Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Biotechnological Strategies to Enhance Nutraceutical Properties
2.3.1. Sprouting
2.3.2. Enzymatic Hydrolysis
2.4. Nutritional Characterization
2.5. Phenolic Extract Preparation
2.5.1. Release of Free Phenolic Compounds (FP)
2.5.2. Release of Bound Phenolic Compounds (BP)
2.6. Determination of Total Phenolics (TPs)
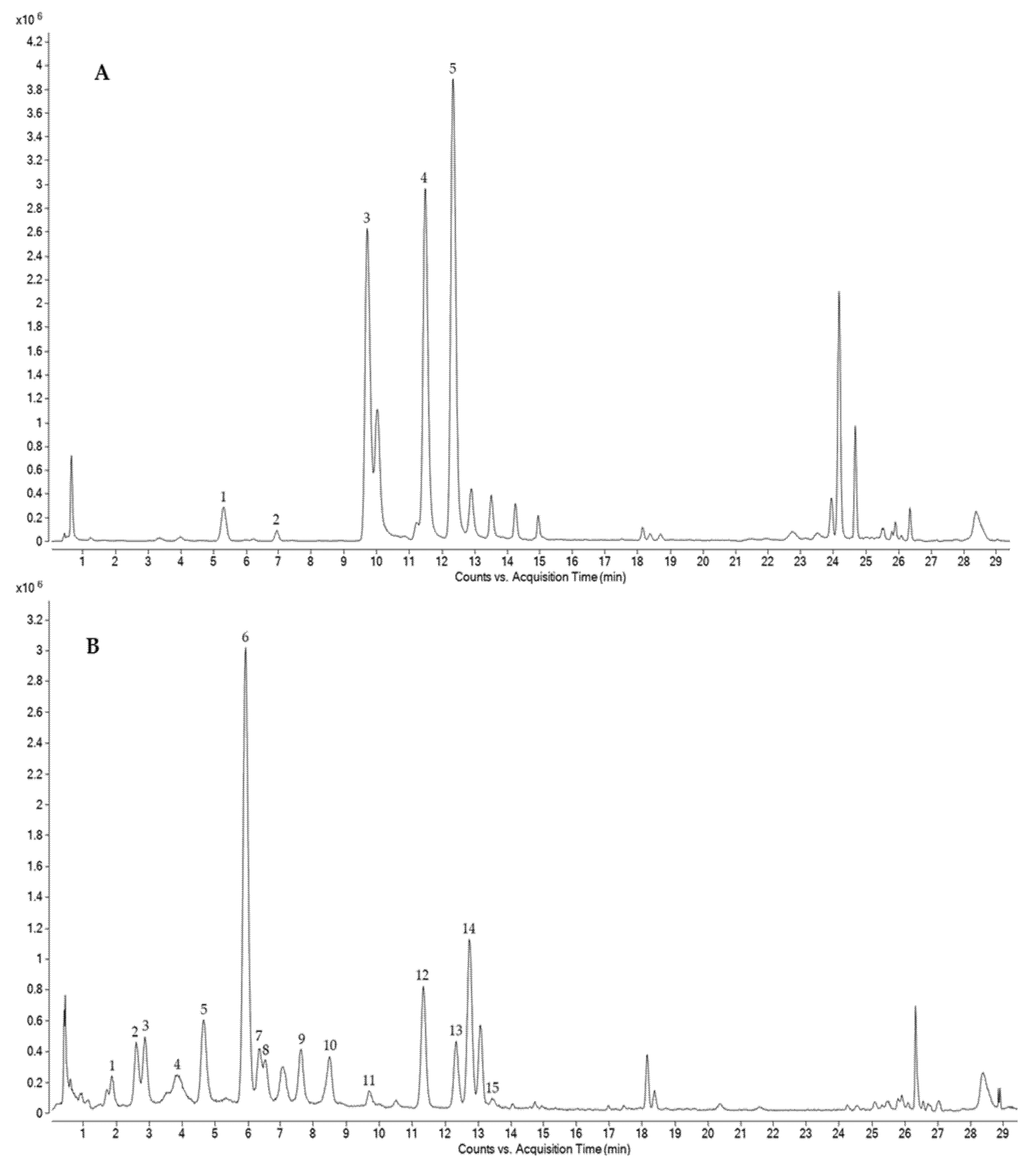
2.7. Characterization of Phenolic Compounds by HPLC-ESI-QTOF-MS
2.8. Total Antioxidant Capacity (TAC)
2.8.1. ABTS•+ Radical Cation Scavenging Activity (ABTS•+)
2.8.2. Oxygen Radical Absorbance Capacity (ORAC)
2.8.3. Ferric Reducing Antioxidant Power (FRAP)
2.9. Glycemic Index (GI)
2.10. Determination of Anti-Inflammatory Activity (AIA)
2.11. Statistical Analysis
3. Results
3.1. Nutritional Characterization
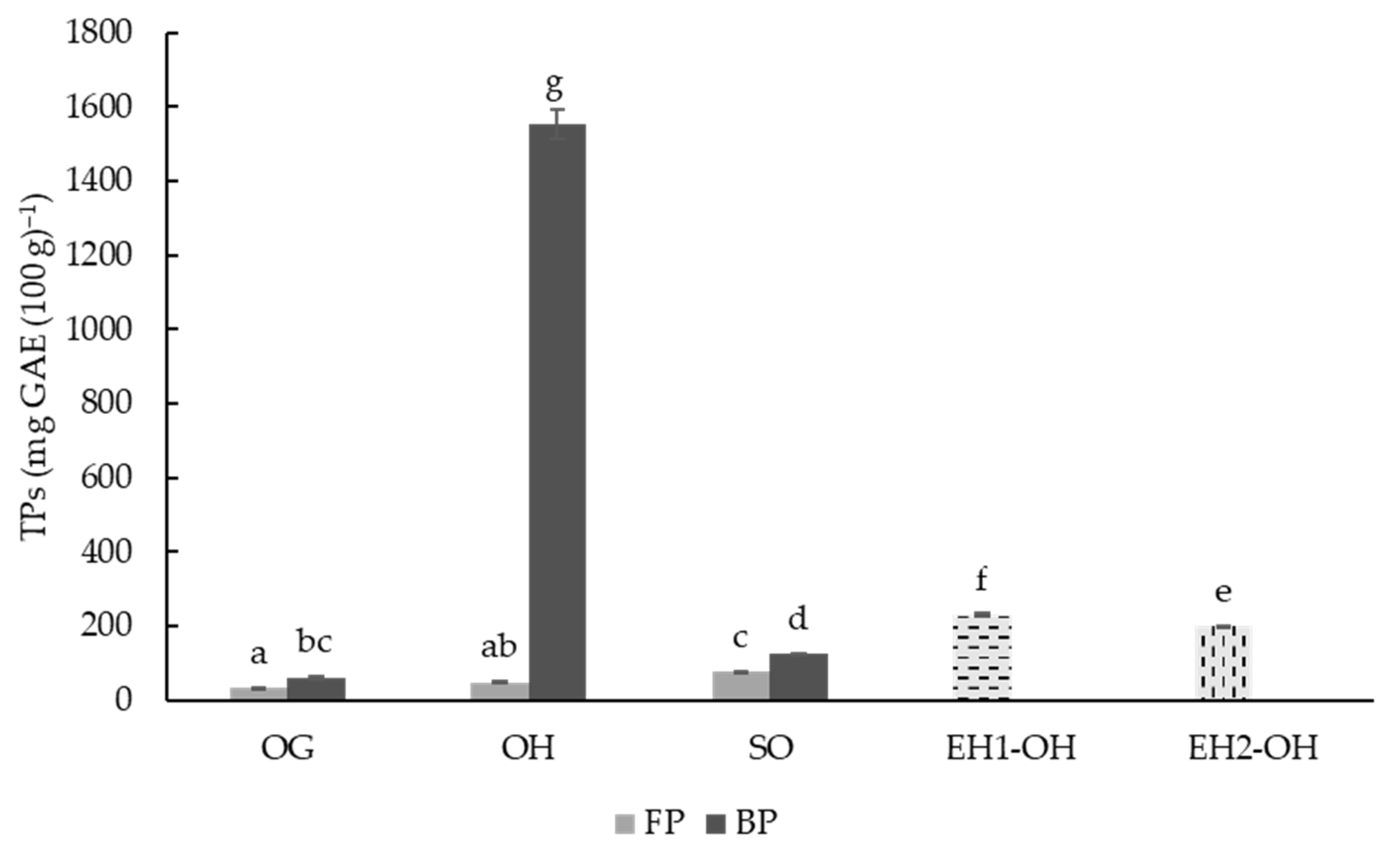
3.2. Determination and Characterization of Total Phenolic Compounds (TPC)
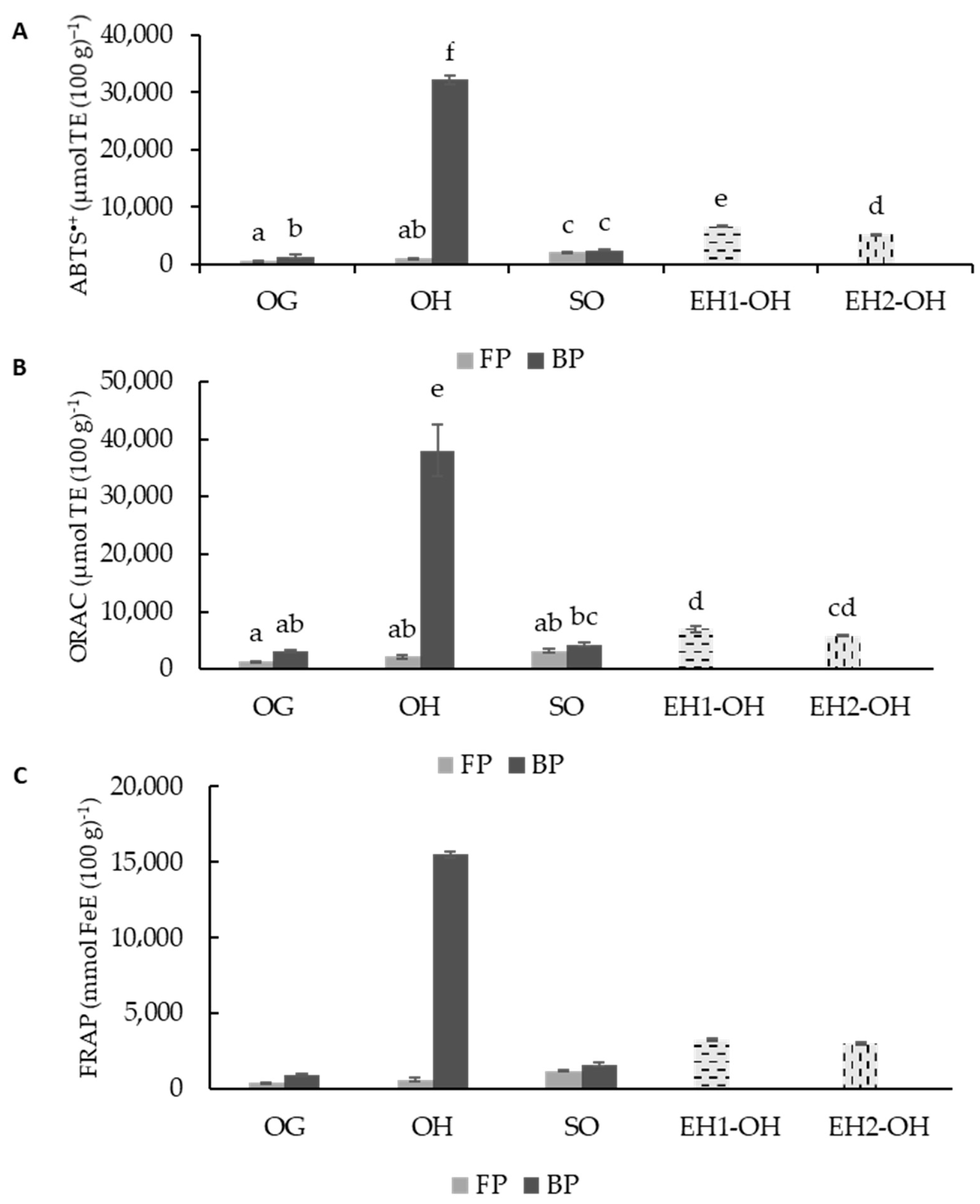
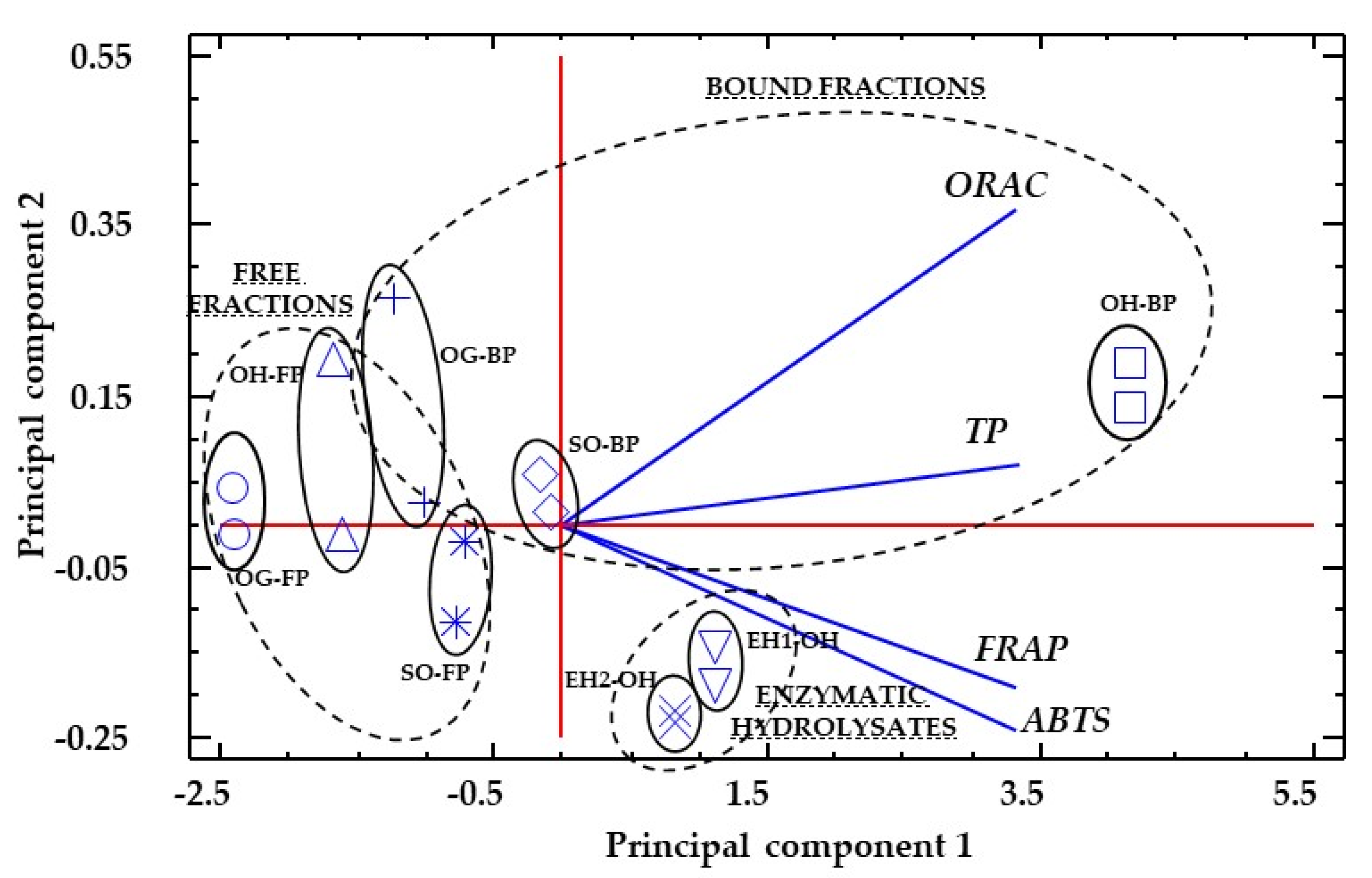
3.3. Total Antioxidant Capacity (TAC)
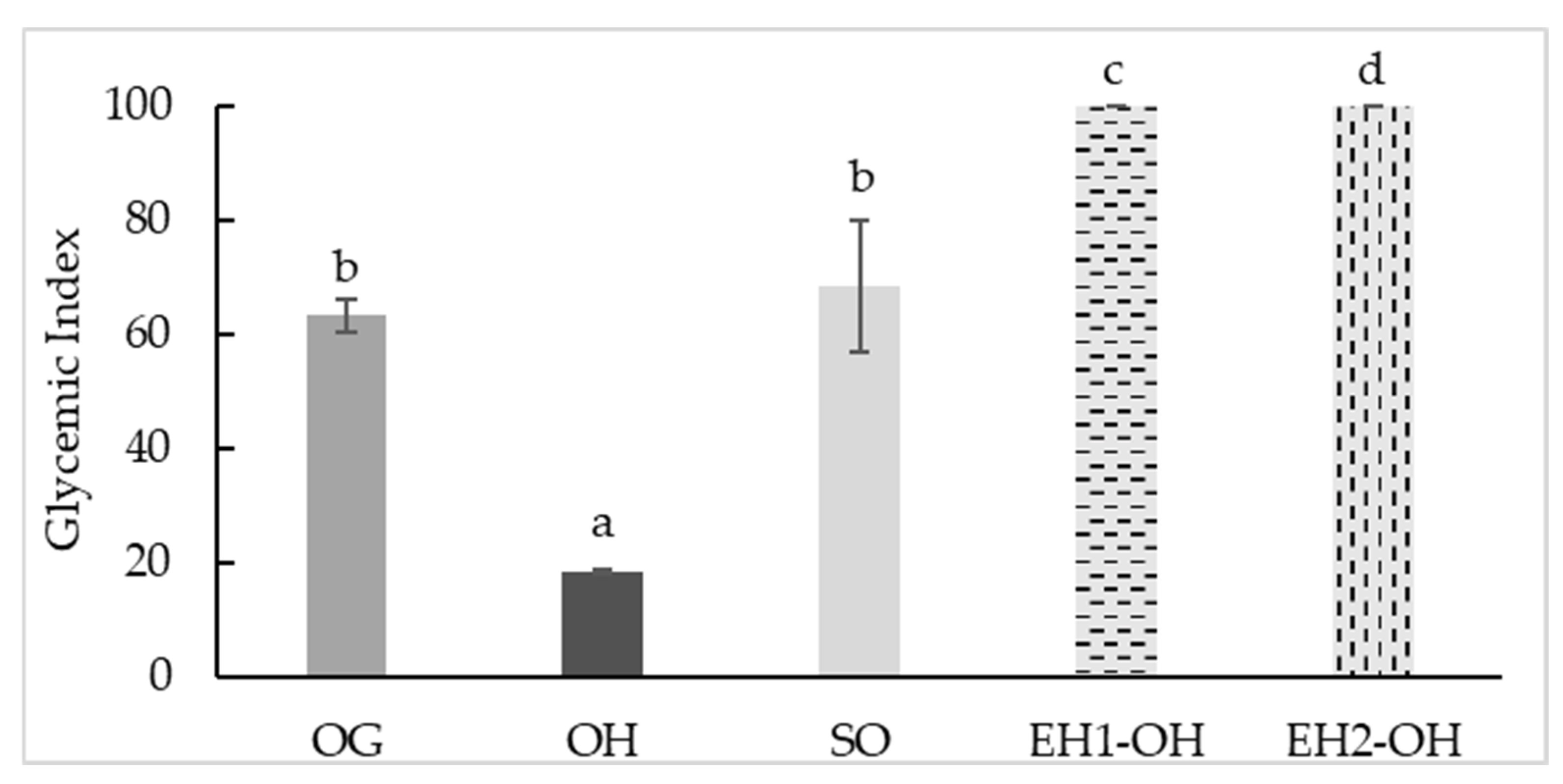
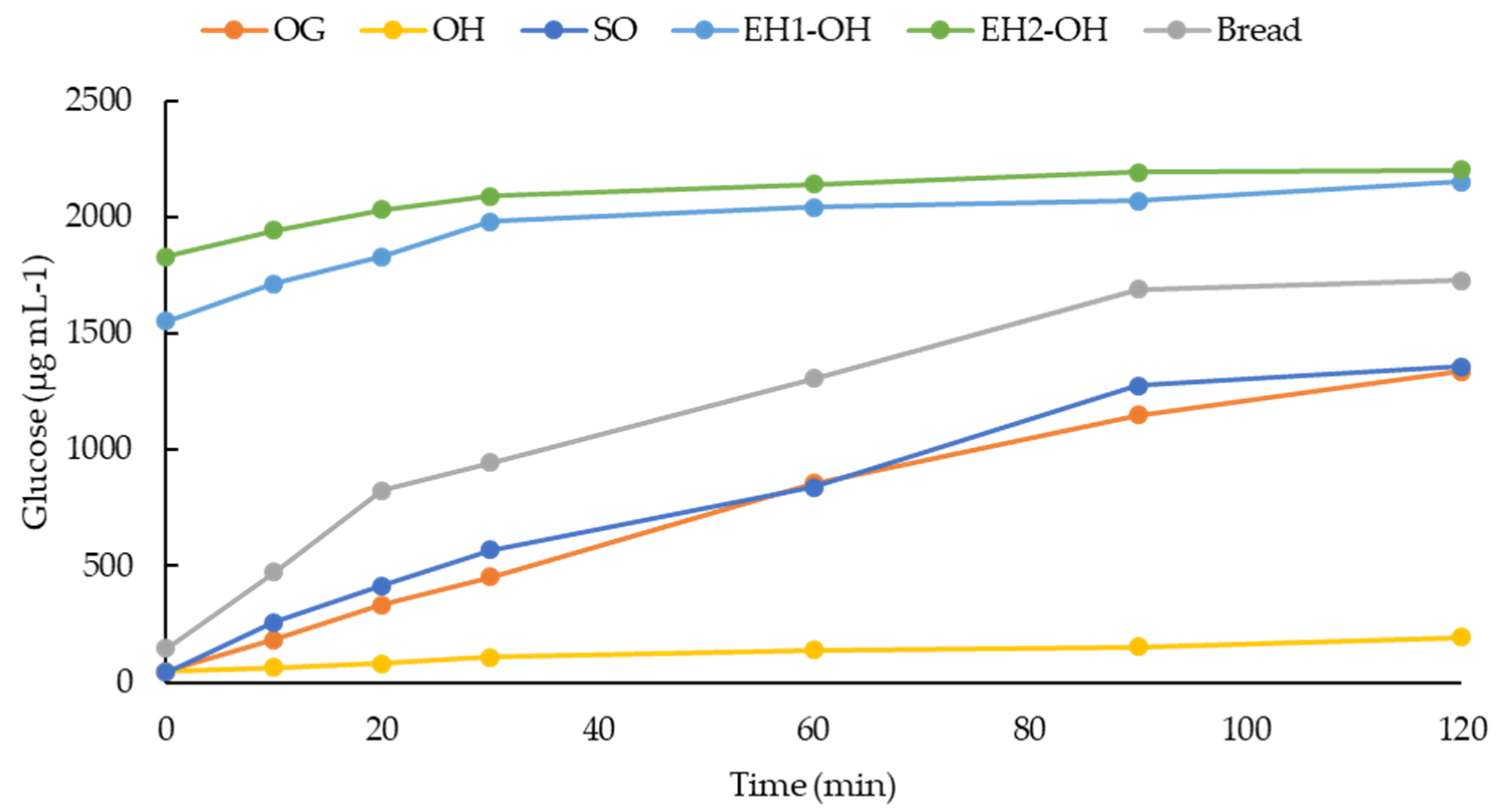
3.4. Glycemic Index (GI)
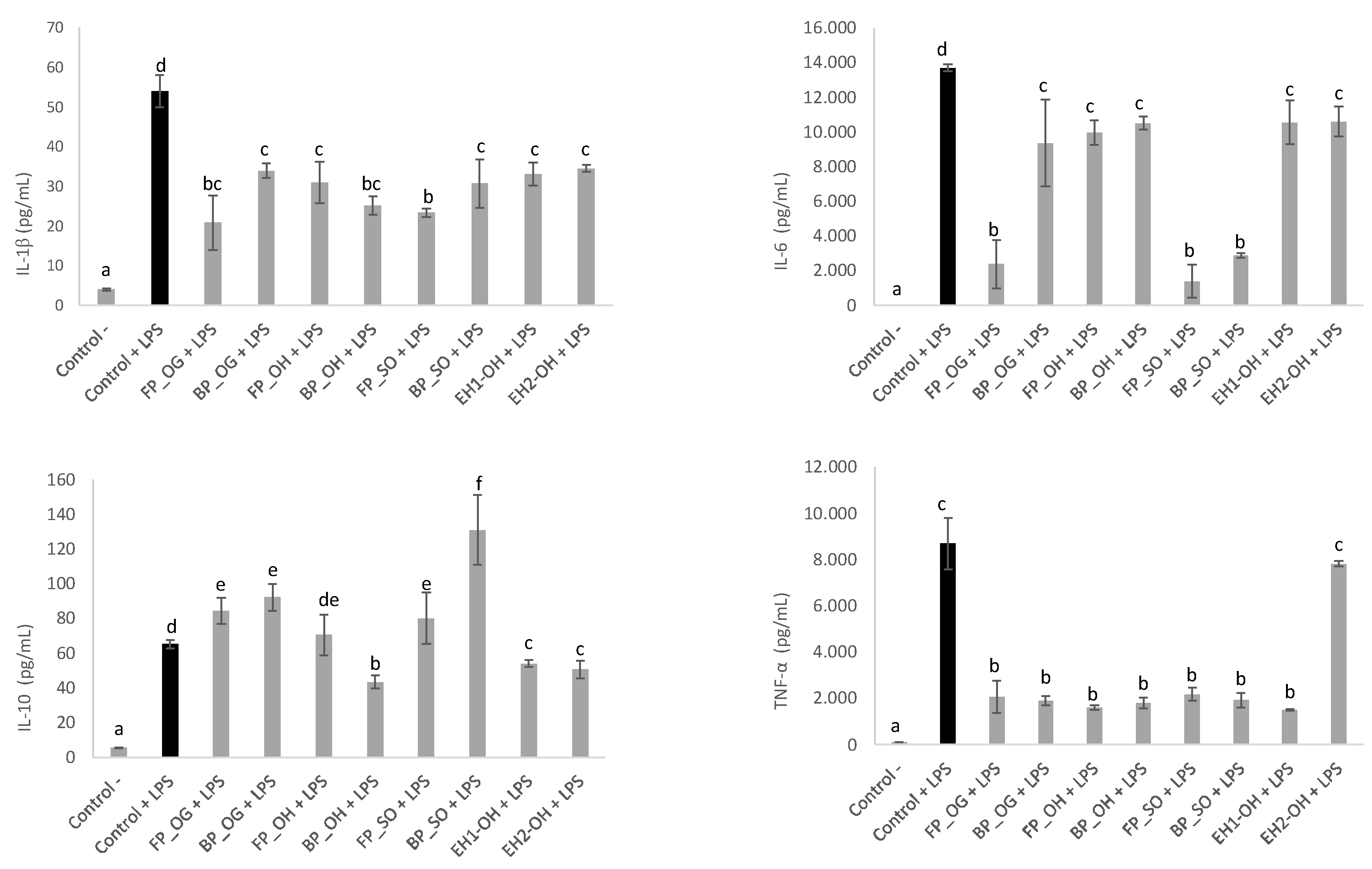
3.5. Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Poole, N.; Donovan, J. Role of staple cereals in human nutrition: Separating the wheat from the chaff in the infodemics age. Trends Food Sci. Technol. 2022, 119, 508–513. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Agri-nutrition research: Revisiting the contribution of maize and wheat to human nutrition and health. Food Policy 2021, 100, 101976. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; van Rooy, G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse Reactions to Wheat or Wheat Components. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1437–1452. [Google Scholar] [CrossRef]
- Fuldkornspartnerskabet. The Danish Whole Grain Partnership. Available online: https://fuldkorn.dk/english/ (accessed on 12 June 2022).
- American Association of Cereal Chemists. AACC Members Agree on Definition of Whole Grain. Available online: https://www.cerealsgrains.org/resources/definitions/Documents/WholeGrains/wgflyer.pdf (accessed on 24 June 2022).
- Helnæs, A.; Kyrø, C.; Andersen, I.; Lacoppidan, S.; Overvad, K.; Christensen, J.; Tjønneland, A.; Olsen, A. Intake of whole grains is associated with lower risk of myocardial infarction: The Danish Diet, Cancer and Health Cohort. Am. J. Clin. Nutr. 2016, 103, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kuang, Q.; Wang, K.; Zhou, S.; Wang, S.; Liu, X.; Wang, S. Insights into molecular structure and digestion rate of oat starch. Food Chem. 2016, 220, 25–30. [Google Scholar] [CrossRef]
- Perrelli, A.; Goitre, L.; Salzano, A.M.; Moglia, A.; Scaloni, A.; Retta, S.F. Biological Activities, Health Benefits, and Therapeutic Properties of Avenanthramides: From Skin Protection to Prevention and Treatment of Cerebrovascular Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 6015351. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. Food Labeling: Health Claims; Soluble Fiber from Certain Foods and Risk of Coronary Heart Disease. Available online: https://www.govinfo.gov/content/pkg/FR-2005-12-23/pdf/05-24387.pdf (accessed on 12 June 2022).
- FSANZ (Food Standards Australia New Zealand). Australia New Zealand Food Standards Code. Schedule 4. Nutrition, Health and Related Claims. Available online: https://www.foodstandards.gov.au/code/Documents/Sched%204%20Nutrition%20and%20health%20claims%20v159.pdf (accessed on 12 June 2022).
- European Commission. Commission Regulation (EU) No 1160/2011 of 14 November 2011 on the Authorisation and Refusal of Authorisation of Certain Health Claims Made on Foods and Referring to the Reduction of Disease Risk. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:296:0026:0028:EN:PDF (accessed on 12 June 2022).
- European Commission. Commission Regulation (EU) No 1048/2012 of 8 November 2012 on the Authorisation of a Health Claim Made on Foods and Referring to the Reduction of Disease Risk. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R1048&from=EN (accessed on 12 June 2022).
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Derm. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Redaelli, R.; Berardo, N. Prediction of fibre components in oat hulls by near infrared reflectance spectroscopy. J. Sci. Food Agric. 2007, 87, 580–585. [Google Scholar] [CrossRef]
- Xing, Y.; White, P.J. Identification and function of antioxidants from oat groats and hulls. J. Am. Oil Chem. Soc. 1997, 74, 303–307. [Google Scholar] [CrossRef]
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Expósito, S.; Tomé-Sánchez, I.; Martín-Diana, A.B.; Frias, J.; Peñas, E.; Rico, D.; Casas, M.J.G.; Martínez-Villaluenga, C. Enzyme Selection and Hydrolysis under Optimal Conditions Improved Phenolic Acid Solubility, and Antioxidant and AntiInflammatory Activities of Wheat Bran. Antioxidants 2020, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Kviesis, J.; Lazdina, D.; Valdovska, A.; Vallejo, F.; Lacis, G. Lignocellulose-Degrading Enzymes: A Biotechnology Platform for Ferulic Acid Production from Agro-Industrial Side Streams. Foods 2021, 10, 3056. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górna´s, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Guzzo, F.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Sprouted oat as a potential gluten-free ingredient with en- hanced nutritional and bioactive properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; García, M.d.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Bautista-Expósito, S.; Frias, J.; Rico, D.; González-Maillo, L.; Martinez-Vil-laluenga, C. Soluble phenolic composition tailored by germination conditions accompany antioxidant and anti-Inflammatory properties of wheat. Antioxidants 2020, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Eyzaguirre, R.Z.; Nienaltowska, K.; de Jong, L.E.; Hasenack, B.B.; Nout, M.R. Effect of food processing of pearl millet (Pennisetum glaucum) IKMP-5 on the level of phenolics, phytate, iron and zinc. J. Sci. Food Agric. 2006, 86, 1391–1398. [Google Scholar] [CrossRef]
- Frias, J.; Fernandez-Orozco, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Vidal-Valverde, C. Effect of germination on the content of vitamin C and E in lentils. Pol. J. Food Nutr. Sci. 2002, 11, 76–78. [Google Scholar]
- Martín-Diana, A.B.; Tomé-Sánchez, I.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Rico, D. A Novel Strategy to Produce a Soluble and Bioactive Wheat Bran Ingredient Rich in Ferulic Acid. Antioxidants 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, G.; Segura-Carretero, A.; Di Silvestro, R.; Marotti, I.; Arráez-Román, D.; Benedettelli, S.; Ghiselli, L.; Fernadez-Gutierrez, A. Profiles of phenolic compounds in modern and old common wheat varieties determined by liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A. 2011, 1218, 7670–7681. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Izquierdo, N.; Albertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of Carob’s Germ and Seed Peel as Natural Antioxidant Ingredients in Gluten-Free Crackers. J. Food Process. Preserv. 2016, 41, e12770. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pulido, I.J.; Rico, D.; Pérez-Jiménez, J.; Martínez-Villaluenga, C.; de Luis, D.; Martín-Diana, A.B. Impact of protein content on antioxidant, anti-inflammatory properties and glycemic index of wheat and wheat bran. Foods 2022, 1, 2049. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.; Rosell, C. Physicochemical properties and enzymatic hydrolysis of different starches in the presence of hydrocolloids. Carbohydr. Polym. 2011, 85, 237–244. [Google Scholar] [CrossRef]
- Granfeldt, Y. Foods Factors Affecting Metabolic Responses to Cereal Products. Ph.D. Thesis, University of Lund, Lund, Sweden, 1994. [Google Scholar]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and Oat Brans as Sources of Polyphenol Compounds for Development of Antioxidant Nutraceutical Ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Teshager, M.A.; Assegie, A.A.; Amare, E. Proximate, mineral and anti-nutrient compositions of oat grains (Avena sativa) cultivated in Ethiopia: Implications for nutrition and mineral bioavailability. Heliyon 2021, 7, e07722. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Godara, P.; Kaur, M.; Punia, S. Effect of toasting on physical, functional and antioxidant properties of flour from oat (Avena sativa L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 197–203. [Google Scholar] [CrossRef]
- Sangwan, S.; Singh, R.; Tomar, S.K. Nutritional and functional properties of oats: An update. J. Innov. Biol. 2014, 1, 3–14. [Google Scholar]
- Ethiopian Health and Nutrition Research Institute (EHNRI). Food Composition Table for Use in Ethiopia Part IV; Ethiopian Health and Nutrition Research Institute: Addis Ababa, Ethiopia, 1998. [Google Scholar]
- Krapf, J.; Kandzia, F.; Brühan, J.; Walther, G.; Flöter, E. Sprouting of oats: A new approach to quantify compositional changes. Cereal Chem. 2019, 96, 994–1003. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef]
- Granese, T.; Cardinale, F.; Cozzolino, A.; Pepe, S.; Ombra, M.; Nazzaro, F.; Coppola, R.; Fratianni, F. Variation of Polyphenols, Anthocyanins and Antioxidant Power in the Strawberry Grape (Vitis labrusca) after Simulated Gastro-Intestinal Transit and Evaluation of in vitro Antimicrobial Activity. Food Nutr. Sci. 2014, 5, 60–65. [Google Scholar] [CrossRef]
- Joint FAO/WHO Food Standards Programme, Secretariat of the CODEX Alimentarius Commission: CODEX Alimentarius (CODEX) Guidelines on Nutrition Labeling CAC/GL 2–1985 as Last Amended 2010; FAO: Rome, Italy, 2010.
- Šterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical Composition of Covered and Naked Spring Barley Varieties and Their Potential for Food Production. Pol. J. Food Nutr. Sci. 2017, 67, 151–158. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anttila, H.; Sontag-Strohm, T.; Salovaara, H. Viscosity of beta-glucan in oat products. Agric. Food Sci. 2004, 13, 80–87. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT—Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Tarasiuk, W.; Różyło, R. Fiber Preparation from Micronized Oat By-Products: Antioxidant Properties and Interactions between Bioactive Compounds. Molecules 2022, 27, 2621. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Plaami, S.; Kumpulainen, J. Genetic and environmental variation in β-glucan content of oats cultivated or tested in Finland. J. Cereal Sci. 1992, 16, 279–290. [Google Scholar] [CrossRef]
- Saastamoinen, M. Effects of environmental factors on the β-glucan content of two oat varieties. Acta Agric. Scand. Sect., B. Soil Plant. Sci. 1995, 45, 181–187. [Google Scholar] [CrossRef]
- Wilhelmson, A.; Oksman-Caldentey, K.M.; Laitila, A.; Suortti, T.; Kaukovirta-Norja, A.; Poutanen, K. Development of a germination process for producing high beta-glucan, whole food ingredients from oat. Cereal Chem. 2001, 78, 715–720. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Kviesis, J.; Valdovska, A. An Environmentally Friendly Approach for the Release of Essential Fatty Acids from Cereal By-Products Using Cellulose-Degrading Enzymes. Biology 2022, 11, 721. [Google Scholar] [CrossRef]
- Hosseini, E.; Kadivar, M.; Shahedi, M. Optimization of Enzymatic Activities in Malting of Oat. World Acad. Sci. Eng. Technol. 2010, 43, 766–771. [Google Scholar]
- Youngs, V.L. Oat lipids and lipid-related enzymes. In Oats: Chemistry and Technology; Webster, F.H., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1986; pp. 205–226. [Google Scholar]
- Lockhart, H.B.; Hurt, H.D. Nutrition of oats. In Oats: Chemistry and Technology; Webster, F.H., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1986; pp. 297–308. [Google Scholar]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT—Food Sci. Technol. 2007, 40, 860–870. [Google Scholar] [CrossRef]
- Outinen, M. Mallastuksen Vaikutus Kauran Lipideihin (Influence of Germination on Oat Lipids). Master’s Thesis, Helsinki University of Technology, Espoo, Finland, 1999. [Google Scholar]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Changes in protein profile, bioactive potential and enzymatic activities of gluten-free flours obtained from hulled and dehulled oat varieties as affected by germination conditions. LWT 2020, 134, 109955. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Mannerstedt-Fogelfors, B.; Kamal-Eldin, A.; Andersson, R.; Dimberg, L.H. Lipids and antioxidants in groats and hulls of Swedish oats (Avena sativa L). J. Sci. Food Agric. 2002, 82, 606–614. [Google Scholar] [CrossRef]
- Halima, N.B.; Saad, R.B.; Khemakhem, B.; Fendri, I.; Abdelkafi, S. Oat (Avena sativa L.): Oil and Nutriment Compounds Valor-ization for Potential Use in Industrial Applications. J. Oleo Sci. 2015, 64, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: Intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef]
- Chajès, V.; Hultén, K.; Van Kappel, A.L.; Winkvist, A.; Kaaks, R.; Hallmans, G.; Lenner, P.; Riboli, E. Fatty-acid composition in serum phospholipids and risk of breast cancer: An incident case-control study in Sweden. Int. J. Cancer 1999, 83, 585–590. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Schuchmann, H.; Schuchmann, H. Getreide und Getreideprodukte. In Lebensmittelverfahrenstechnik, 1st ed.; Karbstein, H.P., Schuchmann, H., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 61–72. [Google Scholar]
- Larsson, M.; Sandberg, A.-S. Phytate reduction in oats during malting. J. Food Sci. 1992, 57, 994–997. [Google Scholar] [CrossRef]
- Fredlund, K.; Asp, N.-G.; Larsson, M.; Marklinder, I.; Sandberg, A.-S. Phytate reduction in whole grains of wheat, rye, barley and oats after hydrothermal treatment. J. Cereal Sci. 1997, 25, 83–91. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Obadi, M.; Jiang, Y.; Chen, Z.; Jiang, S.; Xu, B. Effect of steaming and defatting treatments of oats on the processing and eating quality of noodles with a high oat flour content. J. Cereal Sci. 2019, 89, 102794. [Google Scholar] [CrossRef]
- Tian, B.; Xie, B.; Shi, J.; Wu, J.; Cai, Y.; Xu, T.; Xue, S.; Deng, Q. Physicochemical changes of oat seeds during germination. Food Chem. 2010, 119, 1195–1200. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Kaukovirta-Norja, A.; Heiniö, R.L.; Kleemola, T.; Mikola, M.; Sontag-Strom, T.; Lehtinen, P.; Pihlava, J.M.; Poutanen, P. Kauran biotekninen prosessointi uusiksi elintarvikkeiksi. (Biotechnical processing of oat for novel food ingredients). In Kaurasta Elinvoimaa; Salovaara, H., Sontag-Strom, T., Eds.; EKT-sarja 1221; Helsingin yliopisto, Elintarviketeknologian laitos: Helsinki, Finland, 2001; pp. 85–108. [Google Scholar]
- Zilic, S.; Basic, Z.; Sukalovic, V.H.T.; Maksimovic, V.; Jankovic, M.; Filipovic, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Piatkowska, E.; Witkowicz, R.; Pisulewska, E. Antioxidant properties of selected cultivars of oats. Food Sci. Technol. Qual. 2010, 14, 100–107. [Google Scholar] [CrossRef]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural Study of Phenolic Acids by Triple Quadrupole Mass Spectrometry with Electrospray Ionization in Negative Mode and H/D Isotopic Exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Jágr, M.; Dvořáček, V.; Čepková, P.H.; Doležalová, J. Comprehensive analysis of oat avenanthramides using hybrid quadrupole-Orbitrap mass spectrometry: Possible detection of new compounds. Rapid Commun. Mass Spectrom. 2020, 34, e8718. [Google Scholar] [CrossRef]
- Buksa, K.; Praznik, W.; Loeppert, R.; Nowotna, A. Characterization of water and alkali extractable arabinoxylan from wheat and rye under standardized conditions. J. Food Sci. Technol. 2016, 53, 1389–1398. [Google Scholar] [CrossRef]
- Juhnevica-Radenkova, K.; Kviesis, J.; Moreno, D.A.; Seglina, D.; Vallejo, F.; Valdovska, A.; Radenkovs, V. Highly-efficient release of ferulic acid from agro-industrial by-products via enzymatic hydrolysis with cellulose-degrading enzymes: Part I–The superiority of hydrolytic enzymes versus conventional hydrolysis. Foods 2021, 10, 782. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Theander, O.; Lingnert, H. Avenanthramides—A group of phenolic antioxidants in oats. Cereal Chem. 1992, 70, 637–641. [Google Scholar]
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J. Cereal Sci. 2008, 48, 294–303. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Hübner, F.; Arendt, E.K. Germination of cereal grains as a way to improve the nutritional value: A review. Crit Rev. Food Sci Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Olaerts, H.; Courtin, C.M. Impact of Preharvest Sprouting on Endogenous Hydrolases and Technological Quality of Wheat and Bread: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 698–713. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Karlberg, E. A Study of Avenanthramides in Oats for Future Applications; Uppsala University School of Engineering: Uppsala, Sweden, 2010; ISSN 1401-2138. [Google Scholar]
- Tang, S.; Mao, G.; Yuan, Y.; Weng, Y.; Zhu, R.; Cai, C. Optimization of oat seed steeping and germination temperatures to maximize nutrient content and antioxidant activity. J. Food Process. Preserv. 2020, 44, e14683. [Google Scholar] [CrossRef]
- Xue, Y.; Teng, Y.; Chen, M.; Li, Z.; Wang, G. Antioxidant Activity and Mechanism of Avenanthramides: Double H+/e- Processes and Role of the Catechol, Guaiacyl, and Carboxyl Groups. J. Agric. Food Chem. 2021, 69, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kruma, Z.; Tomsone, L.; Galoburda, R.; Straumite, E.; Kronberga, A.; Åssveen, M. Total phenols and antioxidant capacity of hull-less barley and hull-less oats. Agron. Res. 2016, 14, 1361–1371. [Google Scholar]
- Durazzo, A.; Casale, G.; Melini, V.; Maiani, G.; Acquistucci, R. Evaluation of Antioxidant Properties in Cereals: Study of Some Traditional Italian Wheats. Foods 2015, 4, 391–399. [Google Scholar] [CrossRef]
- Hossain, M.M.; Tovar, J.; Cloetens, L.; Florido, M.T.S.; Petersson, K.; Prothon, F.; Nilsson, A. Oat polar lipids improve cardiometabolic-related markers after breakfast and a subsequent standardized lunch: A randomized crossover study in healthy young adults. Nutrients 2021, 13, 988. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.M.; Debeaufort, F. Antioxidant activity and release kinetics of caffeic and p-coumaric acids from hydrocolloid-based active films for healthy packaged food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, C.W.; Anh, N.H.; Kim, S.W.; Park, S.; Kwon, S.W.; Lee, S.J. Effects of Oats (Avena sativa L.) on Inflammation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2021, 8, 722866. [Google Scholar] [CrossRef]
- Kang, C.; Shin, W.S.; Yeo, D.; Lim, W.; Zhang, T.; Ji, L.L. Anti-inflammatory effect of avenanthramides via NF-κB pathways in C2C12 skeletal muscle cells. Free Radic. Biol. Med. 2018, 117, 30–36. [Google Scholar] [CrossRef]
| OG | OH | SO | |
|---|---|---|---|
| Ash | 2.5 ± 0.09 a | 4.3 ± 0.00 b | 2.41 ± 0.20 a |
| Carbohydrates | 79.07 ± 0.12 b | 92.04 ± 0.07 c | 76.62 ± 0.89 a |
| TDF | 12.63 ± 0.13 b | 89.64 ± 0.10 c | 8.81 ± 0.77 a |
| β-glucan | 4.46 ± 0.16 c | 0.11 ± 0.06 a | 1.55 ± 0.04 b |
| TSC | 55.07 ± 1.10 b | 2.89 ± 0.48 a | 59.01 ± 2.70 c |
| Fat | 6.73 ± 0.08 b | 0.61 ± 0.01 a | 9.45 ± 0.75 c |
| SFA | 18.00 ± 0.00 a | 34.83 ± 1.65 c | 20.41 ± 0.00 b |
| MUFA | 37.00 ± 0.00 a | 40.30 ± 0.42 b | 42.86 ± 0.00 c |
| PUFA | 45.00 ± 0.00 c | 24.87 ± 1,23 a | 36.73 ± 0.00 b |
| Palmitic acid (C16:0) | 15.00 ± 0.00 a | 34.83 ± 1.65 c | 16.33 ± 0.00 b |
| Stearic acid (C18:0) | n.d. | n.d. | 2.04 ± 0.00 a |
| Oleic acid (C18:1) | 37.00 ± 0.00 a | 40.30 ± 0.42 b | 42.86 ± 0.00 c |
| Linoleic acid (C18:2) | 45.00 ± 0.00 c | 24.87 ± 1.23 a | 36.73 ± 0.00 b |
| Behenic acid (C122:0) | n.d. | n.d. | 2.04 ± 0.00 a |
| C18:1/C18:2 | 0.82 ± 0.00 a | 1.62 ± 0,06 c | 1.17 ± 0.00 b |
| Protein | 11.69 ± 0.04 c | 3.06 ± 0.07 a | 11.52 ± 0.06 b |
| Moisture | 10.16 ± 0.01 c | 8.03 ± 0.02 b | 6.93 ± 0.01 a |
| PA | 0.94 ± 0.01 c | 0.07 ± 0.00 a | 0.83 ± 0.01 b |
| Class | Sub-class | Compound | Formula | Fragments | OG | OH | SO | EH1-OH | EH2-OH | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Error | Fraction | Error | Fraction | Error | Fraction | Error | Error | |||||
| Phenolic acids | Hydroxybenzoic acids | Protocatechuic acid | C7H6O4 | 151, 136, 112 | -4.34 | FP | n.d. | n.d. | 3.46 | BP | n.d. | n.d. |
| Hydroxybenzoic acid | C7H6O3 | 135, 125, 121 | -3.49 | BP | -2.05 | FP | 4.47 | BP | n.d. | 3.75 | ||
| Hydroxycinnamic acids | Ferulic acid | C10H10O4 | 178, 149, 134 | -1.89 | BP | 57.88 | BP | 0.17 | BP | 2.23 | 1.2 | |
| p-Coumaric acid | C9H8O3 | 119 | n.d. | n.d. | -2.64 | BP | n.d. | n.d. | 1.63 | -2.03 | ||
| Sinapic acid | C11H12O5 | 208 | -2.24 | BP | n.d. | n.d. | -0.91 | BP | n.d. | n.d. | ||
| Avenantrahamide 2c | C16H13NO6 | 270, 178, 161 | n.d. | n.d. | -0.28 | FP | 0.99 | FP-BP | n.d. | n.d. | ||
| Avenanthramide 2p | C16H13NO5 | 254, (226), (160) | n.d. | n.d. | -4.03 | FP | 9.01 | FP | n.d. | n.d. | ||
| Avenanthramide 2f | C17H15NO6 | 284 | -2.25 | FP | -2.55 | FP | 1.4 | FP-BP | n.d. | n.d. | ||
| Diferulic isomer 1 | C20H18O8 | 341, (282), 193, (112) | -1.58 | BP | n.d. | n.d. | 1.79 | BP | n.d. | n.d. | ||
| Diferulic isomer 2 | C20H18O8 | 359, 341, 326 | -3.65 | BP | n.d. | n.d. | -1.58 | BP | n.d. | n.d. | ||
| Diferulic isomer 4 | C20H18O8 | 341, 326, 282, 248, 227 | -3.39 | BP | n.d. | n.d. | -2.35 | BP | n.d. | n.d. | ||
| Diferulic isomer 5 | C20H18O8 | 347, 313, 261, 217, 193, 178 | -4.69 | BP | n.d. | n.d. | -0.54 | BP | n.d. | n.d. | ||
| Diferulic isomer 6 | C20H18O8 | 341, 303, 239, 193, 178 | -0.54 | BP | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| Caffeic acid | C9H8O4 | (165), 135, 127 | -5.1 | FP-BP | 7.68 | BP | -1.76 | BP | -2.32 | -5.1 | ||
| Isoferulic acid | C10H10O4 | 178, 149, 134 | -1.38 | BP | n.d. | n.d. | -0.35 | BP | n.d. | n.d. | ||
| 1-O-Sinapoyl-beta-D-glucose | C17H22O10 | 216, 162, 119 | -0.98 | FP | n.d. | n.d. | -0.46 | FP | n.d. | n.d. | ||
| Flavonoids | Flavones | Apigenin-6-C-arabinoside-8-C-hexoside III | C26H28O14 | 221, 137 | n.d. | n.d. | -1.19 | FP | 0.05 | FP | n.d. | n.d. |
| Others | Hydroxybenzaldehyde acids | 4-Hydroxybenzaldehyde | C7H6O2 | (112) | 0.84 | BP | -3.25 | FP-BP | 0.84 | BP | 1.66 | -8.99 |
| Class | Sub-class | Compound | OG | OH | SO | EH1-OH | EH2-OH | |||
|---|---|---|---|---|---|---|---|---|---|---|
| FP | BP | FP | BP | FP | BP | |||||
| Phenolic acids | Hydroxybenzoic acids | Protocatechuic acid | 1.05 ± 0.04 a | n.d. | n.d. | n.d. | n.d. | 1.71 ± 0.17 b | n.d. | n.d. |
| Hydroxybenzoic acid | n.d. | 0.75 ± 0.01 c | 0.53 ± 0.02 a | n.d. | n.d. | 0.66 ± 0.10 b | n.d. | 0.74 c | ||
| Hydroxycinnamic acids | Ferulic acid | 0.36 + 0.01 a | 28.30 ± 1.56 d | 0.50 + 0.13 a | 213.76 ± 4.39 f | n.d. | 32.36 ± 3.09 e | 6.15 ± 0.87 c | 3.38 b | |
| p-Coumaric acid | n.d. | n.d. | n.d. | 130.67 ± 1.43 c | n.d. | n.d. | 11.47 ± 2.11 b | 7.32 a | ||
| Sinapic acid | n.d. | 2.93 ± 0.18 b | n.d. | n.d. | n.d. | 2.50 ± 0.14 a | n.d. | n.d. | ||
| Avenanthramide 2c | n.d. | n.d. | 0.21 ± 0.02 a | n.d. | 13.40 ± 0.34 b | 0.19 ± 0.04 a | n.d. | n.d. | ||
| Avenanthramide 2p | n.d. | n.d. | 1.58 ± 0.14 a | n.d. | 14.35 ± 0.27 b | n.d. | n.d. | n.d. | ||
| Avenanthramide 2f | 0.23 ± 0.07 a | n.d. | 1.48 ± 0.10 b | n.d. | 19.24 ± 0.19 d | 1.67 ± 0.01 c | n.d. | n.d. | ||
| Diferulic isomer 1 | n.d. | 4.42 ± 0.22 b | n.d. | n.d. | n.d. | 3.80 ± 0.38 a | n.d. | n.d. | ||
| Diferulic isomer 2 | n.d. | 5.02 ± 0.63 b | n.d. | n.d. | n.d. | 3.85 ± 0.38 a | n.d. | n.d. | ||
| Diferulic isomer 4 | n.d. | 5.70 ± 0.39 a | n.d. | n.d. | n.d. | 8.35 ± 0.82 b | n.d. | n.d. | ||
| Diferulic isomer 5 | n.d. | 6.83 ± 0.59 a | n.d. | n.d. | n.d. | 7.30 ± 0.62 a | n.d. | n.d. | ||
| Diferulic isomer 6 | n.d. | 0.74 ± 0.06 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| Caffeic acid | 2.32 ± 0.01 c | 1.50 ± 0.11 a | n.d. | 6.42 ± 0.26 e | n.d. | 3.44 ± 0.41 d | 2.41 ± 0.25 c | 1.72 b | ||
| Isoferulic acid | n.d. | 3.36 ± 0.67 a | n.d. | n.d. | n.d. | 2.96 ± 0.39 a | n.d. | n.d. | ||
| 1-O-Sinapoyl-beta-D-glucose | 1.55 ± 0.02 a | n.d. | n.d. | n.d. | 2.45 ± 0.02 b | n.d. | n.d. | n.d. | ||
| Flavonoids | Flavones | Apigenin-6-C-arabinoside-8-C-hexoside III | n.d. | n.d. | < LOD | n.d. | < LOD | n.d. | n.d. | n.d. |
| Others | Hydroxybenzaldehide acids | 4-Hydroxybenzaldehyde | n.d. | 1.10 ± 0.11 a | 4.17 ± 0.06 c | 27.88 ± 1.44 e | n.d. | 1.71 ± 0.11 b | 9.10 ± 1.05 d | 9.91 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Pulido, I.J.; Rico, D.; Martinez-Villaluenga, C.; Pérez-Jiménez, J.; Luis, D.D.; Martín-Diana, A.B. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods 2022, 11, 2769. https://doi.org/10.3390/foods11182769
Jiménez-Pulido IJ, Rico D, Martinez-Villaluenga C, Pérez-Jiménez J, Luis DD, Martín-Diana AB. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods. 2022; 11(18):2769. https://doi.org/10.3390/foods11182769
Chicago/Turabian StyleJiménez-Pulido, Iván Jesús, Daniel Rico, Cristina Martinez-Villaluenga, Jara Pérez-Jiménez, Daniel De Luis, and Ana Belén Martín-Diana. 2022. "Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull" Foods 11, no. 18: 2769. https://doi.org/10.3390/foods11182769
APA StyleJiménez-Pulido, I. J., Rico, D., Martinez-Villaluenga, C., Pérez-Jiménez, J., Luis, D. D., & Martín-Diana, A. B. (2022). Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods, 11(18), 2769. https://doi.org/10.3390/foods11182769










