, E.; Augusto, P.E.D.; Calori-Domingues, M.A. Ozone Technology to Reduce Zearalenone Contamination in Whole Maize Flour: Degradation Kinetics and Impact on Quality. J. Sci. Food Agric. 2019, 99, 6814–6821. [Google Scholar] [CrossRef]
- Reid, L.M.; Zhu, X. Screening Corn for Resistance to Common Diseases in Canada; Agiculture and Agri-Food Canada: Ottawa, ON, Canada, 2005; Available online: https://publications.gc.ca/site/eng/9.687532/publication.html (accessed on 31 May 2022).
- American Association of Cereal Chemists. AACC International Method 44-15.02. Approved Methods of Analysis, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Herrman, T. Sampling: Procedures for Feed; Kansas State University Agricultural Experiment Station and Cooperative Extension Service: Manhattan, KS, USA, 2001; Available online: http://www.ksre.k-state.edu/bookstore/pubs/mf2036.pdf (accessed on 30 August 2022).
- Oliveira, C.A.F.; Gonçalves, N.B.; Rosim, R.E.; Fernandes, A.M. Determination of Aflatoxins in Peanut Products in the Northeast Region of São Paulo, Brazil. Int. J. Mol. Sci. 2009, 10, 174–183. [Google Scholar] [CrossRef]
- Abramovic, B.; Jajic, I.; Juric, V.; Gaál, F. Optimization of the Determination of Deoxynivalenol in Corn Samples by Liquid Chromatography and a Comparison of Two Clean-up Principles. J. Serbian Chem. Soc. 2005, 70, 1005–1013. [Google Scholar] [CrossRef]
- British Standard (BS). BS EN 15792:2009. British Standard (BS); BSI Group: London, UK, 2009. [Google Scholar]
- Association of Official Agricultural Chemists. AOAC Method No. 920.85. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- American Oil Chemists’ Society. AOCS Official Method No. Ce 2-66; Official Methods and Recommended Practices of the American Oil Chemists’ Society, AOCS: Champaign, IL, USA, 1992. [Google Scholar]
- Kovats, E.S. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 1958, 41, 1915–1932. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/hlca.19580410703 (accessed on 29 August 2022). [CrossRef]
- McCullagh, P. Regression Models for Ordinal Data. J. R. Stat. Soc. Ser. B Methodol. 1980, 42, 109–142. [Google Scholar] [CrossRef]
- Tutz, G. Regression for Categorical Data; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780511842061. [Google Scholar] [CrossRef]
- Hilbe, J.M. Logistic Regression Models; Chapman and Hall/CRC: Boca Raton, FL, USA, 2009; ISBN 9780429149139. [Google Scholar] [CrossRef]
- Aitchson, J. The Statistical Analysis of Compositional Data; Chapman & Hall: London, UK, 1986; ISBN 9781930665781. Available online: https://www.worldcat.org/title/statistical-analysis-of-compositional-data/oclc/13215689 (accessed on 30 May 2022).
- Pearson, K. Mathematical contributions to the theory of evolution—On a form of spurious correlation which may arise when indices are used in the measurement of organs. Proc. R. Soc. Lond. 1897, 60, 489–502. [Google Scholar] [CrossRef]
- Aitchison, J.; Greenacre, M. Biplots of Compositional Data. J. R. Stat. Soc. Appl. Stat. Ser. C 2002, 51, 375–392. [Google Scholar] [CrossRef]
- Santiago, R.; Cao, A.; Butrón, A. Genetic Factors Involved in Fumonisin Accumulation in Maize Kernels and Their Implications in Maize Agronomic Management and Breeding. Toxins 2015, 7, 3267–3296. [Google Scholar] [CrossRef] [PubMed]
- Annual Bulletin for Serbia the Year of 2021; Republic Hydrometeorlogical Service of Serbia: Belgrade, Serbia, 2022; pp. 1–21. Available online: https://www.hidmet.gov.rs/data/klimatologija/eng/2021.pdf (accessed on 29 August 2022).
- Mesterhazy, A.; Szieberth, D.; Toldine, E.T.; Nagy, Z.; Szabó, B.; Herczig, B.; Bors, I.; Tóth, B. Updating the Methodology of Identifying Maize Hybrids Resistant to Ear Rot Pathogens and Their Toxins—Artificial Inoculation Tests for Kernel Resistance to Fusarium Graminearum, F. Verticillioides, and Aspergillus Flavus. J. Fungi 2022, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC). No 1881/2006 of the European Parliament and of the Council of 19 December 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 31 May 2022).
- Sujayasree, O.J.; Chaitanya, A.K.; Bhoite, R.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Ozone: An Advanced Oxidation Technology to Enhance Sustainable Food Consumption through Mycotoxin Degradation. Ozone Sci. Eng. 2021, 44, 17–37. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Wang, Y.; Chen, Z. Detoxification of aflatoxin in corn flour by ozone. J. Sci. Food Agric. 2014, 94, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Porto, Y.D.; Trombete, F.M.; Freitas-Silva, O.; de Castro, I.M.; Direito, G.M.; Ascheri, J.L.R. Gaseous Ozonation to Reduce Aflatoxins Levels and Microbial Contamination in Corn Grits. Microorganisms 2019, 7, 220. [Google Scholar] [CrossRef]
- Sanjeev, P.; Chaudhary, D.P.; Sreevastava, P.; Saha, S.; Rajenderan, A.; Sekhar, J.C.; Chikkappa, G.K. Comparison of Fatty Acid Profile of Specialty Maize to Normal Maize. J. Am. Oil Chem. Soc. 2014, 91, 1001–1005. [Google Scholar] [CrossRef]
- Dupont, J.; White, P.J.; Carpenter, M.P.; Schaefer, E.J.; Meydani, S.N.; Elson, C.E.; Woods, M.; Gorbach, S.L. Food Uses and Health Effects of Corn Oil. J. Am. Coll. Nutr. 1990, 9, 438–470. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J. 2009, 1176, 1–11. [Google Scholar] [CrossRef]
- Obadi, M.; Zhu, K.-X.; Peng, W.; Noman, A.; Mohammed, K.; Zhou, H.-M. Characterization of Oil Extracted from Whole Grain Flour Treated with Ozone Gas. J. Cereal Sci. 2018, 79, 527–533. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Popović, M. Biohemija Biljaka; Poljoprivredni fakultet u Novom Sadu: Novi Sad, Serbia, 2005; ISBN 9788680735948. Available online: http://polj.uns.ac.rs/sr/node/1910 (accessed on 30 May 2022).
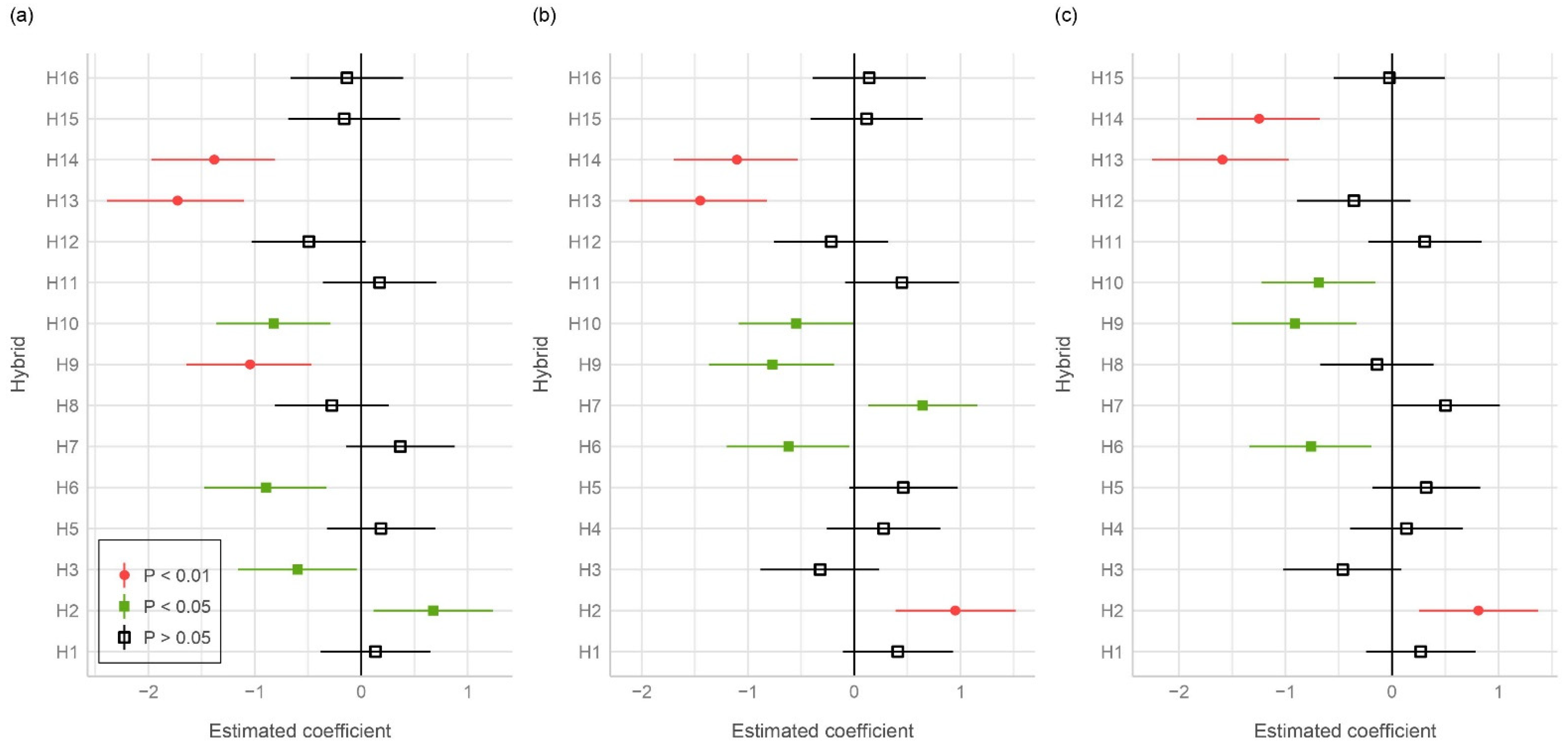
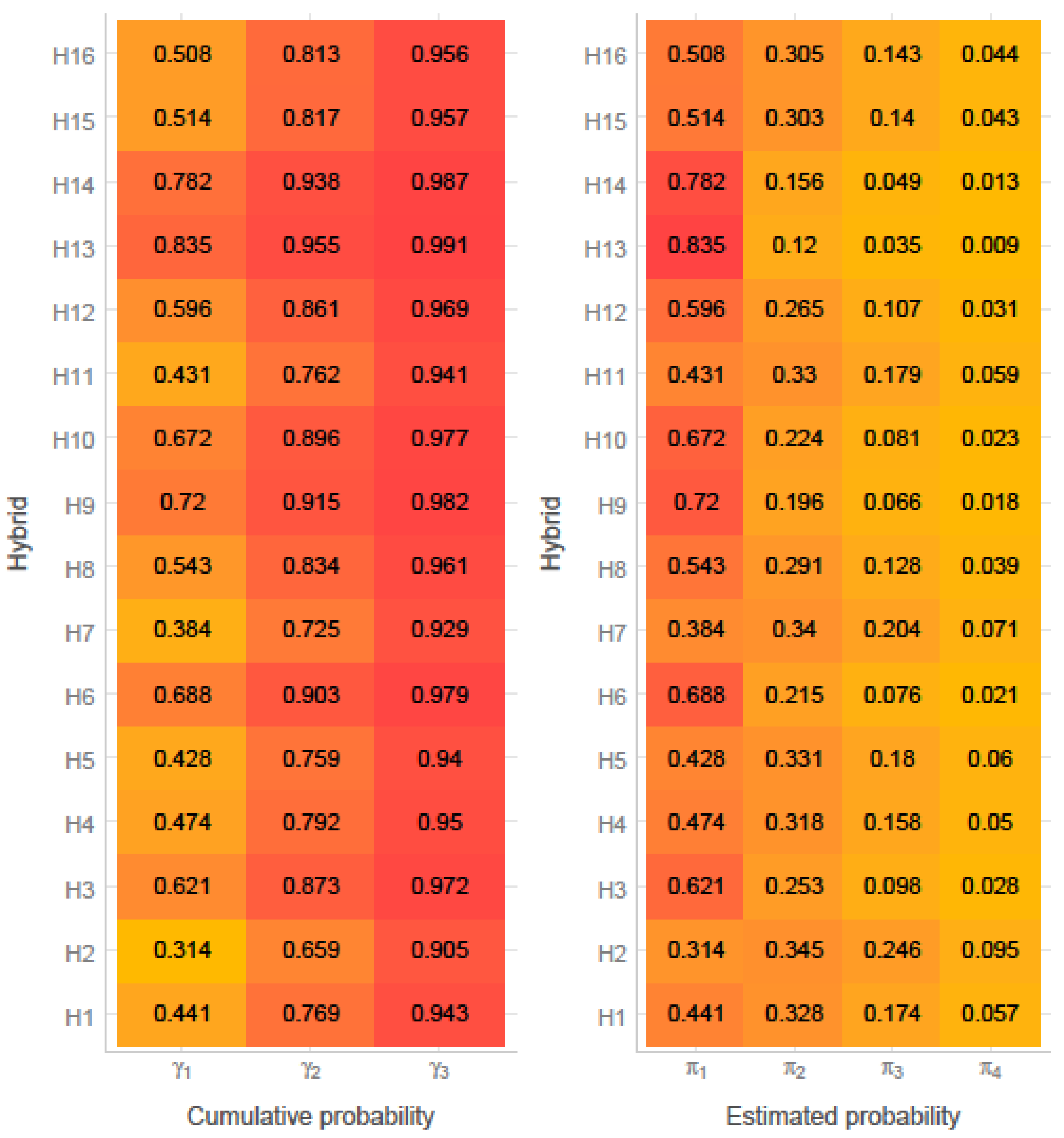
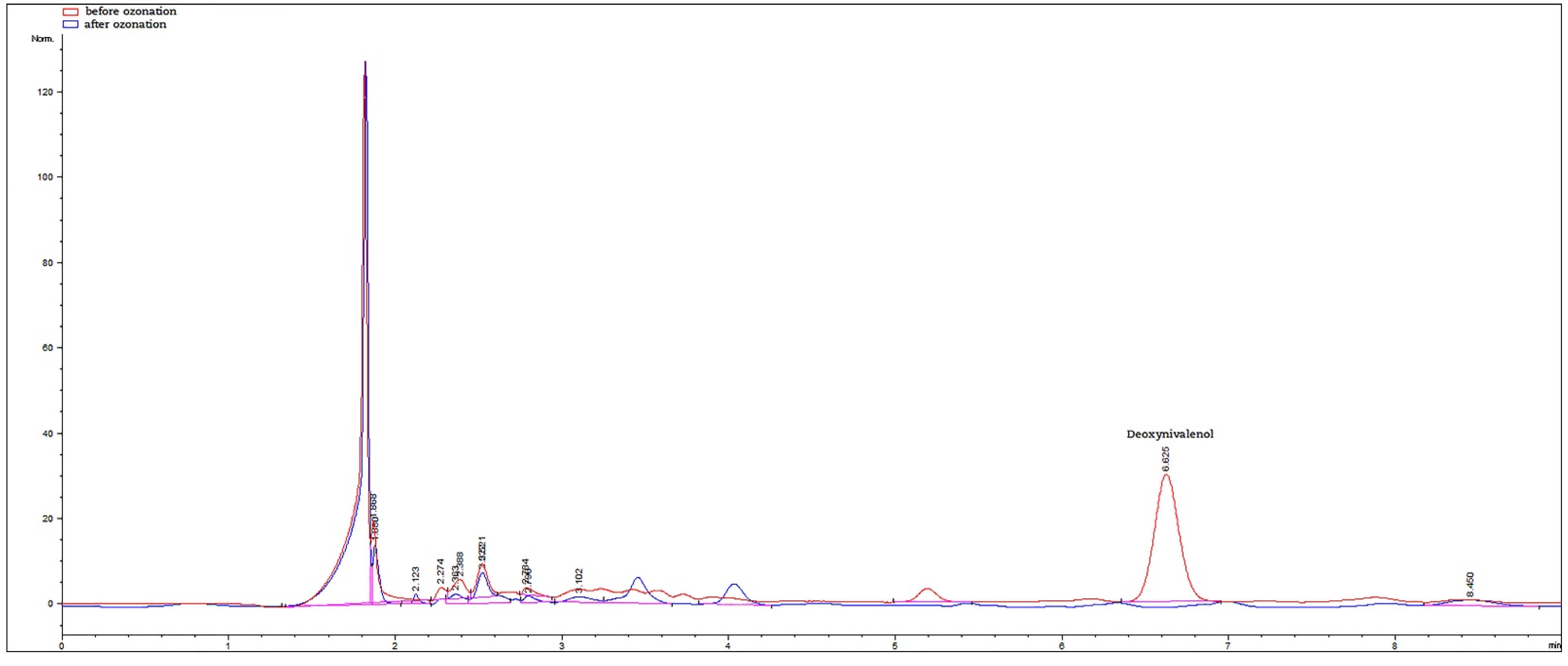
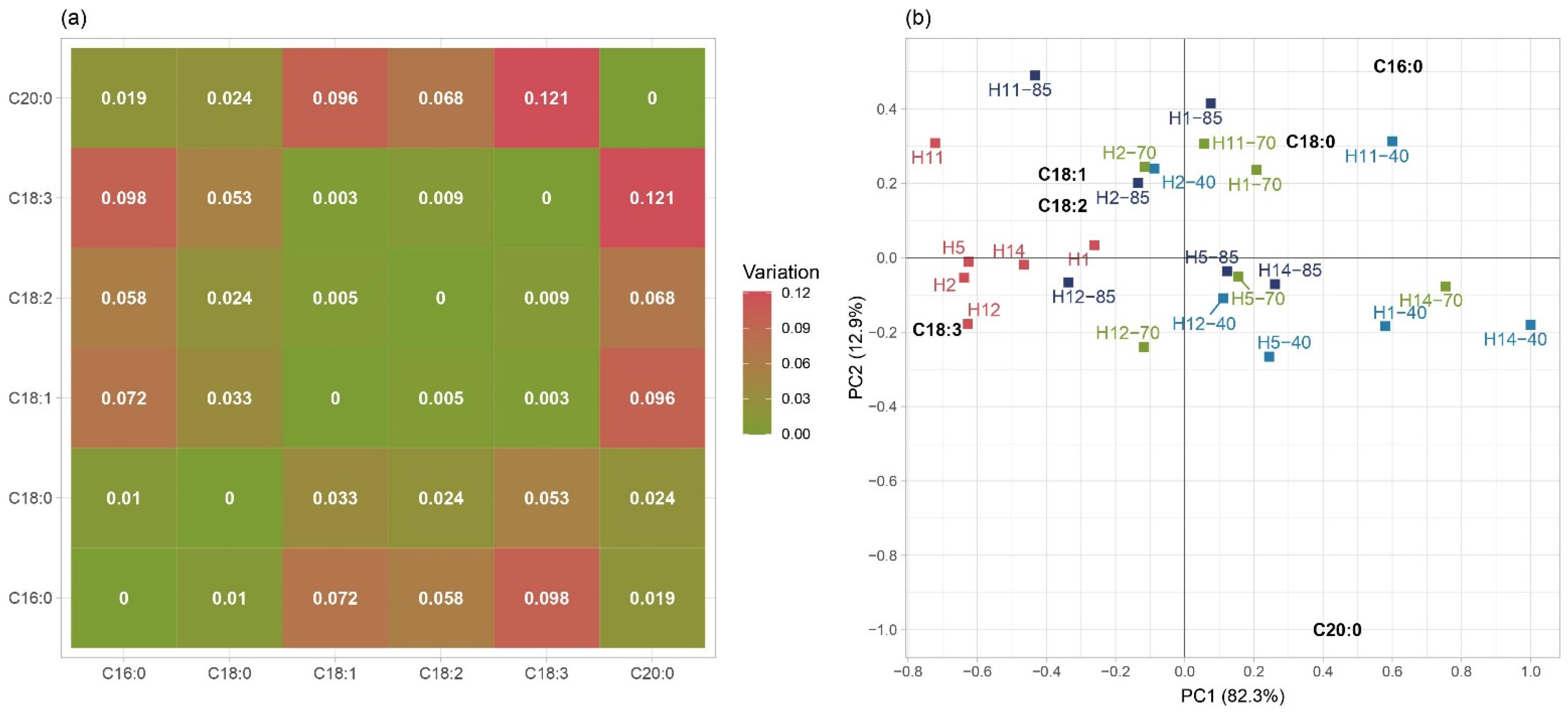
| Maize Hybrid | DON (µg/kg) | ZEN (µg/kg) | AFB1 (µg/kg) | AFG1 (µg/kg) | AFB2 (µg/kg) | AFG2 (µg/kg) | AFB1 + AFG1 + AFB2 + AFG2 (µg/kg) |
|---|---|---|---|---|---|---|---|
| H1 | 1399.5 ± 3.5 | <LOQ | 98.6 ± 2.7 | 5.4 ± 0.1 | 9.2 ± 0.1 | <LOQ | 113.2 ± 2.8 |
| H2 | 839.0 ± 2.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H3 | 656.0 ± 12.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H4 | 403.0 ± 17.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H5 | 321.0 ± 4.0 | <LOQ | 89.2 ± 6.9 | 3.4 ± 0.2 | 4.0 ± 0.2 | <LOQ | 96.5 ± 6.9 |
| H6 | 353.0 ± 16.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H7 | 362.0 ± 1.0 | <LOQ | 2.3 ± 0.1 | <LOQ | <LOQ | <LOQ | 2.3 ± 0.1 |
| H8 | 340.0 ± 14.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H9 | 444.0 ± 11.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H10 | 350.0 ± 9.0 | <LOQ | 1.1 ± 0 | <LOQ | <LOQ | <LOQ | 1.1 ± 0 |
| H11 | 467.5 ± 9.5 | <LOQ | 328.5 ± 2.5 | 5.4 ± 0.1 | 23.7 ± 0.4 | <LOQ | 357.6 ± 2.2 |
| H12 | 746.0 ± 18.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H13 | 533.0 ± 22.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| H14 | <LOQ | <LOQ | 43.4 ± 1.8 | <LOQ | 2.7 ± 0.1 | <LOQ | 46.1 ± 1.9 |
| H15 | 487.0 ± 6.0 | <LOQ | 8.9 ± 0.8 | <LOQ | 1.2 ± 0.1 | <LOQ | 10.0 ± 0.7 |
| H16 | 400.5 ± 6.5 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purar, B.; Djalovic, I.; Bekavac, G.; Grahovac, N.; Krstović, S.; Latković, D.; Janić Hajnal, E.; Živančev, D. Changes in Fusarium and Aspergillus Mycotoxin Content and Fatty Acid Composition after the Application of Ozone in Different Maize Hybrids. Foods 2022, 11, 2877. https://doi.org/10.3390/foods11182877
Purar B, Djalovic I, Bekavac G, Grahovac N, Krstović S, Latković D, Janić Hajnal E, Živančev D. Changes in Fusarium and Aspergillus Mycotoxin Content and Fatty Acid Composition after the Application of Ozone in Different Maize Hybrids. Foods. 2022; 11(18):2877. https://doi.org/10.3390/foods11182877
Chicago/Turabian StylePurar, Božana, Ivica Djalovic, Goran Bekavac, Nada Grahovac, Saša Krstović, Dragana Latković, Elizabet Janić Hajnal, and Dragan Živančev. 2022. "Changes in Fusarium and Aspergillus Mycotoxin Content and Fatty Acid Composition after the Application of Ozone in Different Maize Hybrids" Foods 11, no. 18: 2877. https://doi.org/10.3390/foods11182877
APA StylePurar, B., Djalovic, I., Bekavac, G., Grahovac, N., Krstović, S., Latković, D., Janić Hajnal, E., & Živančev, D. (2022). Changes in Fusarium and Aspergillus Mycotoxin Content and Fatty Acid Composition after the Application of Ozone in Different Maize Hybrids. Foods, 11(18), 2877. https://doi.org/10.3390/foods11182877








