A Comparative Study on the Effect of Ultrasound-Treated Apple Pomace and Coffee Silverskin Powders as Phosphate Replacers in Irish Breakfast Sausage Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredient Preparation
2.2. Sausage Production
- Formulation 1: 0.20% STPP + 0.22% AP + 0.58% CSS
- Formulation 2: 0.20% STPP + 0.00% AP + 0.80% CSS
- Formulation 3: 0.06% STPP + 0.94% AP + 0.00% CSS
2.3. Proximate Analysis
2.4. WHC and Cook Loss
2.5. LF-NMR Analysis of Bound Water
2.6. Emulsion Stability
2.7. Texture Profile Analysis (TPA)
2.8. Color Evaluation
2.9. Lipid Oxidation
2.10. Sausage Scoring System
2.11. Statistical Analysis
3. Results and Discussions
3.1. Proximate Composition Analysis
3.2. Emulsion Stability
3.3. Water Mobility Measured Using LF-NMR
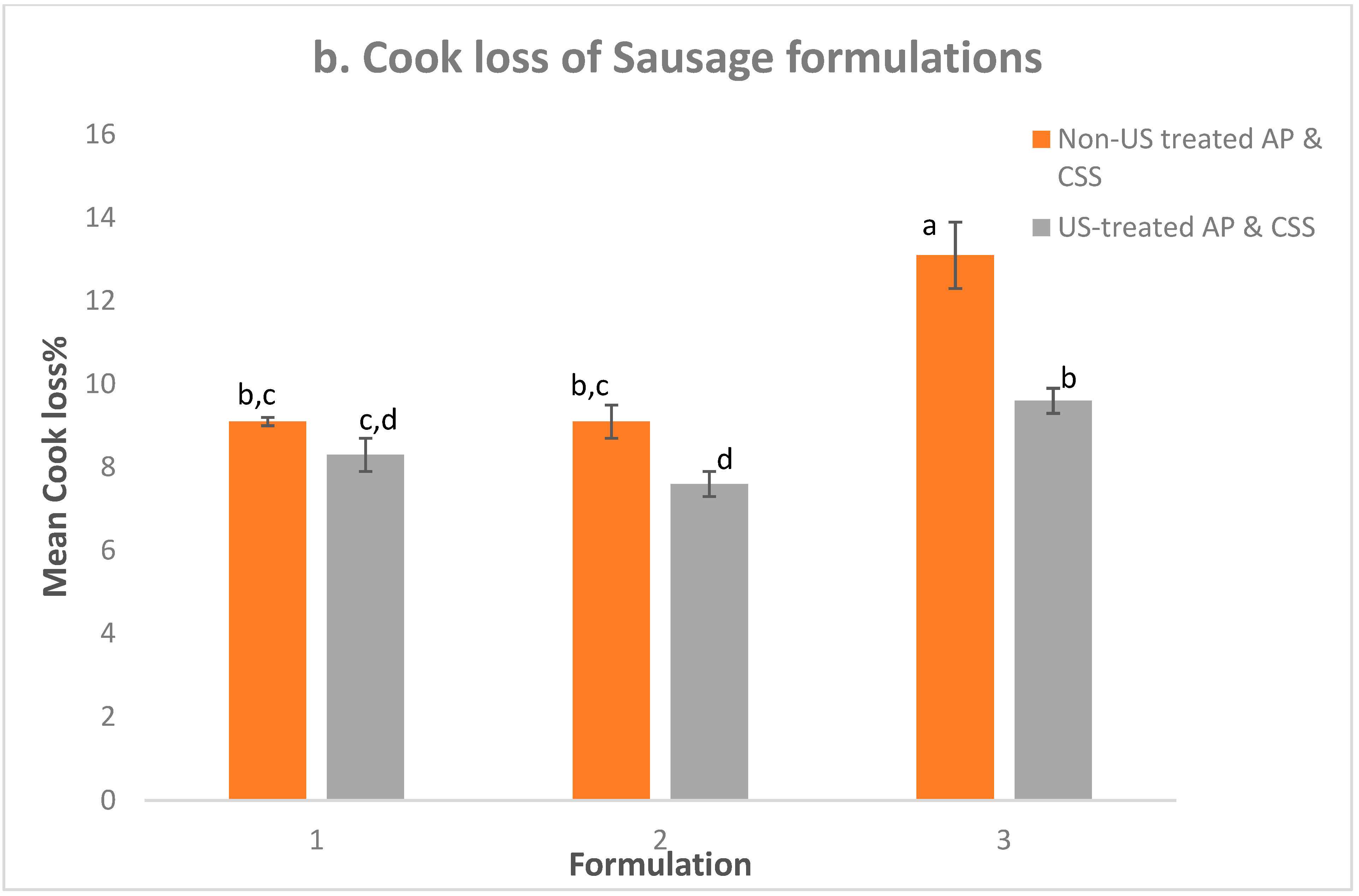
3.4. WHC and Cook Loss
3.5. Color
3.6. Texture Profile Analysis (TPA)
3.7. TBARS Analysis of Sausages
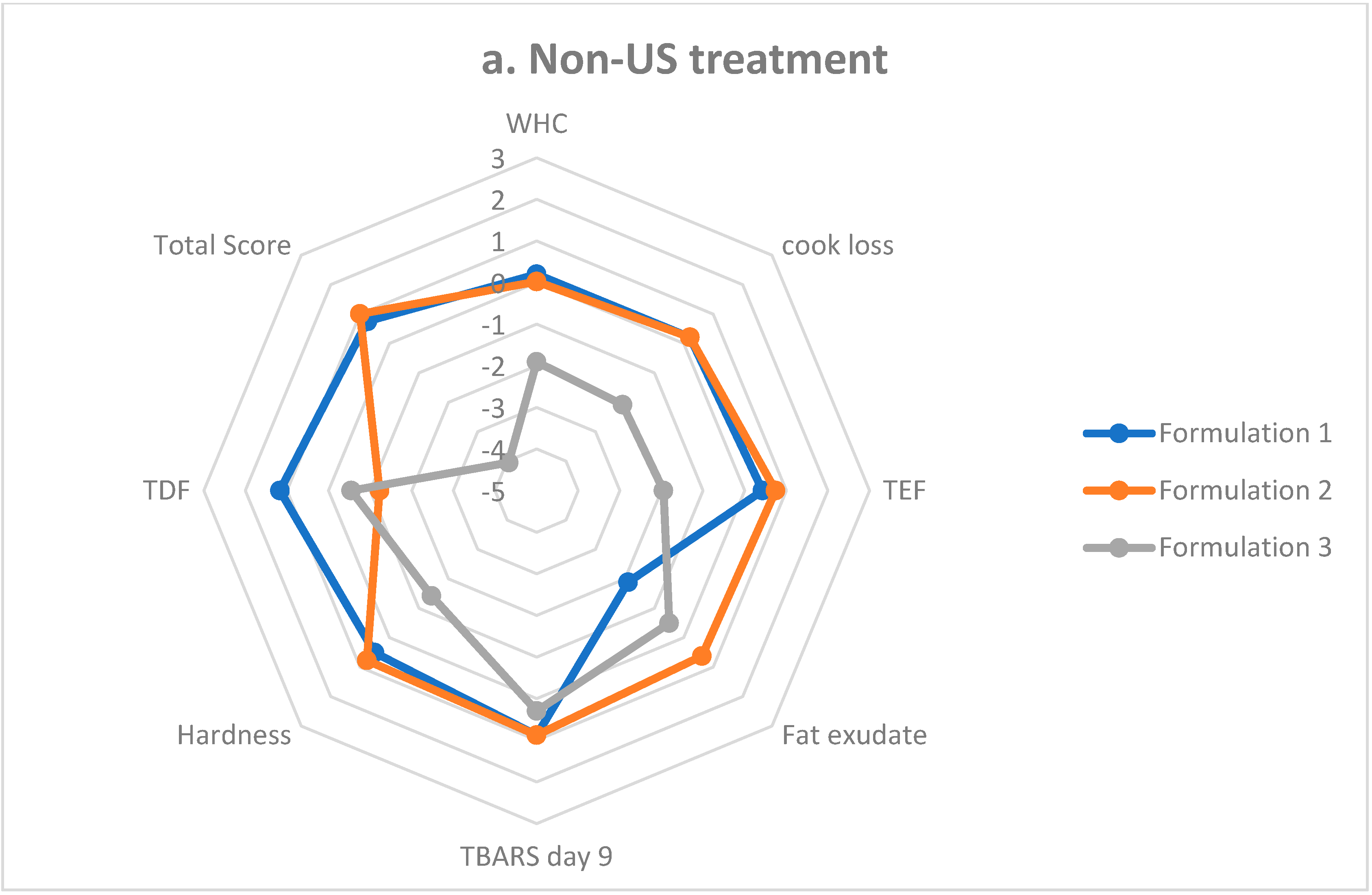
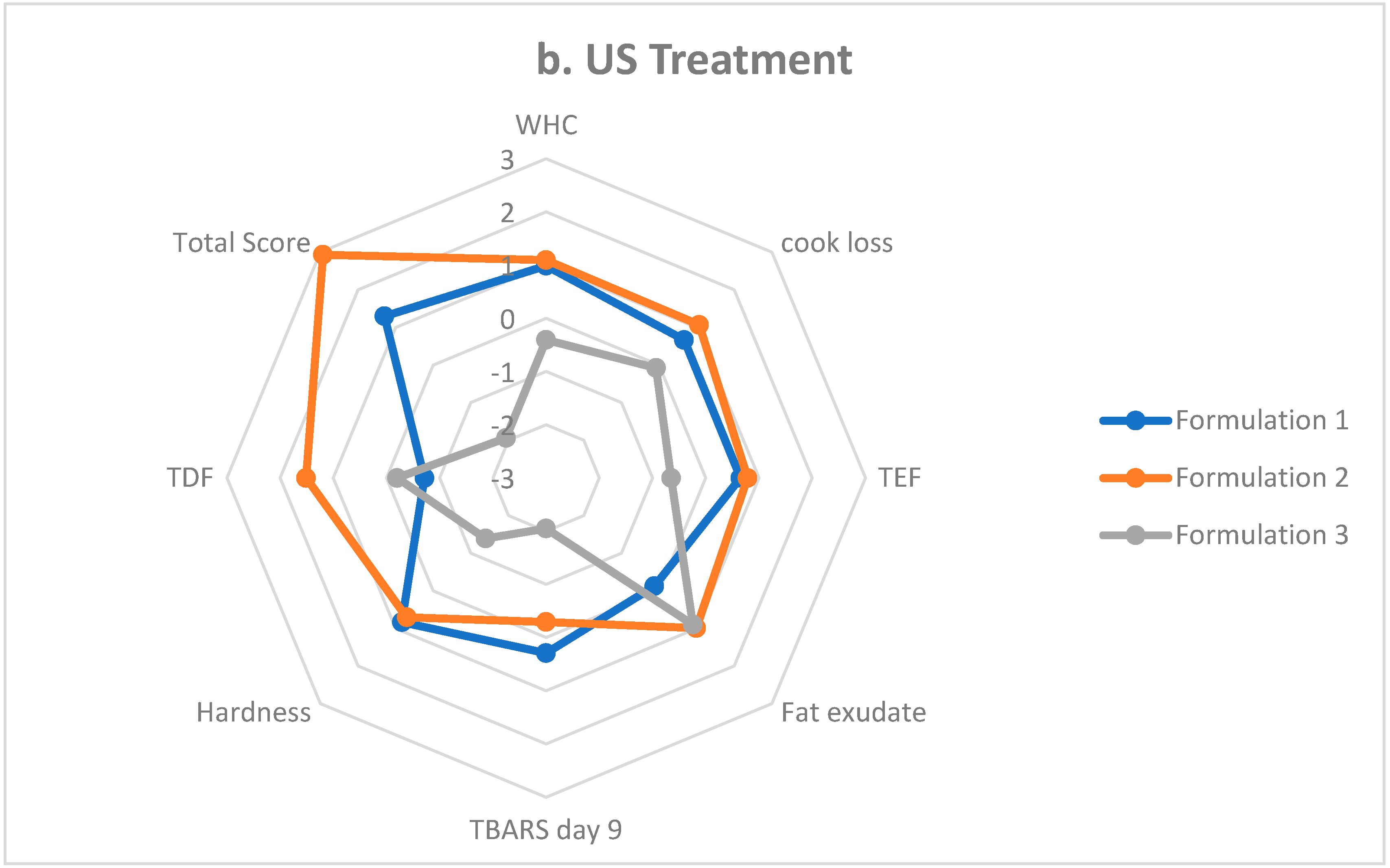
3.8. Sausage Formulation Scoring
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Illippangama, A.U.; Jayasena, D.D.; Jo, C.; Mudannayake, D.C. Inulin as a functional ingredient and their applications in meat products. Carbohydr. Polym. 2022, 275, 118706. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem. Toxicol. 2017, 105, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montes, A.; Rangel-Vargas, E.; Lorenzo, J.M.; Romero, L.; Santos, E.M. Edible mushrooms as a novel trend in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 37, 118–124. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Kerry, J.P.; Tiwari, B.K.; McDonnell, C.K. Novel processing technologies and ingredient strategies for the reduction of phosphate additives in processed meat. Trends Food Sci. Technol. 2019, 94, 43–53. [Google Scholar] [CrossRef]
- Pinton, M.B.; dos Santos, B.A.; Lorenzo, J.M.; Cichoski, A.J.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Curr. Opin. Food Sci. 2021, 40, 1–5. [Google Scholar]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Re-evaluation of phosphoric acid–phosphates—Di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [PubMed]
- Winger, R.J.; Uribarri, J.; Lloyd, L. Phosphorus-containing food additives: An insidious danger for people with chronic kidney disease. Trends Food Sci. Technol. 2012, 24, 92–102. [Google Scholar] [CrossRef]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Increasing dietary phosphorus intake from food additives: Potential for negative impact on bone health. Adv. Nutr. 2014, 5, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Hathwar, S.C.; Rai, A.K.; Modi, V.K.; Narayan, B. Characteristics and consumer acceptance of healthier meat and meat product formulations—A review. J. Food Sci. Technol. 2012, 49, 653–664. [Google Scholar]
- Ren, F.; Feng, Y.; Zhang, H.; Wang, J. Effects of modification methods on microstructural and physicochemical characteristics of defatted rice bran dietary fiber. Food Sci. Technol. 2021, 151, 112161. [Google Scholar] [CrossRef]
- Talukder, S. Effect of dietary fiber on properties and acceptance of meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1005–1011. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Okuro, P.K.; Santos, M.; Paglarini, C.d.S.; da Cunha, R.L.; Ruiz-Capillas, C.; Herrero, A.M.; Pollonio, M.A.R. Understanding the role of chia (Salvia hispanica L.) mucilage on olive oil-based emulsion gels as a new fat substitute in emulsified meat products. Eur. Food Res. Technol. 2020, 246, 909–922. [Google Scholar] [CrossRef]
- El Zeny, T.; Essa, R.; Bisar, B.; Metwalli, S. Effect of using chicory roots powder as a fat replacer on beef burger quality. Slov. Vet. Zb. 2019, 56 (Suppl. 22), 509–514. [Google Scholar] [CrossRef]
- Han, M.; Bertram, H.C. Designing healthier comminuted meat products: Effect of dietary fibers on water distribution and texture of a fat-reduced meat model system. Meat Sci. 2017, 133, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, F.; Hurtado, M.A.L.; Estèvez, A.M.A.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Belmiro, R.H.; Oliveira, L.d.C.; Tribst, A.A.L.; Cristianini, M. Techno-functional properties of coffee by-products are modified by dynamic high pressure: A case study of clean label ingredient in cookies. LWT 2022, 154, 112601. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Tiwari, B.K.; Kerry, J.P.; McDonnell, C.K.; Álvarez, C. Phosphate replacing potential of apple pomace and coffee silver skin in Irish breakfast sausage using a mixture design approach. Meat Sci. 2022, 185, 108722. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Hernández-Santo, B.; Rodríguez-Miranda, J. Effect of ultrasound on the techno-functional properties of food components/ingredients: A review. Ultrason. Sonochem. 2020, 61, 104787. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Tiwari, B.K.; Kerry, J.P.; Álvarez, C. A comprehensive study on the characterisation properties of power ultrasound-treated apple pomace powder and coffee silverskin powder. Eur. Food Res. Technol. 2022, 248, 1939–1949. [Google Scholar] [CrossRef]
- AOAC 992.15 Association of Official Analytical Chemists (AOAC) official method 992.15. Crude protein in meat and meat products. Combustion method. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1992.
- AOAC 985.14 Association of Official Analytical Chemists (AOAC) official method 985.14. Moisture in Meat and Poultry Products—Rapid. By Microwave drying. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1991.
- AOAC 2008.06 Association of Official Analytical Chemists (AOAC) official method 2008.06. Moisture and fat in meats. Microwave and nuclear magnetic resonance analysis. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 2008.
- AOAC 920.153 Association of Official Analytical Chemists (AOAC) official method 920.153. Ash of meat. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1920.
- AOAC 935.47 Association of Official Analytical Chemists (AOAC) official method 935.47. Salt (chlorine as sodium chloride) in meat. Volumetric method. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1987.
- AOAC 991.43 Association of Official Analytical Chemists (AOAC) official method 991.43. Total, Soluble and Insoluble dietary fiber in foods. By Enzymatic-Gravimetric method, MES–TRIS buffer. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1994.
- Lianji, M.; Chen, N. Research in Improving the Water Holding Capacity (WHC) of Meat in Sausage Products; Paper presented at the ICoMST; Danish Meat Research Institute: Copenhagen, Denmark, 1989. [Google Scholar]
- McDonnell, C.K.; Allen, P.; Duggan, E.; Arimi, J.M.; Casey, E.; Duane, G.; Lyng, J.G. The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: A low field NMR study. Meat Sci. 2013, 95, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Mullen, A.M.; Troy, D.J. Effects of fat level, tapioca starch and whey protein on frankfurters formulated with 5% and 12% fat. Meat Sci. 1998, 48, 169–180. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 33, 62–66. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, Sensitive, and Specific Thiobarbituric Acid Method for Measuring Lipid Peroxidation in Animal Tissue, Food, and Feedstuff Samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Álvarez, C.; Drummond, L.; Mullen, A.M. Protein recovered from meat co-products and processing streams as pork meat replacers in Irish breakfast sausages formulations. LWT 2018, 96, 679–685. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Yang, H.; Tao, F.; Cao, G.; Han, M.; Xu, X.; Zhou, G.; Shen, Q. Stability improvement of reduced-fat reduced-salt meat batter through modulation of secondary and tertiary protein structures by means of high pressure processing. Meat Sci. 2021, 176, 108439. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Role of interfacial protein membrane in oxidative stability of vegetable oil substitution emulsions applicable to nutritionally modified sausage. Meat Sci. 2015, 109, 56–65. [Google Scholar] [CrossRef]
- Yum, H.-W.; Seo, J.-K.; Jeong, J.-Y.; Kim, G.-D.; Rahman, M.S.; Yang, H.-S. The Quality Improvement of Emulsion-type Pork Sausages Formulated by Substituting Pork Back fat with Rice Bran Oil. Korean J. Food Sci. Anim. Resour. 2018, 38, 123–134. [Google Scholar]
- Zhang, C.; Liu, H.; Xia, X.; Sun, F.; Kong, B. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein. LWT 2021, 142, 111016. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Zhou, G.; Xu, X.; Qi, J.; Shi, P.; Xia, T. Meat quality and cooking attributes of thawed pork with different low field NMR T21. Meat Sci. 2012, 92, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Mullen, A.M.; Hamill, R.; O’Neill, E.; Álvarez, C. Chapter Three—Dry-aging of beef as a tool to improve meat quality. Impact of processing conditions on the technical and organoleptic meat properties. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 95, pp. 97–130. [Google Scholar]
- Warner, R.D. Chapter 14—The Eating Quality of Meat—IV Water-Holding Capacity and Juiciness. In Lawrie´s Meat Science, 8th ed.; Toldrá, F., Ed.; Woodhead Publishing: Thorston, UK, 2017; pp. 419–459. [Google Scholar]
- Glorieux, S.; Goemaere, O.; Steen, L.; Fraeye, I. Phosphate Reduction in Emulsified Meat Products: Impact of Phosphate Type and Dosage on Quality Characteristics. Food Technol. Biotechnol. 2017, 55, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.; Tatol, M. Colour difference∆ E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Zhang, Y.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef]

| Formulation 1 | Formulation 2 | Formulation 3 | Interaction Sig. * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | ||
| Moisture (%) | 62.8 ± 0.5 | 62.3 ± 0.9 | ns | 62.6 ± 0.5 | 62.7 ± 0.5 | ns | 63.7 ± 0.6 | 62.6 ± 0.9 | ns | ns |
| Fat (%) | 13.3 ± 0.1 | 13.2 ± 0.3 | ns | 13.5 ± 0.2 | 13.5 ± 0.4 | ns | 12.8 ± 0.5 | 13.5 ± 0.6 | ns | ns |
| Protein (%) | 16.0 ± 0.4 | 16.3 ± 0.7 | ns | 16.4 ± 0.1 | 16.0 ± 0.5 | ns | 16.0 ± 0.5 | 16.2 ± 0.5 | ns | ns |
| Ash (%) | 1.8 ± 0.1 | 1.8 ± 0.1 | ns | 1.8 ± 0.0 | 1.8 ± 0.0 | ns | 1.6 ± 0.0 | 1.6 ± 0.1 | ns | ns |
| TDF (%) | 7.6 ± 2.7 | 6.5 ± 4.0 | ns | 6.2 ± 2.0 | 7.8 ± 1.9 | ns | 6.6 ± 2.7 | 6.8 ± 1.5 | ns | ns |
| Salt (NaCl) (%) | 0.7 ± 0.1 | 0.7 ± 0.2 | ns | 0.9 ± 0.2 | 0.8 ± 0.2 | ns | 0.9 ± 0.2 | 0.8 ± 0.2 | ns | ns |
| TEF (%) | 8.3 ± 0.3 c | 7.7 ± 0.1 c | * | 7.5 ± 0.4 c | 7.4 ± 0.2 c | ns | 13.5 ± 0.6 a | 10.6 ± 0.4 b | * | * |
| Fat Exudate (%) | 7.4 ± 0.6 | 5.5 ± 0.2 | * | 4.3 ± 1.1 | 4.3 ± 2.9 | ns | 5.9 ± 1.5 | 4.4 ± 0.9 | ns | ns |
| T2b (ms) | 3.9 ± 0.6 | 2.5 ± 0.3 | * | 3.8 ± 0.4 | 2.4 ± 0.2 | * | 3.0 ± 0.5 | 2.1 ± 0.1 | * | ns |
| T21 (ms) | 36.5 ± 2.1 | 38.9 ± 0.0 | ns | 37.7 ± 2.1 | 37.7 ± 2.1 | ns | 37.7 ± 2.1 | 38.9 ± 0.0 | ns | ns |
| T22 (ms) | 227.3 ± 24.4 | 257.6 ± 14.1 | ns | 220.0 ± 21.1 | 257.6 ± 14.1 | ns | 234.0 ± 12.8 | 283.6 ± 15.5 | * | ns |
| P2b (%) | 3.4 ± 0.1 a | 2.4 ± 0.1 b | * | 3.2 ± 0.4 a | 2.4 ± 0.1 b | * | 1.8 ± 0.1 b | 2.2 ± 0.3 b | ns | * |
| P21 (%) | 89.9 ± 0.6 d | 92.1 ± 1.0 b,c,d | * | 90.3 ± 0.8 c,d | 92.3 ± 0.1 a,b,c | * | 94.6 ± 1.2 a | 93.2 ± 1.2 a,b | ns | * |
| P22 (%) | 6.8 ± 0.6 a | 5.5 ± 1.0 a,b,c | ns | 6.5 ± 0.8 a,b | 5.3 ± 0.1 a,b,c | ns | 3.5 ± 1.0 b,c | 4.5 ± 0.9 c | ns | * |
| Formulation 1 | Formulation 2 | Formulation 3 | Interaction Sig. * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | Non-US Treated AP and CSS | US-Treated AP and CSS | Sig. * | |||
| L* | 61.8 ± 2.0 | 61.3 ± 1.8 | ns | 59.9 ± 1.5 | 59.9 ± 2.1 | ns | 64.9 ± 2.2 | 64.1 ± 1.0 | ns | ns | |
| a* | 6.3 ± 0.2 | 6.6 ± 0.4 | ns | 6.5 ± 0.1 | 6.9 ± 0.2 | * | 6.3 ± 0.4 | 6.5 ± 0.2 | ns | ns | |
| b* | 18.4 ± 1.3 | 18.9 ± 1.3 | ns | 18.9 ± 0.7 | 19.4 ± 0.8 | ns | 20.1 ± 1.3 | 20.1 ± 0.6 | ns | ns | |
| ΔE | 0.82 | 0.60 | 0.89 | ||||||||
| Hardness (N) | 26.6 ± 5.0 | 27.7 ± 2.6 | ns | 27.4 ± 8.4 | 27.2 ± 3.7 | ns | 20.7 ± 1.7 | 20.8 ± 3.2 | ns | ns | |
| Chewiness (J) | 40.2 ± 21.8 | 31.4 ± 6.9 | ns | 38.0 ± 21.6 | 33.7 ± 14.7 | ns | 14.2 ± 1.0 | 13.0 ± 2.8 | ns | ns | |
| Cohesive force (no unit) | 0.7 ± 0.0 | 0.7 ± 0.0 | ns | 0.8 ± 0.0 | 0.8 ± 0.0 | ns | 0.8 ± 0.0 | 0.8 ± 0.0 | ns | ns | |
| Springiness (mm) | 6.6 ± 0.3 | 6.4 ± 0.3 | ns | 6.7 ± 0.9 | 6.1 ± 0.8 | ns | 4.5 ± 0.1 | 4.4 ± 0.3 | ns | ns | |
| TBARS (mg MDA/kg) | day 0 | 0.2 ± 0.0 | 0.2 ± 0.0 | ns | 0.2 ± 0.0 | 0.2 ± 0.0 | ns | 0.2 ± 0.0 | 0.2 ± 0.0 | ns | ns |
| day 3 | 0.2 ± 0.0 | 0.2 ± 0.0 | ns | 0.2 ± 0.0 | 0.3 ± 0.1 | ns | 0.2 ± 0.0 | 0.3 ± 0.0 | ns | ns | |
| day 6 | 0.3 ± 0.1 | 0.3 ± 0.1 | ns | 0.3 ± 0.1 | 0.3 ± 0.1 | ns | 0.4 ± 0.2 | 0.4 ± 0.1 | ns | ns | |
| day 9 | 0.3 ± 0.0 b,c | 0.4 ± 0.0 b | ns | 0.3 ± 0.0 c | 0.4 ± 0.0 b | * | 0.4 ± 0.1 b | 0.8 ± 0.1 a | * | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangavelu, K.P.; Tiwari, B.; Kerry, J.P.; Álvarez, C. A Comparative Study on the Effect of Ultrasound-Treated Apple Pomace and Coffee Silverskin Powders as Phosphate Replacers in Irish Breakfast Sausage Formulations. Foods 2022, 11, 2763. https://doi.org/10.3390/foods11182763
Thangavelu KP, Tiwari B, Kerry JP, Álvarez C. A Comparative Study on the Effect of Ultrasound-Treated Apple Pomace and Coffee Silverskin Powders as Phosphate Replacers in Irish Breakfast Sausage Formulations. Foods. 2022; 11(18):2763. https://doi.org/10.3390/foods11182763
Chicago/Turabian StyleThangavelu, Karthikeyan Palanisamy, Brijesh Tiwari, Joseph P. Kerry, and Carlos Álvarez. 2022. "A Comparative Study on the Effect of Ultrasound-Treated Apple Pomace and Coffee Silverskin Powders as Phosphate Replacers in Irish Breakfast Sausage Formulations" Foods 11, no. 18: 2763. https://doi.org/10.3390/foods11182763
APA StyleThangavelu, K. P., Tiwari, B., Kerry, J. P., & Álvarez, C. (2022). A Comparative Study on the Effect of Ultrasound-Treated Apple Pomace and Coffee Silverskin Powders as Phosphate Replacers in Irish Breakfast Sausage Formulations. Foods, 11(18), 2763. https://doi.org/10.3390/foods11182763









