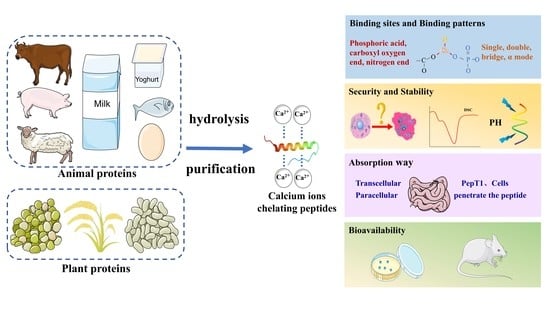
The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium–Peptide Chelates
Abstract
:1. Introduction
2. Sources of Calcium-Binding Peptides
2.1. Plant Proteins
2.2. Animal Proteins
3. Preparation of Calcium Chelating Peptides
3.1. Hydrolysis
3.2. Purification
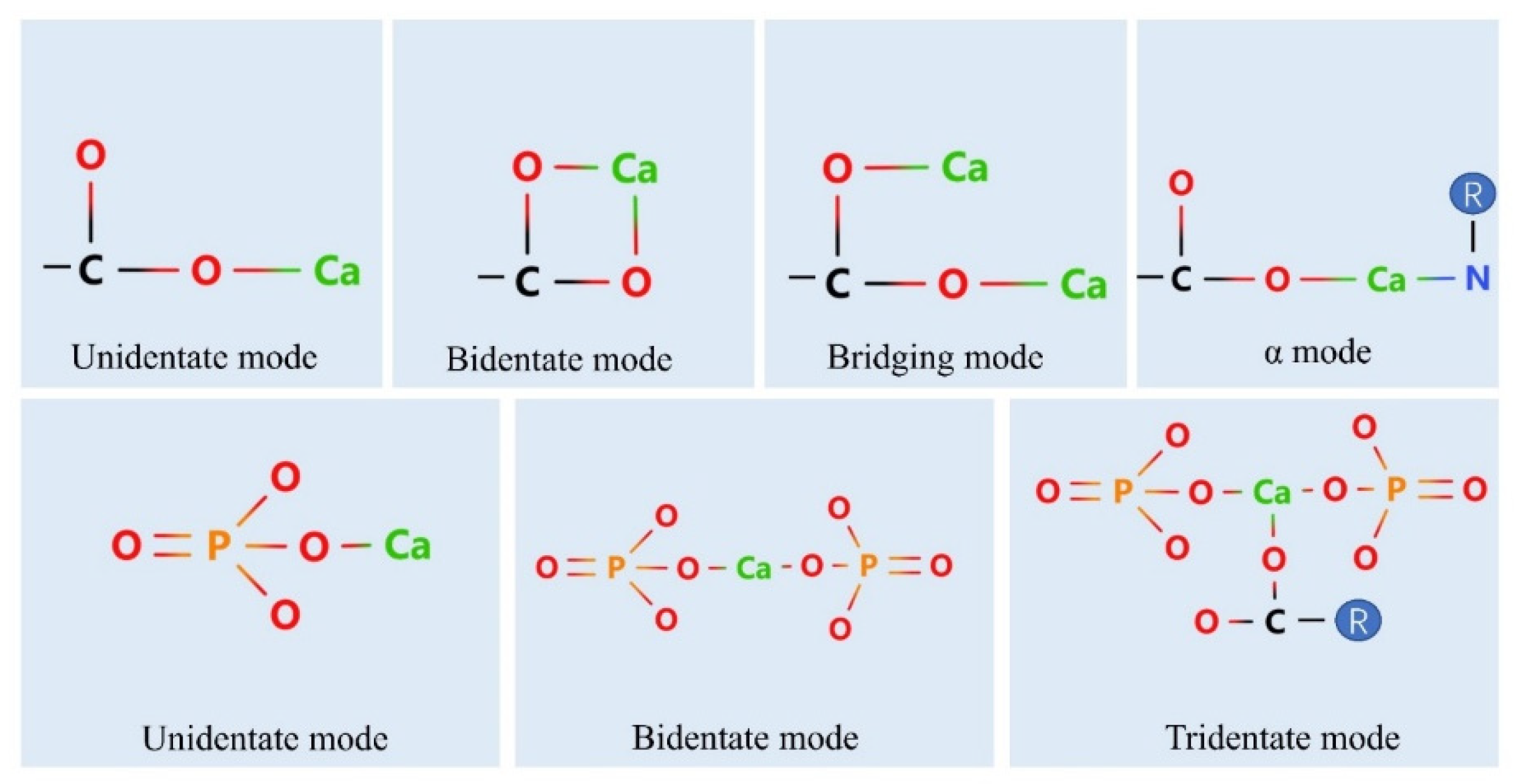
4. Calcium Binding Sites on Peptides
4.1. Phosphate Group
4.2. Carboxylate Oxygen Atoms
4.3. Nitrogen Atoms
4.4. Binding of Peptides to Calcium Ions
5. The Security and Stability of Calcium–Peptide Chelates
5.1. Security
5.2. Stability
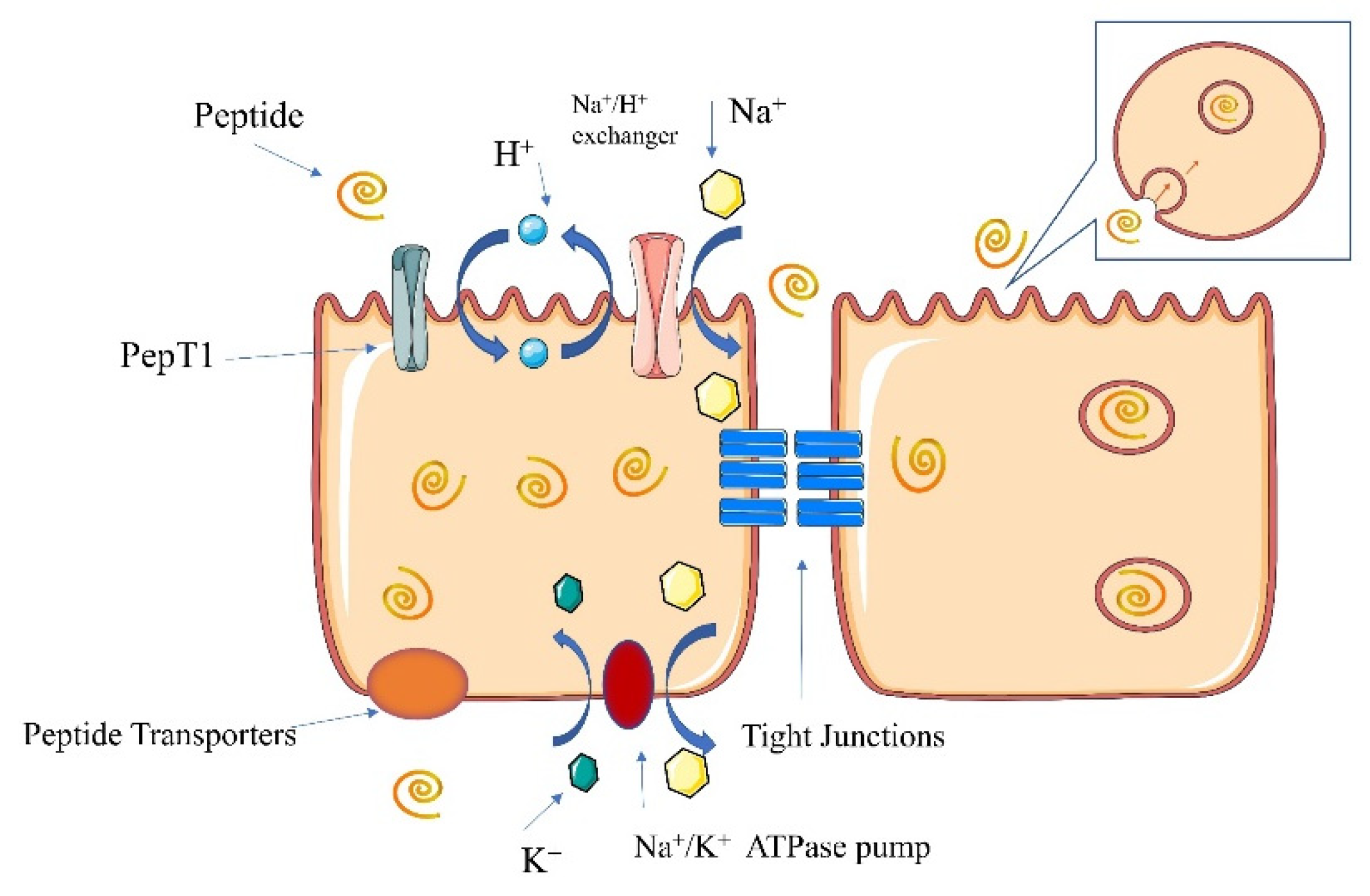
6. Absorption Pathways of Calcium–Peptide Chelates
6.1. Pathways of Calcium Absorption
6.1.1. Transcellular Pathway
Facilitated Diffusion
Vesicular Transport
Endoplasmic Reticulum Transport
6.1.2. Paracellular Pathway
6.2. Pathways of Peptide Absorption
6.2.1. PepT1 Pathway
6.2.2. Cell-Penetrating Peptide Pathway
6.2.3. Paracellular Pathway
7. Bioavailability of Calcium–Peptide Chelates
7.1. In Vitro Bioavailability
7.2. In Vivo Bioavailability
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daengprok, W.; Garnjanagoonchorn, W.; Naivikul, O.; Pornsinlpatip, P.; Issigonis, K.; Mine, Y. Chicken eggshell matrix proteins enhance calcium transport in the human intestinal epithelial cells, Caco-2. J. Agric. Food Chem. 2003, 51, 6056–6061. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A. The role of dietary calcium in bone health. Proc. Nutr. Soc. 2003, 62, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; He, H.; Guo, D.J.; Zhang, X.; Jia, L.; Hou, T.; Ma, A.M. Chitosan oligosaccharides-tripolyphosphate microcapsules as efficient vehicles for desalted duck egg white peptides-calcium: Fabrication, entrapment mechanism and in vivo calcium absorption studies. Lebensm. Wiss. Technol. 2022, 154, 112869. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, C.B.; Chen, Z.; Liu, X.Y. Editorial: The Role of Calcium Channels in Human Health and Disease. Front. Mol. Biosci. 2022, 9, 834108. [Google Scholar] [CrossRef]
- Mori, M.; Tanifuji, S.; Mochida, S. Kinetic organization of Ca2+ signals that regulate synaptic release efficacy in sympathetic neurons. Mol. Pharmacol. 2014, 86, 297–305. [Google Scholar] [CrossRef]
- Guo, D.J.; He, H.; Zhao, M.G.; Zhang, G.Q.; Hou, T. Desalted duck egg white peptides promoted osteogenesis via wnt/β-catenin signal pathway. J. Food Sci. 2020, 85, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Bierman, J.; Hale, C.A.; Bendich, A. Absorbability and cost effectiveness in calcium supplementation. J. Am. Coll. Nutr. 2001, 20, 239–246. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Vavrusova, M.; Skibsted, L.H. Calcium nutrition. Bioavailability and fortification. Lebensm. Wiss. Technol. 2014, 59, 1198–1204. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Hernandez-Mendoza, A.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wu, H.T.; Du, M.; Tang, Y.; Liu, H.W.; Fu, Y.H.; Zhu, B.W. Food protein-derived calcium chelating peptides: A review. Trends Food Sci. Technol. 2016, 58, 140–148. [Google Scholar] [CrossRef]
- Wu, W.F.; Li, B.F.; Hou, H.; Zhang, H.W.; Zhao, X. Isolation and identification of calcium-chelating peptides from Pacific cod skin gelatin and their binding properties with calcium. Food Funct. 2017, 8, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Wang, X.P.; Guo, X.N. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Jeon, S.J.; Lee, J.H.; Song, K.B. Isolation of a Calcium-binding Peptide from Chlorella Protein Hydrolysates. Prev. Nutr. Food Sci. 2010, 15, 282–286. [Google Scholar] [CrossRef]
- Cai, X.X.; Lin, J.P.; Wang, S.Y. Novel peptide with specific calcium-binding capacity from Schizochytrium sp. protein hydrolysates and calcium bioavailability in Caco-2 Cells. Mar. Drugs 2016, 15, 3. [Google Scholar] [CrossRef]
- Wang, X.; Gao, A.; Chen, Y.; Zhang, X.Y.; Li, S.H.; Chen, Y. Preparation of cucumber seed peptide-calcium chelate by liquid state fermentation and its characterization. Food Chem. 2017, 229, 487–494. [Google Scholar] [CrossRef]
- Kheeree, N.; Kuptawach, K.; Puthong, S.; Sangtanoo, P.; Srimongkol, P.; Boonserm, P.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Discovery of calcium-binding peptides derived from defatted lemon basil seeds with enhanced calcium uptake in human intestinal epithelial cells, Caco-2. Sci. Rep. 2022, 12, 4659. [Google Scholar] [CrossRef]
- Lv, Y.; Bao, X.L.; Liu, H.; Ren, J.H.; Guo, S.T. Purification and characterization of calcium-binding soybean protein hydrolysates by Ca2+/Fe3+ immobilized metal affinity chromatography (IMAC). Food Chem. 2013, 141, 1645–1650. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Z.J.; Liu, C.H.; Sun, H.; Liu, Y.F. Investigating the calcium binding characteristics of black bean protein hydrolysate. Food Funct. 2020, 11, 8724–8734. [Google Scholar] [CrossRef]
- Budseekoad, S.; Yupanqui, C.T.; Sirinupong, N.; Alashi, A.M.; Aluko, R.E.; Youravong, W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods 2018, 49, 333–341. [Google Scholar] [CrossRef]
- Jung, W.K.; Karawita, R.; Heo, S.J.; Lee, B.J.; Kim, S.K.; Jeon, Y.J. Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem. 2006, 41, 2097–2100. [Google Scholar] [CrossRef]
- Charoenphun, N.; Cheirsilp, B.; Sirinupong, N.; Youravong, W. Calcium-binding peptides derived from tilapia (Oreochromis niloticus) protein hydrolysate. Eur. Food Res. Technol. 2012, 236, 57–63. [Google Scholar] [CrossRef]
- Choi, D.W.; Lee, J.H.; Chun, H.H.; Bin Song, K. Isolation of a calcium-binding peptide from bovine serum protein hydrolysates. Food Sci. Biotechnol. 2012, 21, 1663–1667. [Google Scholar] [CrossRef]
- Chen, M.; Ji, H.W.; Zhang, Z.W.; Zeng, X.G.; Su, W.M.; Liu, S.C. A novel calcium-chelating peptide purified from Auxis thazard rotein hydrolysate and its binding properties with calcium. J. Funct. Foods 2019, 60, 103447. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, Z.; Xu, H.Y.; Li, X.Y.; Hao, X.D. Preparation of sheep bone collagen peptide-calcium chelate using enzymolysis-fermentation methodology and its structural characterization and stability analysis. RSC Adv. 2020, 10, 11624–11633. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, K.B. Isolation of a calcium-binding peptide from enzymatic hydrolysates of porcine blood plasma protein. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 290–294. [Google Scholar] [CrossRef]
- Huang, G.; Ren, L.; Jiang, J. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur. Food Res. Technol. 2010, 232, 281–287. [Google Scholar] [CrossRef]
- Vo, T.D.L.; Pham, K.T.; Le, L.T.; Nguyen, T.T.H. Identification of a new calcium-binding peptide from enzymatic proteolysate of Acetes japonicus. J. Food Process. Preserv. 2018, 42, e13837. [Google Scholar] [CrossRef]
- Kumagai, H.; Shizawa, Y.; Sakurai, H.; Kumagai, H. Influence of Phytate Removal and Structural Modification on the Calcium-binding Properties of Soybean Globulins. Biosci. Biotechnol. Biochem. 1998, 62, 341–346. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.; Ren, J.H.; Li, X.; Guo, S.T. The positive effect of soybean protein hydrolysates-calcium complexes on bone mass of rapidly growing rats. Food Funct. 2013, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of Peptide-calcium complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T.; Tanaka, S.; Yokochi, T.; Nakahara, T.; Higashihara, T. Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J. Am. Oil Chem. Soc. 1997, 74, 1431–1434. [Google Scholar] [CrossRef]
- Wu, W.M.; He, L.C.; Li, C.L.; Zhao, S.L.; Liang, Y.H.; Yang, F.; Zhang, M.; Jin, G.F.; Ma, M.H. Phosphorylation of porcine bone collagen peptide to improve its calcium chelating capacity and its effect on promoting the proliferation, differentiation and mineralization of osteoblastic MC3T3-E1 cells. J. Funct. Foods 2020, 64, 103701. [Google Scholar] [CrossRef]
- Li, H.Z.; Duan, S.L.; Yuan, P.; Liu, J.; Wang, X.; Liu, Y.F.; Peng, Y.X.; Pan, C.; Xia, K. Preparation of casein phosphopeptides calcium complex and the promotion in calcium cellular uptake through transcellular transport pathway. J. Food Biochem. 2021, 45, e14001. [Google Scholar] [CrossRef]
- Bennett, T.; Desmond, A.; Harrington, M.; McDonagh, D.; FitzGerald, R.; Flynn, A.; Cashman, K.D. The effect of high intakes of casein and casein phosphopeptide on calcium absorption in the rat. Br. J. Nutr. 2000, 83, 673–680. [Google Scholar] [CrossRef]
- Tian, L.J.; Xiong, D.D.; Jia, J.; Liu, X.B.; Zhang, Y.; Duan, X. Mechanism study on enhanced emulsifying properties of phosvitin and calcium-binding capacity of its phosphopeptides by lactic acid bacteria fermentation. Lebensm. Wiss. Technol. 2022, 155, 113002. [Google Scholar] [CrossRef]
- Liu, B.T.; Zhuang, Y.L.; Sun, L.P. Identification and characterization of the peptides with calcium-binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. J. Food Sci. 2020, 85, 114–122. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.F.; Chen, Q.R.; Zhang, Z.H.; Zhao, X.; Hou, H. Functional calcium binding peptides from pacific cod (Gadus macrcephalus) bone: Calcium bioavailability enhancing activity and anti-osteoporosis effects in the ovariectomy-induced osteoporosis rat model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhao, L.Y.; Shen, Q.S.; Qi, L.W.; Jiang, S.; Guo, Y.J.; Zhang, C.H.; Richel, A. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability. Lebensm. Wiss. Technol. 2021, 144, 111264. [Google Scholar] [CrossRef]
- Cui, P.B.; Sun, N.; Jiang, P.F.; Wang, D.; Lin, S.Y. Optimised condition for preparing sea cucumber ovum hydrolysate-calcium complex and its structural analysis. Int. J. Food Sci. Technol. 2017, 52, 1914–1922. [Google Scholar] [CrossRef]
- Lin, Y.L.; Cai, X.X.; Wu, X.P.; Lin, S.N.; Wang, S.Y. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake. J. Funct. Foods 2020, 65, 103717. [Google Scholar] [CrossRef]
- Wu, J.H.; Cai, X.X.; Tang, M.R.; Wang, S.Y. Novel calcium-chelating peptides from octopus scraps and their corresponding calcium bioavailability. J. Sci. Food Agric. 2019, 99, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Dong, C.; Li, W.J.; Zhang, F.W. Optimization of preparation process of peptides chelated calcium from Bacillus natto fermentation by response surface methodology. China Brew. 2021, 40, 119–123. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J.P. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Tian, Q.J.; Fan, Y.; Hao, L.; Wang, J.; Xia, C.S.; Wang, J.F.; Hou, H. A comprehensive review of calcium and ferrous ions chelating peptides: Preparation, structure and transport pathways. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Liao, W.W.; Chen, H.; Jin, W.G.; Yang, Z.N.; Cao, Y.; Miao, J.Y. Three newly isolated calcium-chelating peptides from tilapia Bone collagen hydrolysate enhance calcium absorption activity in intestinal Caco-2 cells. J. Agric. Food Chem. 2020, 68, 2091–2098. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.Y.; Zhang, X.X.; Li, Y.F.; Wang, R.; Luo, X.H.; Li, Y.N.; Li, J.; Chen, Z.X. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef]
- Hou, T.; Liu, Y.S.; Guo, D.J.; Li, B.; He, H. Collagen peptides from crucian skin improve calcium bioavailability and structural characterization by HPLC-ESI-MS/MS. J. Agric. Food Chem. 2017, 65, 8847–8854. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.B.; Lin, S.Y.; Yu, C.P.; Tang, Y.; Wei, Y.; Xiong, Y.L.; Wu, H.T. Characterization of sea cucumber (stichopus japonicus) ovum hydrolysates: Calcium chelation, solubility and absorption into intestinal epithelial cells. J. Sci. Food Agric. 2017, 97, 4604–4611. [Google Scholar] [CrossRef]
- Zhao, L.N.; Huang, S.L.; Cai, X.X.; Hong, J.; Wang, S.Y. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods 2014, 10, 46–53. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Zhang, X.F.; Jia, J.Q.; Kuang, C.; Yang, H.S. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: Thermodynamic parameters, physicochemical properties and bioactivities. Process Biochem. 2018, 67, 46–54. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.F.; Arfat, Y.A. High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. Lebensm. Wiss. Technol. 2019, 100, 231–236. [Google Scholar] [CrossRef]
- Gaberc-Porekar, V.; Menart, V. Perspectives of immobilized-metal affinity chromatography. J. Biochem. Biophys. Methods 2001, 49, 335–360. [Google Scholar] [CrossRef]
- Guo, L.D.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.F.; Hou, H.; FitzGerald, R.J. Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Iwashita, K.; Sakuraba, S.; Shiraki, K.; Arakawa, T.; Kameda, T. Salt-dependent elution of uncharged aromatic solutes in ion-exchange chromatography. J. Chromatogr. A 2018, 1546, 46–55. [Google Scholar] [CrossRef]
- Zhang, X.W.; Jia, Q.; Li, M.Y.; Liu, H.P.; Wang, Q.; Wu, Y.R.; Niu, L.L.; Liu, Z.T. Isolation of a novel calcium-binding peptide from phosvitin hydrolysates and the study of its calcium chelation mechanism. Food Res. Int. 2021, 141, 110169. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.W.; Hou, H.; Zhang, H.W.; Li, B.F. Purification and characterization of a novel calcium-biding decapeptide from Pacific cod (Gadus Macrocephalus) bone: Molecular properties and calcium chelating modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.M.; Huang, H.; Nie, R.Y.; Liu, Z.Y.; Zeng, M.Y. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Sun, N.; Hu, S.J.; Wang, D.; Jiang, P.F.; Zhang, S.M.; Lin, S.Y. Calcium delivery systems assembled using antarctic krill derived heptapeptides: Exploration of the assembly mechanism, In vitro digestion profile, and calcium absorption behavior. J. Agric. Food Chem. 2022, 70, 2018–2028. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 652–685. [Google Scholar] [CrossRef]
- Khare, E.; Holten-Andersen, N.; Buehler, M.J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, L.; Tian, Z.; Chen, L.; Subirade, M. Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study. Food Chem. 2012, 133, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Peng, L.J.; Zhang, S.S.; Lin, Y.; Feng, F.Q. Effects of molecular structure on the calcium-binding properties of phosphopeptides. Eur. Food Res. Technol. 2012, 235, 811–816. [Google Scholar] [CrossRef]
- Ferraretto, A.; Gravaghi, C.; Fiorilli, A.; Tettamanti, G. Casein-derived bioactive phosphopeptides: Role of phosphorylation and primary structure in promoting calcium uptake by HT-29 tumor cells. FEBS Lett. 2003, 551, 92–98. [Google Scholar] [CrossRef]
- Meisel, H.; Frister, H. Chemical characterization of a caseinophosphopeptide isolated from in vivo digests of a casein diet. Biol. Chem. Hoppe Seyler 1988, 369, 1275–1279. [Google Scholar] [CrossRef]
- Jiang, B.; Mine, Y. Preparation of novel functional oligophosphopeptides from hen egg yolk phosvitin. J. Agric. Food Chem. 2000, 48, 990–994. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.X.; Bao, Z.J.; Cui, P.B.; Wang, S.; Lin, S.Y. Calcium binding to herring egg phosphopeptides: Binding characteristics, conformational structure and intermolecular forces. Food Chem. 2020, 310, 125867. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, J.I.; Hong, S.M.; Hahm, D.H.; Lee, S.Y.; Kim, I.H.; Choi, S.Y. Phosphorylation of peptides derived from isolated soybean protein: Effects on calcium binding, solubility and influx into Caco-2 cells. Biofactors 2005, 23, 121–128. [Google Scholar] [CrossRef]
- Bao, X.L.; Lv, Y.; Yang, B.C.; Ren, C.G.; Guo, S.T. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates. J. Food Sci. 2008, 73, C117–C121. [Google Scholar] [CrossRef]
- Sun, N.; Jin, Z.Q.; Li, D.M.; Yin, H.J.; Lin, S.Y. An exploration of the calcium-binding mode of egg White peptide, Asp-His-Thr-Lys-Glu, and In vitro calcium absorption Studies of peptide-calcium complex. J. Agric. Food Chem. 2017, 65, 9782–9789. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.Y.; Chen, H.; Zhu, Z.X.; Han, L.Y.; Dong, X.F.; Du, M.; Li, T.T. Characterization of Chelation and Absorption of Calcium by a Mytilus edulis Derived Osteogenic Peptide. Front. Nutr. 2022, 9, 840638. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.B.; Lin, S.Y.; Han, W.W.; Jiang, P.F.; Zhu, B.W.; Sun, N. Calcium delivery system assembled by a nanostructured peptide derived from the sea cucumber ovum. J. Agric. Food Chem. 2019, 67, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Nara, M.; Morii, H.; Tanokura, M. Coordination to divalent cations by calcium-binding proteins studied by FTIR spectroscopy. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2319–2327. [Google Scholar] [CrossRef]
- Luo, M.N.; Xiao, J.; Sun, S.W.; Cui, F.C.; Li, W.; Liu, G.; Li, Y.Q.; Cao, Y. Deciphering calcium-binding behaviors of casein phosphopeptides by experimental approaches and molecular simulation. Food Funct. 2020, 11, 5284–5292. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.S.; Zheng, J.X.; Bu, T.T.; He, G.Q.; Wu, J.P. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Huang, W.; Lan, Y.Q.; Liao, W.W.; Lin, L.; Liu, G.; Xu, H.M.; Xue, J.P.; Guo, B.Y.; Cao, Y.; Miao, J.Y. Preparation, characterization and biological activities of egg white peptides-calcium chelate. Lebensm. Wiss. Technol. 2021, 149, 112035. [Google Scholar] [CrossRef]
- Cai, X.X.; Zhao, L.N.; Wang, S.Y.; Rao, P.F. Fabrication and characterization of the nano-composite of whey protein hydrolysate chelated with calcium. Food Funct. 2015, 6, 816–823. [Google Scholar] [CrossRef]
- Zhang, P.L.; Bao, Z.J.; Jiang, P.F.; Zhang, S.M.; Zhang, X.M.; Lin, S.Y.; Sun, N. Nanoliposomes for encapsulation and calcium delivery of egg white peptide-calcium complex. J. Food Sci. 2021, 86, 1418–1431. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.V.; Picotto, G.; Carpentieri, A.R.; Rivoira, M.A.; Peralta Lopez, M.E.P.; de Talamoni, N.G.T. Minireview on regulation of intestinal calcium absorption. Emphasis on molecular mechanisms of transcellular pathway. Digestion 2008, 77, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. Regulation of intestinal calcium transport. Annu. Rev. Nutr. 2008, 28, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Recent advances in our understanding of 1,25-dihydroxyvitamin D3 regulation of intestinal calcium absorption. Arch. Biochem. Biophys. 2012, 523, 73–76. [Google Scholar] [CrossRef]
- De Barboza, G.D.; Guizzardi, S.; de Talamoni, N.T. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Petersen, O.H.; Fedirko, N.V. Calcium signalling: Store-operated channel found at last. Curr. Biol. 2001, 11, R520–R523. [Google Scholar] [CrossRef]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Katimba, H.A.; Wang, R.C.; Cheng, C.L. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Fernandez-Musoles, R.; Salom, J.B.; Castello-Ruiz, M.; Contreras, M.D.; Recio, I.; Manzanares, P. Bioavailability of antihypertensive lactoferricin B-derived peptides: Transepithelial transport and resistance to intestinal and plasma peptidases. Int. Dairy J. 2013, 32, 169–174. [Google Scholar] [CrossRef]
- Palm, C.; Jayamanne, M.; Kjellander, M.; Hallbrink, M. Peptide degradation is a critical determinant for cell-penetrating peptide uptake. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E., Jr. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef] [PubMed]
- Gravaghi, C.; Del Favero, E.; Cantu‘, L.; Donetti, E.; Bedoni, M.; Fiorilli, A.; Tettamanti, G.; Ferraretto, A. Casein phosphopeptide promotion of calcium uptake in HT-29 cells—relationship between biological activity and supramolecular structure. FEBS J. 2007, 274, 4999–5011. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lv, Y.; Xu, J.T.; Guo, S.T. Interaction mode of calcium-binding peptides and Caco-2 cell membrane. Food Res. Int. 2017, 102, 225–233. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Yang, R.; Wang, Q.; Wang, G.Z.; Song, H.B.; Geng, F.; Luo, P.; Huang, Q. Identification, characterization and binding sites prediction of calcium transporter-embryo egg-derived egg white peptides. J. Food Meas. Charact. 2022, 16, 2948–2960. [Google Scholar] [CrossRef]
- Bao, Z.J.; Zhang, P.L.; Sun, N.; Lin, S.Y. Elucidating the calcium-binding site, absorption activities, and thermal stability of egg white peptide-calcium chelate. Foods 2021, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, X.L.; Hu, W.D.; Zeng, B.; Liang, R.M.; Liu, H.; Li, Z.X.; Zhang, Z. Phosvitin phosphopeptide preparation using immobilised trypsin and enhancing calcium absorption in growing rats. Czech J. Food Sci. 2017, 34, 325–331. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, Y.H.; Song, S.J.; Wang, X.J.; Qin, Y.Y.; Si, S.Y.; Guo, Y.C. Combined oral administration of bovine collagen peptides with calcium citrate inhibits bone loss in ovariectomized rats. PLoS ONE 2015, 10, e0135019. [Google Scholar] [CrossRef]

| Source | Preparation | Amino Acid Sequence | CaBC (Ca2+/Peptide) | Ca2+ Binding Site |
|---|---|---|---|---|
| Chlorella (Chlorella vulgaris) [15] | Flavourzyme hydrolysis | NSGC | 211 µg/mg | Ser, Gly and Cys residues |
| Schizochytrium sp. [16] | Alcalase and Flavourzyme hydrolysis | FY | 128.77 µg/mg | Carboxyl oxygen atoms and amino nitrogen atoms; nitrogen and oxygen atoms of amido bonds |
| Cucumber seed [17] | Liquid state fermentation with B. subtilis | 191.5 µg/mg | -COOH, -OH, -NH2, -CO-NH- | |
| Lemon basil seeds (Ocimum citriodorum) [18] | Alcalase hydrolysate | AFNRAKSKALNEN, YDSSGGPTPWLSPY | 278.14 µg/mg, 151.88 µg/mg | Amino nitrogen atoms and oxygen atoms on the carboxyl group |
| Soybean [19] | Protease M&Amano enzyme | DEGEQPRPFPFP | 79.24 μg/mg | Glu, Gln, Lys and Pro |
| Black bean [20] | ficin hydrolysate | 77.54 μg/mg | Amino nitrogen atoms and carboxyl oxygen atoms | |
| Mung bean [21] | Enzymatic hydrolysis | LLLG, AIVIL, HADAD | 943.60 μg/mg, 834.87 μg/mg, 809.13 μg/mg | Leucine or isoleucine at the C- or N-terminal |
| Alaska pollack (Theragra chalcogramma) backbone [22] | Pepsinolytic hydrolysis | VLSGGTTMAMYTLV | 160.00 μg/mg | |
| Tilapia [23] | Alcalase (2.4 L) hydrolysis | WEWLHYW | 65 μg/mg | |
| Bovine serum protein [24] | Alcalase, Flavourzyme and Protamex hydrolysis | DNLPNPEDRKNYE | 14.18 μg/mg | |
| Frigate mackerel (Auxis thazard) [25] | Enzymatic hydrolysis and membrane separation | EPAH | 76.8 ± 4.5 μg/mg | Carboxylic group of Glu, carboxylic group and the amino group of His |
| Sheep bone [26] | Enzymatic hydrolysis and Lactobacillus fer-mentation | 56.39 μg/mg | Carboxyl oxygen and amino nitrogen atoms of collagen peptides | |
| Porcine blood [27] | Flavourzyme hydrolysis | VSGVEDVN | 7.758 μg/mg | |
| Shrimp processing by-products [28] | TCH | 11.76 μg/mg | ||
| Akiami paste shrimp (Acetes japonicus) [29] | Flavourzyme | YEIPAEDL | 277.96 ± 20.93 μg/mg | Carboxylate oxygen of Glu and Asp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Zhang, Y.; Ying, Z.; Li, H.; Liu, W.; Wang, J.; Liu, X. The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium–Peptide Chelates. Foods 2022, 11, 2762. https://doi.org/10.3390/foods11182762
An J, Zhang Y, Ying Z, Li H, Liu W, Wang J, Liu X. The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium–Peptide Chelates. Foods. 2022; 11(18):2762. https://doi.org/10.3390/foods11182762
Chicago/Turabian StyleAn, Jiulong, Yinxiao Zhang, Zhiwei Ying, He Li, Wanlu Liu, Junru Wang, and Xinqi Liu. 2022. "The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium–Peptide Chelates" Foods 11, no. 18: 2762. https://doi.org/10.3390/foods11182762
APA StyleAn, J., Zhang, Y., Ying, Z., Li, H., Liu, W., Wang, J., & Liu, X. (2022). The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium–Peptide Chelates. Foods, 11(18), 2762. https://doi.org/10.3390/foods11182762