The Controlled Semi-Solid Fermentation of Seaweeds as a Strategy for Their Stabilization and New Food Applications
Abstract
:1. Introduction
2. Materials and Methods
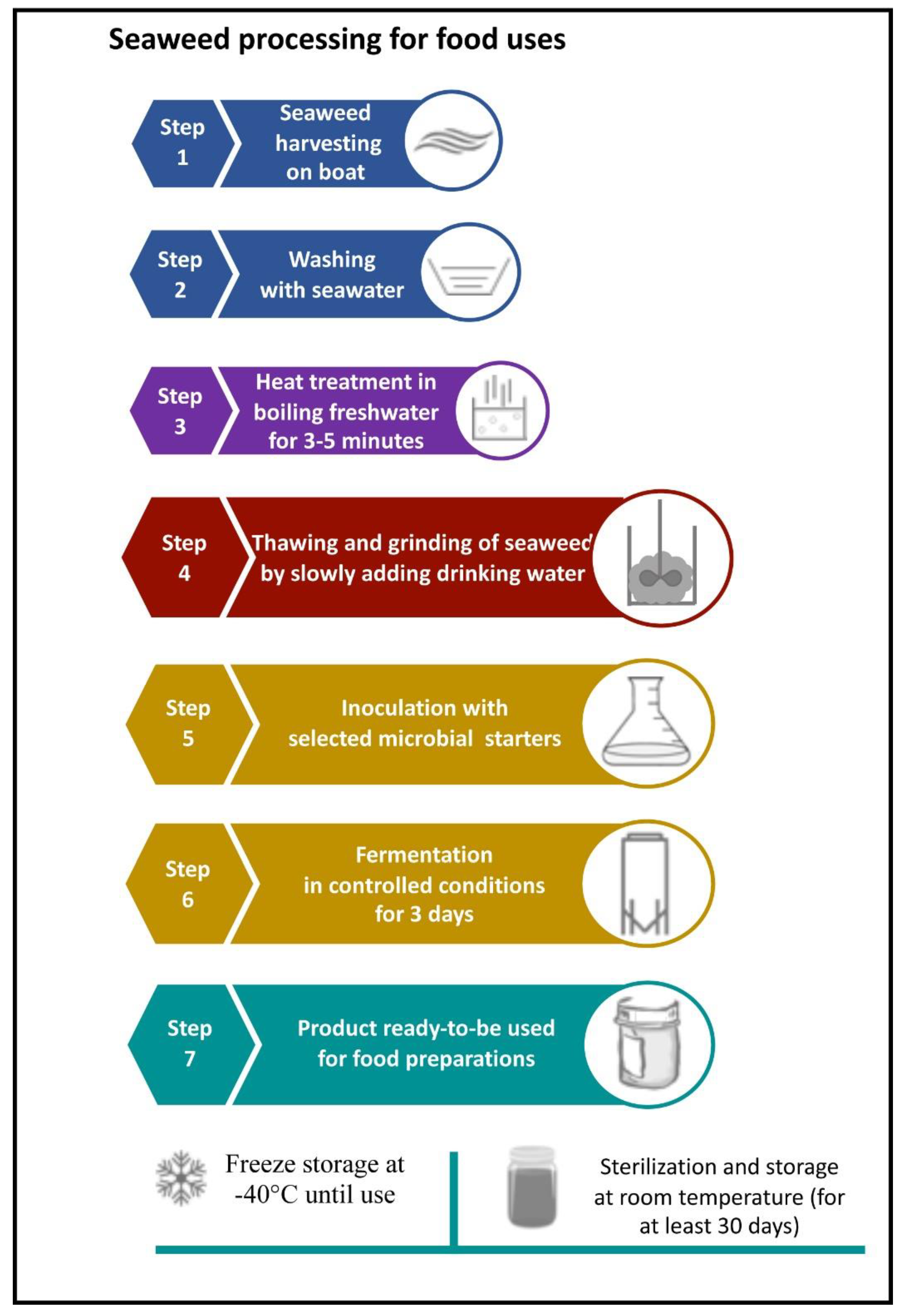
2.1. Sample Collection and Pre-Treatment
2.2. Microbiological Analyses
2.3. Molecular Identification of Bacterial Isolates
2.4. Seaweed Treatment
2.5. Enzyme Activities
2.6. Physicochemical Analyses
2.7. Total Polyphenol Content Analyses and Antioxidant Capacity Methods
2.8. Extraction and Quantification of Proteins
2.9. Determination of Insoluble Indigestible Fraction (IIF) of Seaweeds
2.10. Lipid Extraction and Fatty Acids Analysis
2.11. Calculation of Nutritional Indices for Assessing Fatty Acids
- IA: [C12:0 + (4 × C14:0) + C16:0]/UFA
- IT: (C14:0 + C16:0/[(0.5 × MUFA) +(0.5 × n-6 PUFA) + (3 × n-3 PUFA) + (n-3/n-6)]
- h/H: (MUFA + PUFA)/(C14:0 + C16:0)
- UI: 1 × (% monoenoics) + 2 × (% dienoics) + 3 × (% trienoics) + 4 × (% tetraenoics) + 5 × (% pentaenoics)
- PUFA = polyunsaturated fatty acids
- SFA = saturated fatty acids
- UFA = unsaturated fatty acids
- MUFA = monounsaturated fatty acids
2.12. Isoprenoids Content and Analysis
2.13. Cell Culture and Preparation of Aqueous Extract of Seaweeds
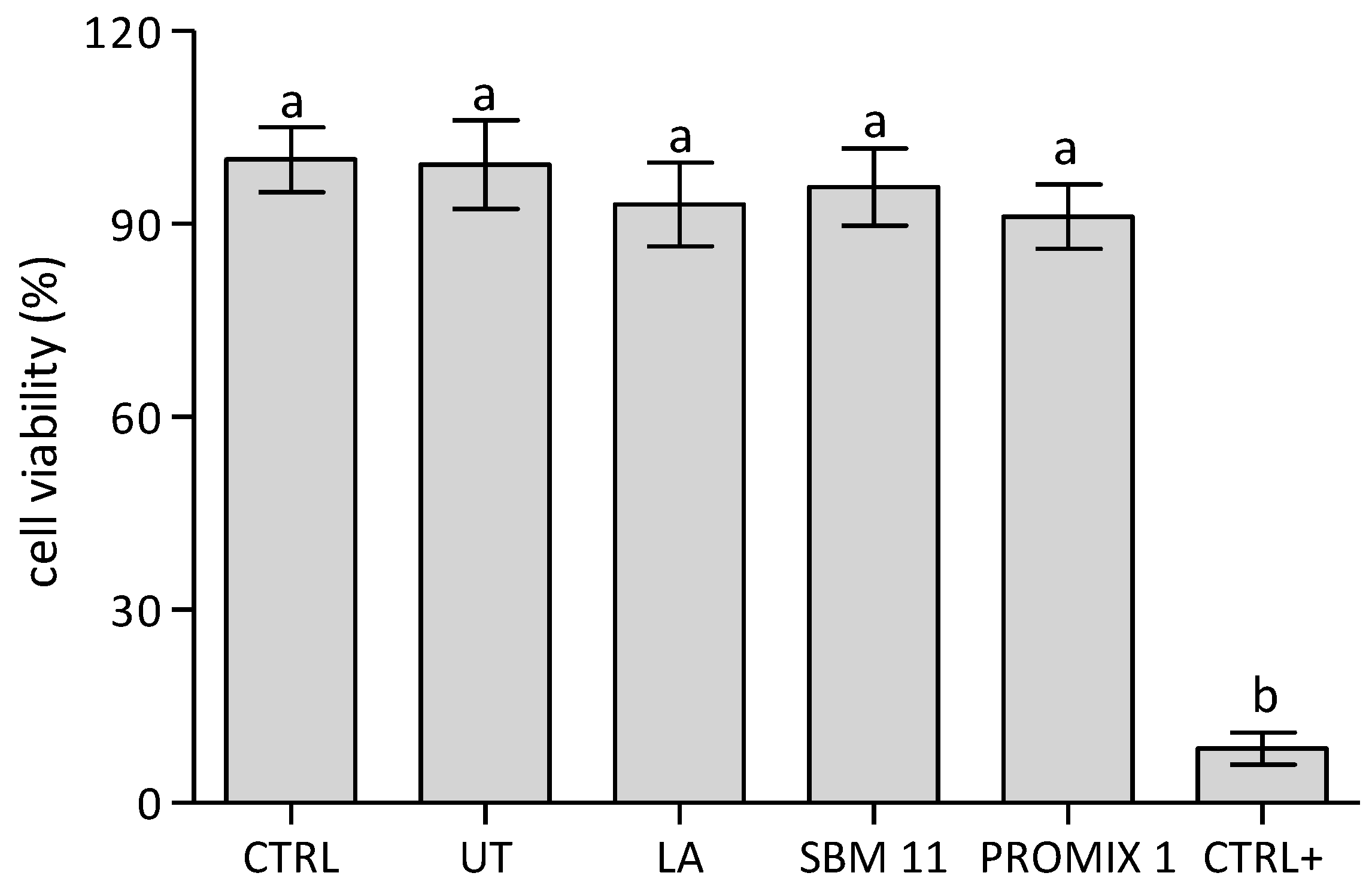
2.14. Cell Viability Assay
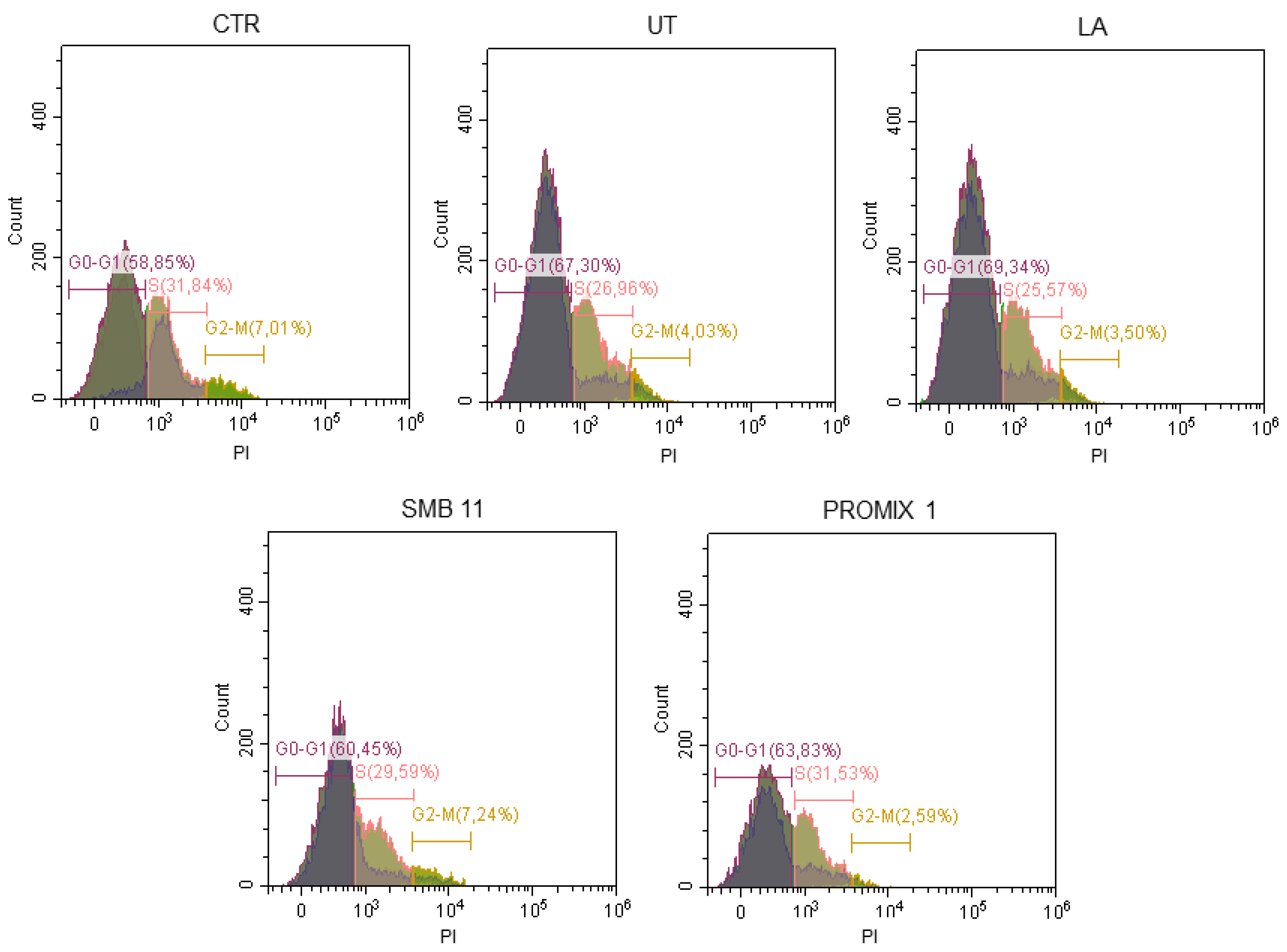
2.15. Cell Cycle Investigation
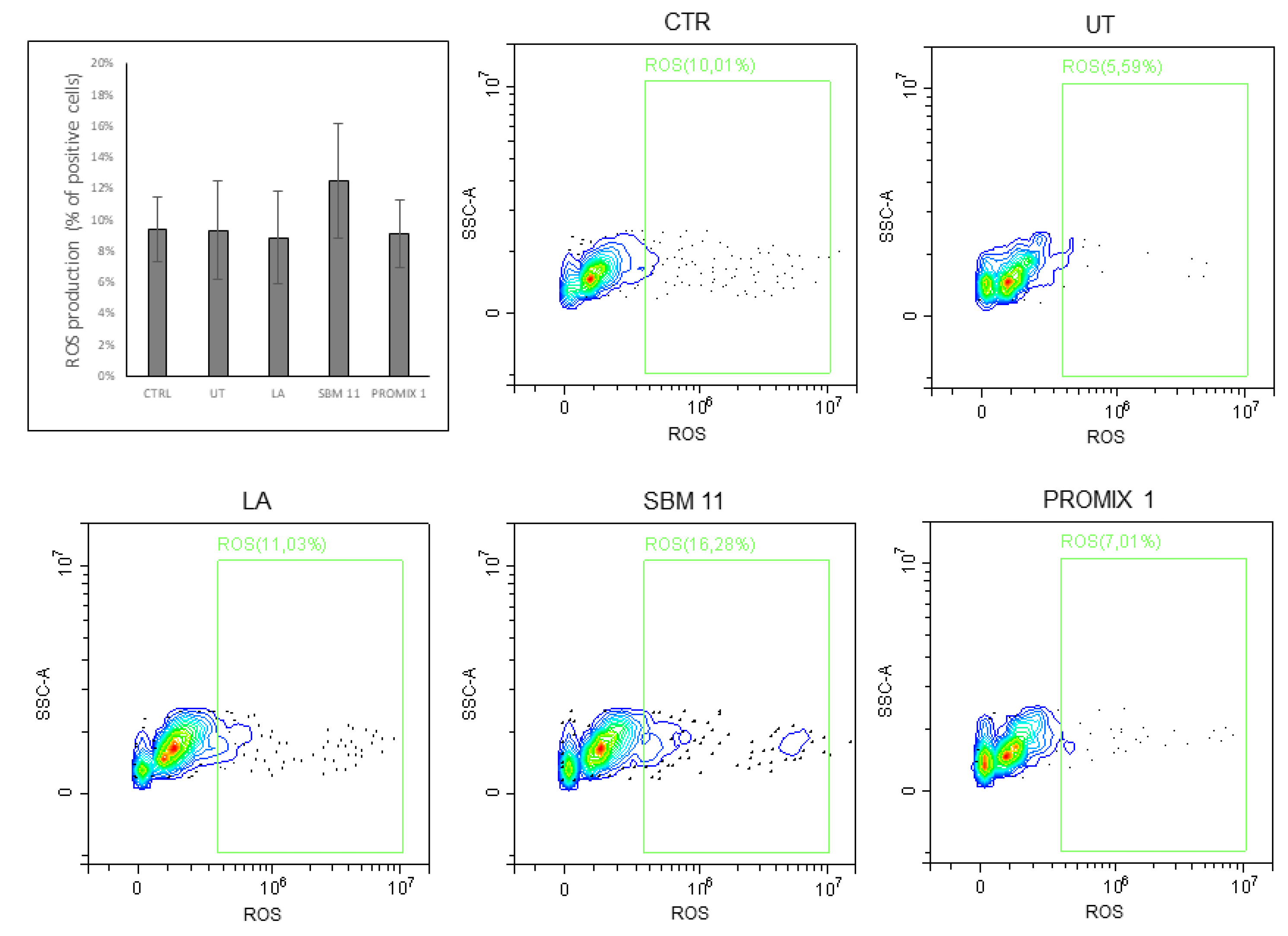
2.16. Cellular ROS Detection Assay
2.17. Statistical Analysis
3. Results
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzachor, A.; Richards, C.E.; Holt, L. Future foods for risk-resilient diets. Nat. Food 2021, 2, 326–329. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Dos Santos Fernandes De Araujo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; EUR 30779 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-40548-1. [Google Scholar] [CrossRef]
- FAO. The Global Status of Seaweed Production, Trade and Utilization; Globefish Research Programme: Rome, Italy, 2018; 120p, Available online: https://www.fao.org/3/ca1121en/ca1121en.pdf (accessed on 20 June 2022)ISBN 978-92-5-130870-7.
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Guiry, M.D.; 2000 – 2022. The Seaweed Site: Information on Marine Algae. Available online: https://www.seaweed.ie/aquaculture/noricultivation.php (accessed on 1 August 2022).
- Fleurence, J. Seaweeds as Food. Seaweed in Health and Disease Prevention; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 149–167. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2283/2015 of the European Parliament and of the Council of 25 November 2015. On Novel Foods, Amending Regulation (EU) N. 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) N. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) N. 1852/2001. Official Journal of the European Communities, L 327/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R2283 (accessed on 1 July 2022).
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, F., Eds.; Springer: Singapore, 2018; pp. 27–66. [Google Scholar]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as a fermentation substrate: A challenge for the food processing industry. Processes 2021, 9, 1953. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. OALib 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent advances in industrial applications of seaweeds. Crit. Rev. Food Sci. Nutr. 2021, 1–30. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Rajauria, G. Effect of heating and probiotic fermentation on the phytochemical content and antioxidant potential of edible Irish brown seaweeds. Bot. Mar. 2012, 55, 527–537. [Google Scholar] [CrossRef]
- Uchida, M.; Kurushima, H.; Ishihara, K.; Murata, Y.; Touhata, K.; Ishida, N.; Niwa, K.; Araki, T. Characterization of fermented seaweed sauce prepared from nori (Pyropia yezoensis). J. Biosci. Bioeng. 2017, 123, 327–332. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal fermentation—The seed for a new fermentation industry of foods and related products. Jpn. Agric. Res. Q. 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Bajury, D.M.; Rawi, M.H.; Sazali, I.H.; Abdullah, A.; Sarbini, S.R. Prebiotic evaluation of red seaweed (Kappaphycus alvarezii) using in vitro colon model. Int. J. Food Sci. Nutr. 2017, 68, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Marrion, O.; Schwertz, A.; Fleurence, J.; Guéant, J.L.; Villaume, C. Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Food/Nahrung 2003, 47, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of lactic acid from seaweed hydrolysates via lactic acid bacteria fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Løvdal, T.; Lunestad, B.T.; Myrmel, M.; Rosnes, J.T.; Skipnes, D. Microbiological food safety of seaweeds. Foods 2021, 10, 2719. [Google Scholar] [CrossRef] [PubMed]
- Terefe, N.S. Food Fermentation. Reference Module in Food Science; Elsevier: Werribee, Australia, 2016. [Google Scholar] [CrossRef]
- Economou, C.N.; Makri, A.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour. Technol. 2010, 101, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Hu, L.; Liu, S.; Yu, H.; Xing, R.; Li, F.; Wang, X.; Li, P. In vitro prebiotic effects of seaweed polysaccharides. J. Oceanol. Limnol. 2017, 36, 926–932. [Google Scholar] [CrossRef]
- Rodrigues, D.; Walton, G.; Sousa, S.; Rocha-Santos, T.A.P.; Duarte, A.C.; Freitas, A.C.; Gomes, A.M.P. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT 2016, 73, 131–139. [Google Scholar] [CrossRef]
- Machado, I.; Teixeira, J.A.; Rodríguez-Couto, S. Semi-solid-state fermentation: A promising alternative for neomycin production by the actinomycete Streptomyces fradiae. J. Biotechnol. 2013, 165, 195–200. [Google Scholar] [CrossRef]
- Bleve, G.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 2019, 8, 263. [Google Scholar] [CrossRef]
- Bleve, G.; Tufariello, M.; Durante, M.; Perbellini, E.; Ramires, F.A.; Grieco, F.; Cappello, M.S.; De Domenico, S.; Mita, G.; Tasioula-Margari, M.; et al. Physico-chemical and microbiological characterization of spontaneous fermentation of Cellina di Nardò and Leccino table olives. Front. Microbiol. 2014, 5, 570. [Google Scholar] [CrossRef]
- Bleve, G.; Lezzi, C.; Chiriatti, M.A.; D’Ostuni, I.; Tristezza, M.; Di Venere, D.; Sergio, L.; Mita, G.; Grieco, F. Selection of non-conventional yeasts and their use in immobilized form for the bioremediation of olive oil mill waste waters. Bioresour. Technol. 2011, 102, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, S.J.; Chung, S.W.; Choi, Y.J.; Yoo, J.Y.; Chung, D.H. Changes of microorganisms, enzyme activities and major components during the fermentation of korean traditional doenjang and kochujang. Korean J. Microbiol. Biotechnol. 1996, 24, 247–253. [Google Scholar]
- Walter, H.E. Proteinases: Methods with Haemoglobin, Casein and Azocoll as Substrates. In Methods of Enzymatic Analysis; Bergmeyer, H.J., Ed.; Wiley-VCH: Weinham, Germany, 1984; Volume V, p. 270. [Google Scholar]
- Moyano, F.J.; Díaz, M.; Alarcón, F.J.; Sarasquete, M.C. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 1996, 15, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.B.; Fraga, L.P.; Fleuri, L.F.; Macedo, G.A. Lipase and esterase: To what extent can this classification be applied accurately? Food Sci. Technol. 2011, 31, 603–613. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kang, S.G.; Woo, J.; Lee, J.; Choi, H.; Kim, S. A new esterase showing similarity to putative dienelactone hydrolase from a strict marine bacterium, Vibrio sp. GMD509. Appl. Microbiol. Biotechnol. 2007, 77, 107–115. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: A comparison between the black carrot and the Apulian Landrace “Polignano” carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro-and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Rupérez, P.; Toledano, G. Indigestible fraction of edible marine seaweeds. J. Sci. Food Agric. 2003, 83, 1267–1272. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; Marrese, P.P.; Rizzi, V.; De Caroli, M.; Piro, G.; Fini, P.; Russo, G.L.; Mita, G. α-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E. A 20-year update on the state of seaweed resources in Italy. Bot. Mar. 2019, 62, 249–264. [Google Scholar] [CrossRef]
- Ozogul, F.; Hamed, I. Lactic Acid Bacteria: Lactobacillus spp.: Lactobacillus acidophilus; Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar] [CrossRef]
- Stavropoulou, D.A.; De Vuyst, L.; Leroy, F. Nonconventional starter cultures of coagulase-negative staphylococci to produce animal-derived fermented foods, a SWOT analysis. J. Appl. Microbiol. 2018, 125, 1570–1586. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- UNI EN ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 1 July 2022).
- European Union. Commission Regulation (EU) No 1441/2007 of 5 December 2007 Amending Regulation (EC) N. 2073/2005 on Microbiological Criteria for Foodstuffs. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/reg/2007/1441/oj (accessed on 1 July 2022).
- European Union. Commission Regulation (EU) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 4 July 2022).
- FCD 2009: Federation des Entreprises de Commerce et de la Distribution (FCD) (2009). Critères Microbiologiques Applicables à Partir de 2010 aux Marques de Distributeurs, Marques Premiers Prix et Matières Premières dans leur Conditionnement Initial Industriel. Available online: http://www.qualtech-groupe.com/wp-content/uploads/2012/10/FCD-criteres-process-31-10-09.pdf (accessed on 11 July 2022).
- AFSSA 2007. Available online: https://www.anses.fr/fr/system/files/MIC2007sa0174.pdf (accessed on 4 July 2022).
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stus—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. ISO: Geneva, Switzerland, 2001. Available online: https://www.iso.org/standard/29824.html (accessed on 11 July 2022).
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/63504.html (accessed on 11 July 2022).
- ANZFA. Safe Food Australia: A Guide to the Food Safety Standards. Commonwealth of Australia, Australia New Zealand Food Authority Publ., Canberra, Australia. 2001. Available online: http://www.anzfa.gov.au (accessed on 11 July 2022).
- ISO 7932:2004/AMD 1:2020; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 Degrees C—Amendment 1: Inclusion of Optional Tests. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/76664.html (accessed on 4 July 2022).
- ISO 7937:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Clostridium Perfringens—Colony-Count Technique. ISO: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/36588.html (accessed on 4 July 2022).
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38282.html (accessed on 4 July 2022).
- Legislative Decree No. 131 of the Italian Parliament of 10 May 2007. Agreement, Pursuant to Article 8, Paragraph 6, of the Law of 5 June 2003, n. 131, between the Government, the Regions and the Autonomous Provinces of Trento and Bolzano on Guidelines Relating to the Application of the EC Regulation. Official Gazette of the Italian Republic, No. 126, General Series of 30 May 2007. Available online: https://www.gazzettau_ciale.it/eli/gu/2007/05/30/124/so/126/sg/pdf (accessed on 18 July 2022).
- NSSP 2017: National Shellfish Sanitation Program Guide for the Control of Molluscan Shellfish: 2017 Revision. Guide for the Control of Molluscan Shellfish 2017 Revision. From the U.S. Food and Drug Administration Website. Available online: https://www.fda.gov/food/federalstate-food-programs/nationalshellfish-sanitation-program-nssp (accessed on 11 July 2022).
- UNI EN ISO 6888-2:1999; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium. ISO: Geneva, Switzerland, 1999. Available online: https://www.iso.org/standard/25571.html (accessed on 11 July 2022).
- ISO 21872-1:2017; Microbiology of the Food Chaflorin—Horizontal Method for the Determination of Vibrio spp.—Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/74112.html (accessed on 11 July 2022).
- Canadian Food Inspection Agency. 2021. Available online: https://www.inspection.gc.ca/eng/1297964599443/1297965645317 (accessed on 4 July 2022).
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0,95. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38275.html (accessed on 11 July 2022).
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0,95. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38276.html (accessed on 11 July 2022).
- Flores, M.; Olivares, A. Flavor. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrà, F., Ed.; John Wiley Sons: Chichester, UK, 2014; pp. 217–226. [Google Scholar]
- Zhao, C.J.; Schieber, A.; Ganzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Al-kaf, H.A.; Huyop, F.; Zainol, N.A. Growth analysis of Lactobacillus Acidophilus using different non-digestible carbohydrates. Int. J. Life Sci. Biotechnol. 2021, 4, 33–45. [Google Scholar] [CrossRef]
- Muller, A.; Reichhardt, R.; Fogarassy, G.; Bosse, R.; Gibis, M.; Weiss, J.; Schmidt, H.; Weiss, A. Safety assessment of selected Staphylococcus carnosus strains with regard to their application as meat starter culture. Food Control 2016, 66, 93–99. [Google Scholar] [CrossRef]
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic profiling of Xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef]
- Kum, S.J.; Yang, S.O.; Lee, S.M.; Chang, P.S.; Choi, Y.H.; Lee, J.J.; Hurh, B.S.; Kim, Y.S. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J. Agric. Food Chem. 2015, 63, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R.; Rastogi, R.P.; Madamwar, D. Antioxidant potential of phycobiliproteins: Role in anti-aging research. Biochem. Anal. Biochem. 2015, 4, 1009–2161. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef]
- Ramires, F.A.; Durante, M.; Maiorano, G.; Migoni, D.; Rampino, P.; Fanizzi, F.P.; Perrotta, C.; Mita, G.; Grieco, F.; Bleve, G. Industrial scale bio-detoxification of raw olive mill wastewaters by the use of selected microbial yeast and bacterial strains to obtain a new source for fertigation. J. Environ. Manag. 2020, 265, 110574. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. 834 Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, micronutrient and physicochemical properties of the dried red seaweeds Gracilaria edulis and Gracilaria corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal changes in the nutritional composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef]
- Michas, G.; Micha, R.; Zampelas, A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 2014, 234, 320–328. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Werenska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Debbarma, J.; Rao, B.M.; Murthy, L.N.; Mathew, S.; Venkateshwarlu, G.; Ravishankar, C.N. Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J. Fish 2016, 63, 81–87. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Jensen, I.-J.; Eilertsen, K.-E. Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs 2016, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal Polysaccharides, Novel Application, and Outlook. In Algae Based Polymers, Blends, and Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Kazłowski, B.; Chiu, Y.H.; Kazłowska, K.; Pan, C.L.; Wu, C.J. Prevention of Japanese encephalitis virus infections by low-degree-polymerisation sulfated saccharides from Gracilaria sp. and Monostroma nitidum. Food Chem. 2012, 133, 866–874. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydr. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Minicante, S.A.; Michelet, S.; Bruno, F.; Castelli, G.; Vitale, F.; Sfriso, A.; Morabito, M.; Genovese, G. Bioactivity of Phycocolloids against the Mediterranean Protozoan Leishmania infantum: An inceptive study. Sustainability 2016, 8, 1131. [Google Scholar] [CrossRef]
- Sudharsan, S.; Subhapradha, N.; Seedevi, P.; Shanmugam, V.; Madeswaran, P.; Shanmugam, A.; Srinivasan, A. Antioxidant and anticoagulant activity of sulfated polysaccharide from Gracilaria debilis (Forsskal). Int. J. Biol. Macromol. 2015, 81, 1031–1038. [Google Scholar] [CrossRef]
- De Casabianca, M.L.; Laugier, T.; Collart, D. Impact of shellfish farming eutrophication on benthic macrophyte communities in the Thau lagoon, France. Aquac. Int. 1997, 5, 301–314. [Google Scholar] [CrossRef]
- Ksouri, J.; Said, R.B. Potentialités en macroalgues: Cartographie et biomasse de l’agarophyte Gracilaria dans le lac de Bizerte. Bull. Inst. Natn. Sci. Technol. Mer. Salammbo 1998, 25, 17–34. [Google Scholar]
- Menéndez, M.; Comin, F.A. Spring and summer proliferation of oating macroalgae in a Mediterranean coastal lagoon (Tancada Lagoon, Ebro Delta, NE Spain). Estuar. Coast. Mar. Sci. 2000, 51, 215–226. [Google Scholar] [CrossRef]
- Sfriso, A.; Wolf, M.A.; Maistro, S.; Sciuto, K.; Moro, I. Spreading and autoecology of the invasive species Gracilaria vermiculophylla (Gracilariales, Rhodophyta) in the lagoons of the north-western Adriatic Sea (Mediterranean Sea, Italy). Estuar. Coast. Shelf Sci. 2012, 114, 192–198. [Google Scholar] [CrossRef]
- Khaled, A.; Hessein, A.; Abdel-Halim, A.M.; Morsy, F.M. Distribution of heavy metals in seaweeds collected along Marsa-Matrouh beaches, Egyptian Mediterranean Sea. Egypt. J. Aquat. Res. 2014, 40, 363–371. [Google Scholar] [CrossRef]
- Mensi, F.; Nasraoui, S.; Bouguerra, S.; Ghedifa, A.B.; Chalghaf, M. Effect of lagoon and sea water depth on Gracilaria gracilis growth and biochemical composition in the Northeast of Tunisia. Sci. Rep. 2020, 10, 10014. [Google Scholar] [CrossRef] [PubMed]
- Belattmania, Z.; Bhaby, S.; Nadri, A.; Khaya, K.; Bentiss, F.; Jama, C.; Reani, A.; Vasconcelos, V.; Sabour, B. Gracilaria gracilis (Gracilariales, Rhodophyta) from Dakhla (Southern Moroccan Atlantic Coast) as source of Agar: Content, chemical characteristics, and gelling properties. Mar. Drugs 2021, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An innovative IMTA system: Polychaetes, sponges and macroalgae co-cultured in a Southern Italian in-shore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]

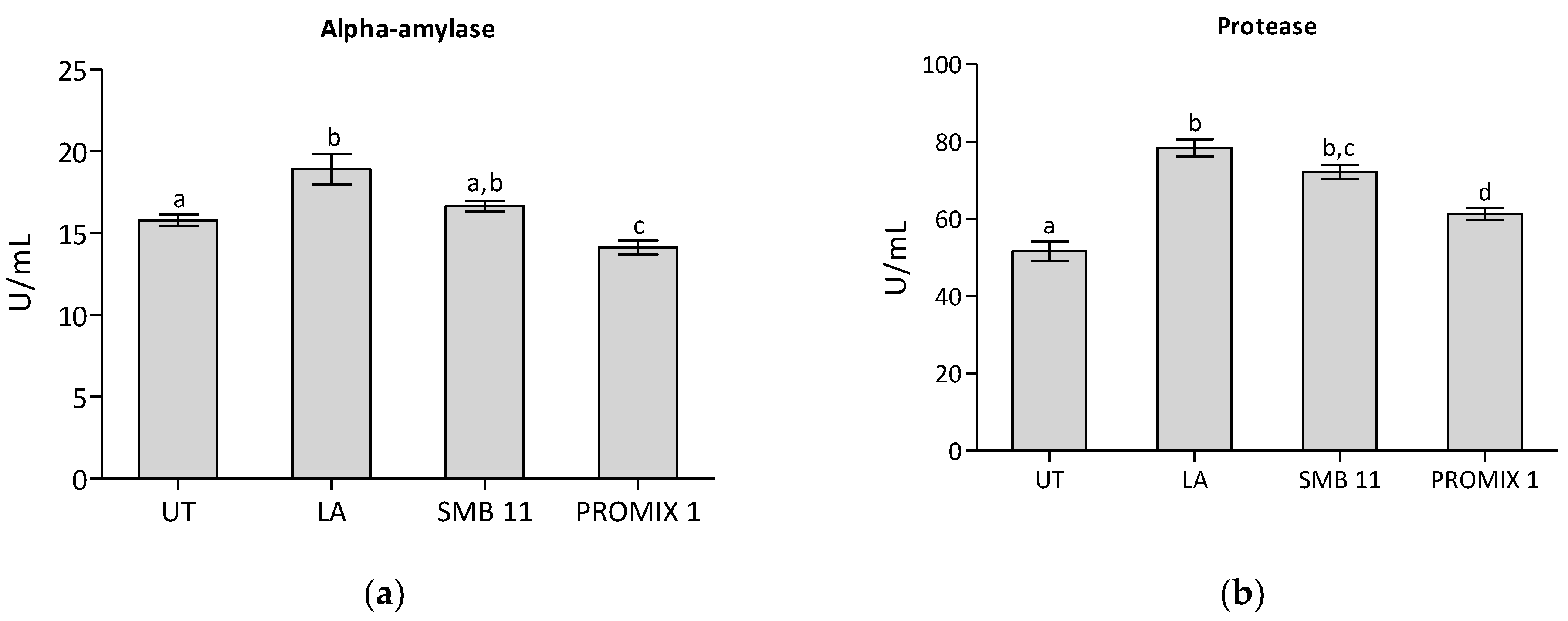
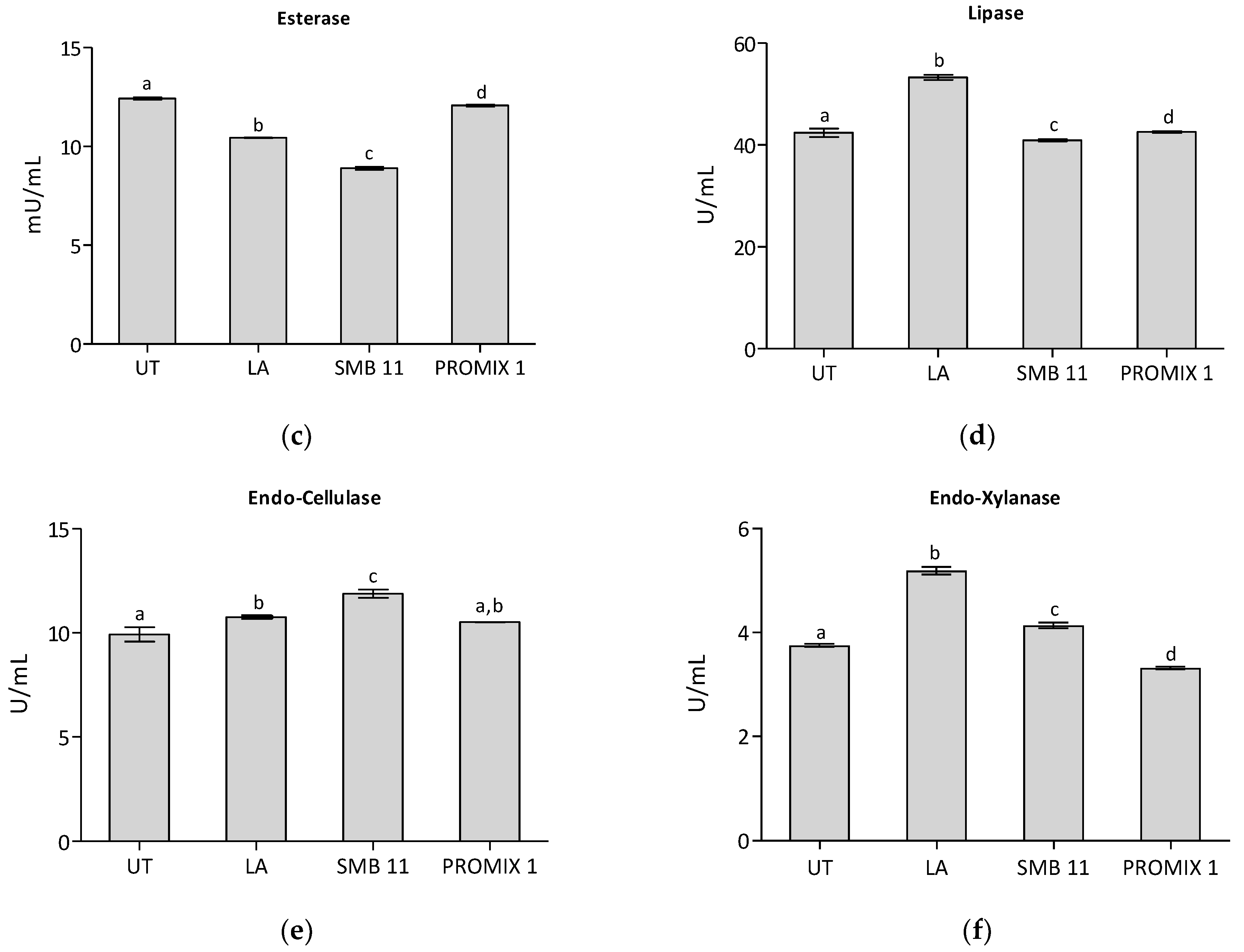
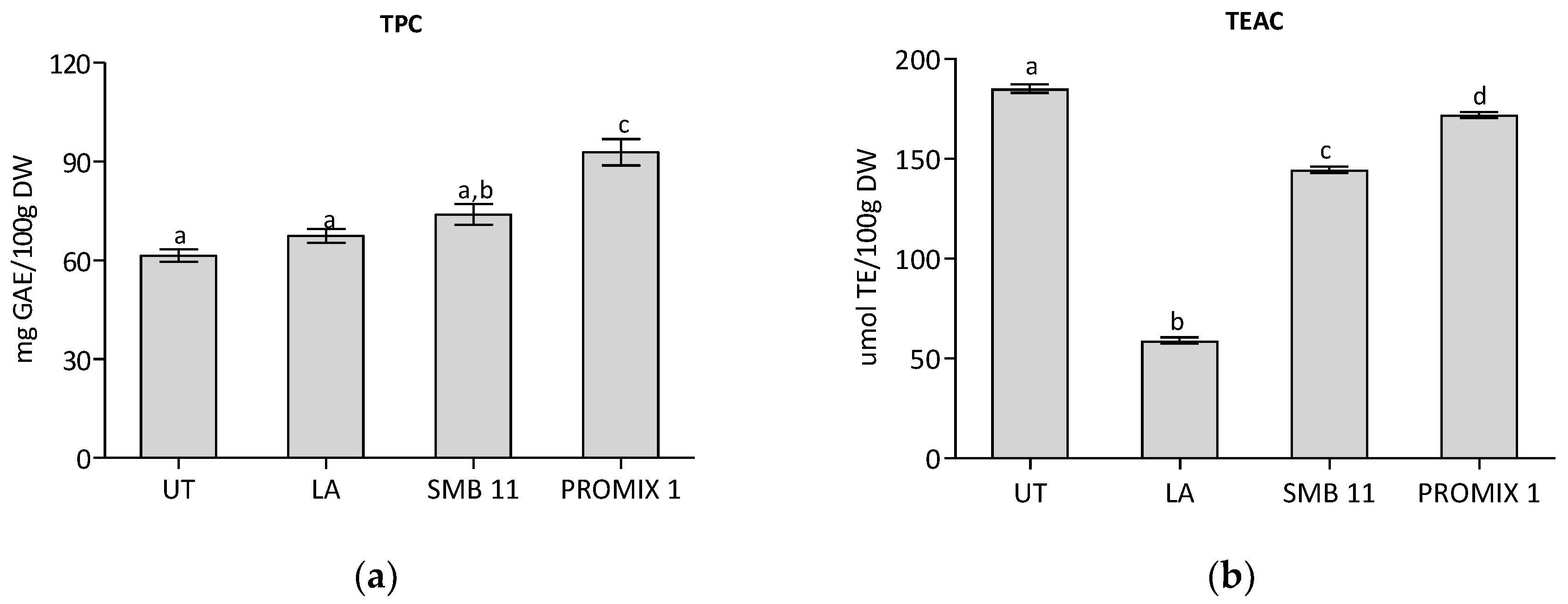
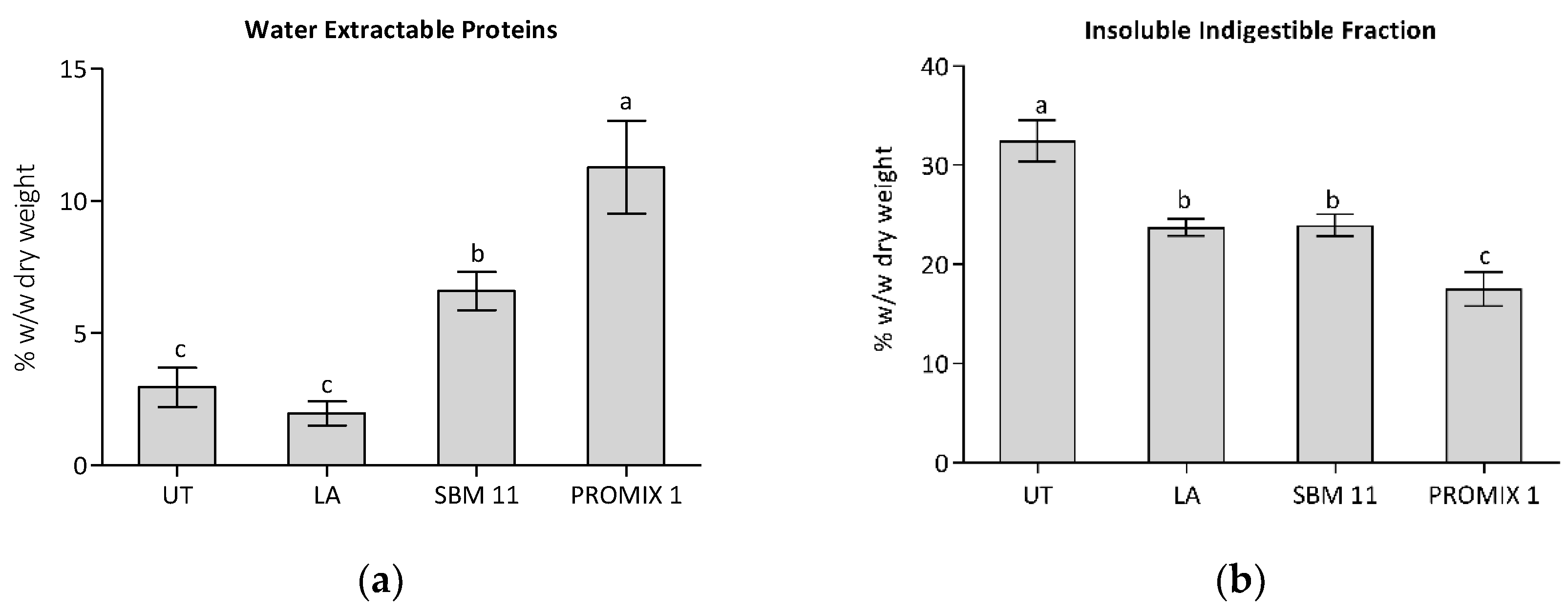
| Microorganisms | Medium | Untreated Sample | LA | PROMIX 1 | SBM-11 |
|---|---|---|---|---|---|
| Mean (CFU/g) | Mean (CFU/g) | Mean (CFU/g) | Mean (CFU/g) | ||
| TBC | PCA | 2.2 × 108 ± 6.8 × 107 (a) | 9.7 × 106 ± 1.6 × 105 (a) | 1.5 v 108 ± 2.6 × 107 (a) | 1.1 × 107 ± 1.4 × 106 (a) |
| sNA | 4.5 × 108 ± 6 × 107 (b) | 3.9 × 106 ± 2.7 × 105 (b) | 9.2 × 107 ± 7.2 × 106 (b) | 1.7 × 107 ± 3.4 × 106 (b) | |
| Bacillus spp. | BCSA | 1.3 × 108 ± 6.5 × 107 (a) | 5 × 105 ± 5 × 104 (c) | 8 × 107 ± 6.9 × 106 (a) | 5.6 × 106 ± 4.2 × 105 (c) |
| Bacillus cereus | 0 (c) | 0 (d) | 0 (c) | 0 (d) | |
| H2S-producing bacteria | IRON AGAR | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Clostridium perfringens | SPS | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Enterobacteriaceae | VRBGA | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Coli-Aerogenes Bacteria | VRBA | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Coagulase positive staphylococci | Baird Parker Agar | 0 (c) | 0(d) | 0 (c) | 0 (d) |
| Pathogenic staphylococci | MSA | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Vibrio spp. | TCBSA | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Lactic acid bacteria | sMRS-Glucose | 1.7 × 108 ± 7.8 × 107 (a) | 7.7 × 107 ± 3.6 × 106 (e) | 1.8 × 108 ± 1.7 × 107 (a) | 1.7 × 107 ± 1.4 × 106 (a) |
| Staphylococci | sMRS-Sucrose | 1.1 × 108 ± 5.2 × 107 (a) | 4.8 × 106 ± 1.1 × 105 (b) | 2.3 × 108 ± 2.4 × 107 (d) | 1.4 × 107 ± 2.5 × 106 (a, b) |
| Yeast/Moulds | DRBC | 0 (c) | 4 × 101 ± 6.4 × 100 (d) | 0 (c) | 0 (d) |
| sSDA | 0 (c) | 0 (d) | 0 (c) | 0 (d) |
| Assays | Limits | Analytical Reference Method | Reference |
|---|---|---|---|
| Aerobic colony count | <105 CFU/g (for multi-ingredient cooked ready to eat preparations) <106 CFU/g (for multi-ingredient not cooked ready to eat preparations) | [46] | [47,48,49,50] |
| β-glucuronidase positive Escherichia coli | <10 CFU/g | [51] | [47,48] |
| Enterobacteriaceae | <102 CFU/g | [52] | [53] |
| Presumptive Bacillus cereus | <102 CFU/g | [54] | [53] |
| Clostridium perfringens | <10 CFU/g | [55] | [49,50] |
| Coliforms | <10 CFU/g <=70 MPN/100 mL | [56] | [57,58] |
| Coagulase positive Staphylococci | <102 CFU/g, 102 < X < 103 CFU/g | [59] | [47,48] |
| Vibrio parahaemolyticus | Absence in 25 g | [60] | [52,61] |
| Vibrio cholearae | Absence in 25 g | [60] | [61] |
| Moulds and yeasts | <102 CFU/g (Marinated octopus, seafood cocktail) | [62,63] | [49] |
| pH | ||||
|---|---|---|---|---|
| 0 | 24 h | 48 h | 72 h | |
| UT | 8.6 | 5.82 | 5.19 | 6.02 |
| LA | 8.6 | 4.9 | 4.41 | 4.2 |
| SMB-11 | 8.6 | 4.96 | 5.08 | 4.7 |
| PROMIX 1 | 8.6 | 5.13 | 4.87 | 4.2 |
| UT | LA | PROMIX 1 | SBM-11 | |
|---|---|---|---|---|
| Lipids % DW | 5.38 ± 0.42 * | 5.28 ± 0.36 | 3.61 ± 1.03 * | 5.41 ± 0.41 |
| % TFA | ||||
| SFA | ||||
| Myristic acid (14:0) | 3.52 ± 0.24 * | 2.46 ± 0.13 * | 3.16 ± 0.33 | 1.23 ± 0.36 * |
| Palmitic acid (16:0) | 21.63 ± 1.91 * | 39.10 ± 2.31 * | 26.11 ± 1.98 * | 20.02 ± 1.85 * |
| Stearic acid (18:0) | 9.20 ± 0.08 * | 39.73 ± 3.21 * | 18.98 ± 1.81 * | 20.06 ± 1.12 * |
| Docosanoic acid (C22:0) | 0.17 ± 0.02 * | 0.24 ± 0.01 * | nd | 0.23 ± 0.01 * |
| Total | 34.52 ± 2.25 * | 81.53 ± 5.66 * | 48.25 ± 4.12 * | 41.54 ± 3.34 * |
| MUFA | ||||
| Palmitoleic acid (16:1 n-7) | 1.05 ± 0.04 * | 0.47 ± 0.02 * | 1.01 ± 0.01 | 0.93 ± 0.02 * |
| Oleic acid (18:1 n-9) | 7.24 ± 0.12 * | 2.80 ± 0.11 * | 5.13 ± 0.21 * | 4.85 ± 0.13 * |
| Vaccenic acid (18:1 n-7) | 1.59 ± 0.01 * | 0.65 ± 0.03 * | 2.39 ± 0.11 * | 1.19 ± 0.01 * |
| Erucic acid (22:1 n-9) | 0.53 ± 0.02 | 0.48 ± 0.01 | 0.63 ± 0.01 | 0.80 ± 0.02 |
| Total | 10.41 ± 0.19 * | 4.40 ± 0.17 * | 9.16 ± 0.34 * | 7.77 ± 0.18 * |
| PUFA | ||||
| 4,7,10,13 hexatetranoic acid (16:4 n-3) | 0.82 ± 0.03 * | 0.72 ± 0.03 * | 0.72 ± 0.04 * | 0.89 ± 0.03 * |
| Linoleic acid (18:2 n-6) | 4.79 ± 0.21 * | 0.38 ± 0.01 * | 1.12 ± 0.01 * | 4.19 ± 0.51 * |
| γ-linolenic acid (18:3 n-3) | 0.25 ± 0.01 * | 0.22 ± 0.01 * | 0.40 ± 0.01 * | 0.25 ± 0.03 |
| α-linolenic acid (18:3 n-6) | 0.19 ± 0.02 * | 0.27 ± 0.02 * | nd | 0.10 ± 0.01 * |
| Stearidonic acid (18:4 n-3) | 0.49 ± 0.02 | 0.50 ± 0.03 | 0.43 ± 0.04 | 0.48 ± 0.02 |
| Dihomo γ linoleic acid (20:3 n-6) | 1.01 ± 0.01 * | 0.35 ± 0.01 * | 0.86 ± 0.02 * | 0.49 ± 0.01 * |
| Arachidononic acid (20:4 n-6) | 46.36 ± 3.21 * | 10.68 ± 0.91 * | 37.59 ± 1.23 * | 43.72 ± 1.54 |
| 8,11,14,17 Eicosatrienoic acid (20:4 n-3) | 0.08 ± 0.01 * | 0.35 ± 0.01 * | 0.04 ± 0.01 * | 0.07 ± 0.01 |
| Eicosapentaenoic acid (20:5 n-3) | 0.73 ± 0.02 * | 0.60 ± 0.05 * | 1.43 ± 0.01 * | 0.50 ± 0.05 * |
| Adrenic acid (22:4 n-6) | 0.35 ± 0.03 | nd | nd | Nd |
| Total | 55.07 ± 3.57 * | 14.07 ± 1.08 * | 42.59 ± 1.37 * | 50.69 ± 2.21 * |
| Nutritional Index | ||||
| P/S | 1.59 ± 0.20 * | 0.17 ± 0.02 * | 0.88 ± 0.10 * | 1.22 ± 0.14 |
| n-6/n-3 | 22.23 ± 1.10 * | 4.88 ± 0.66 * | 13.10 ± 0.89 * | 22.15 ± 2.35 |
| IA | 0.54 ± 0.07 * | 2.65 ± 0.33 * | 0.75 ± 0.09 * | 0.43 ± 0.08 |
| IT | 0.64 ± 0.08 * | 2.05 ± 0.22 * | 0.87 ± 0.10 * | 0.61 ± 0.09 |
| h/H | 2.60 ± 0.37 * | 0.44 ± 0.06 * | 1.77 ± 0.20 * | 2.75 ± 0.40 |
| UI | 213.69 ± 13.82 * | 59.68 ± 4.48 * | 177.45 ± 5.78 * | 201.81 ± 9.01 |
| UT | LA | PROMIX 1 | SBM-11 | |
|---|---|---|---|---|
| mg/100 g DW | ||||
| Tocopherols | ||||
| α-T | 3.90 ± 0.23 * | nd | 0.38 ± 0.10 * | 1.56 ± 0.31 * |
| Carotenoids | ||||
| Violaxanthin | 0.71 ± 0.03 | nd | nd | Nd |
| Fucoxanthin | 8.60 ± 0.06 * | 0.82 ± 0.07 * | 1.09 ± 0.19 * | 0.73 ± 0.14 * |
| Lutein | 0.39 ± 0.04 | 0.36 ± 0.04 | 0.37 ± 0.12 | 0.37 ± 0.11 |
| Zeaxanthin | 16.45 ± 0.33 * | 5.47 ± 0.17 * | 7.98 ± 0.47 * | 9.29 ± 0.94 * |
| α-cryptoxanthin | 13.26 ± 1.03 * | 2.71 ± 0.03 * | 4.64 ± 0.38 * | 4.24 ± 0.98 * |
| β-cryptoxanthin | 14.19 ± 0.44 * | 2.52 ± 0.21 * | 5.70 ± 0.38 * | 6.40 ± 0.40* |
| β-carotene | 9.26 ± 0.99 * | 1.50 ± 0.20 * | 4.06 ± 0.03 * | 5.26 ± 0.15 * |
| 9 cis β-carotene | 1.73 ± 0.42 * | 0.34 ± 0.01 * | 0.49 ± 0.01 * | 0.39 ± 0.06 * |
| Total | 64.59 ± 3.33 * | 13.72 ± 0.73 * | 24.33 ± 0.42 * | 26.68 ± 2.78 * |
| Chlorophylls a + b | 60.93 ± 1.61 * | 21.49 ± 0.60 * | 37.60 ± 2.78 * | 26.75 ± 3.51 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorano, G.; Ramires, F.A.; Durante, M.; Palamà, I.E.; Blando, F.; De Rinaldis, G.; Perbellini, E.; Patruno, V.; Gadaleta Caldarola, C.; Vitucci, S.; et al. The Controlled Semi-Solid Fermentation of Seaweeds as a Strategy for Their Stabilization and New Food Applications. Foods 2022, 11, 2811. https://doi.org/10.3390/foods11182811
Maiorano G, Ramires FA, Durante M, Palamà IE, Blando F, De Rinaldis G, Perbellini E, Patruno V, Gadaleta Caldarola C, Vitucci S, et al. The Controlled Semi-Solid Fermentation of Seaweeds as a Strategy for Their Stabilization and New Food Applications. Foods. 2022; 11(18):2811. https://doi.org/10.3390/foods11182811
Chicago/Turabian StyleMaiorano, Gabriele, Francesca Anna Ramires, Miriana Durante, Ilaria Elena Palamà, Federica Blando, Gianluca De Rinaldis, Ezio Perbellini, Valeria Patruno, Carlo Gadaleta Caldarola, Santa Vitucci, and et al. 2022. "The Controlled Semi-Solid Fermentation of Seaweeds as a Strategy for Their Stabilization and New Food Applications" Foods 11, no. 18: 2811. https://doi.org/10.3390/foods11182811
APA StyleMaiorano, G., Ramires, F. A., Durante, M., Palamà, I. E., Blando, F., De Rinaldis, G., Perbellini, E., Patruno, V., Gadaleta Caldarola, C., Vitucci, S., Mita, G., & Bleve, G. (2022). The Controlled Semi-Solid Fermentation of Seaweeds as a Strategy for Their Stabilization and New Food Applications. Foods, 11(18), 2811. https://doi.org/10.3390/foods11182811









