3.1. Viability of Microbial Load and pH
The survival of microorganisms during processing and storage in fermented milk is highly important [
40]. As can be seen in
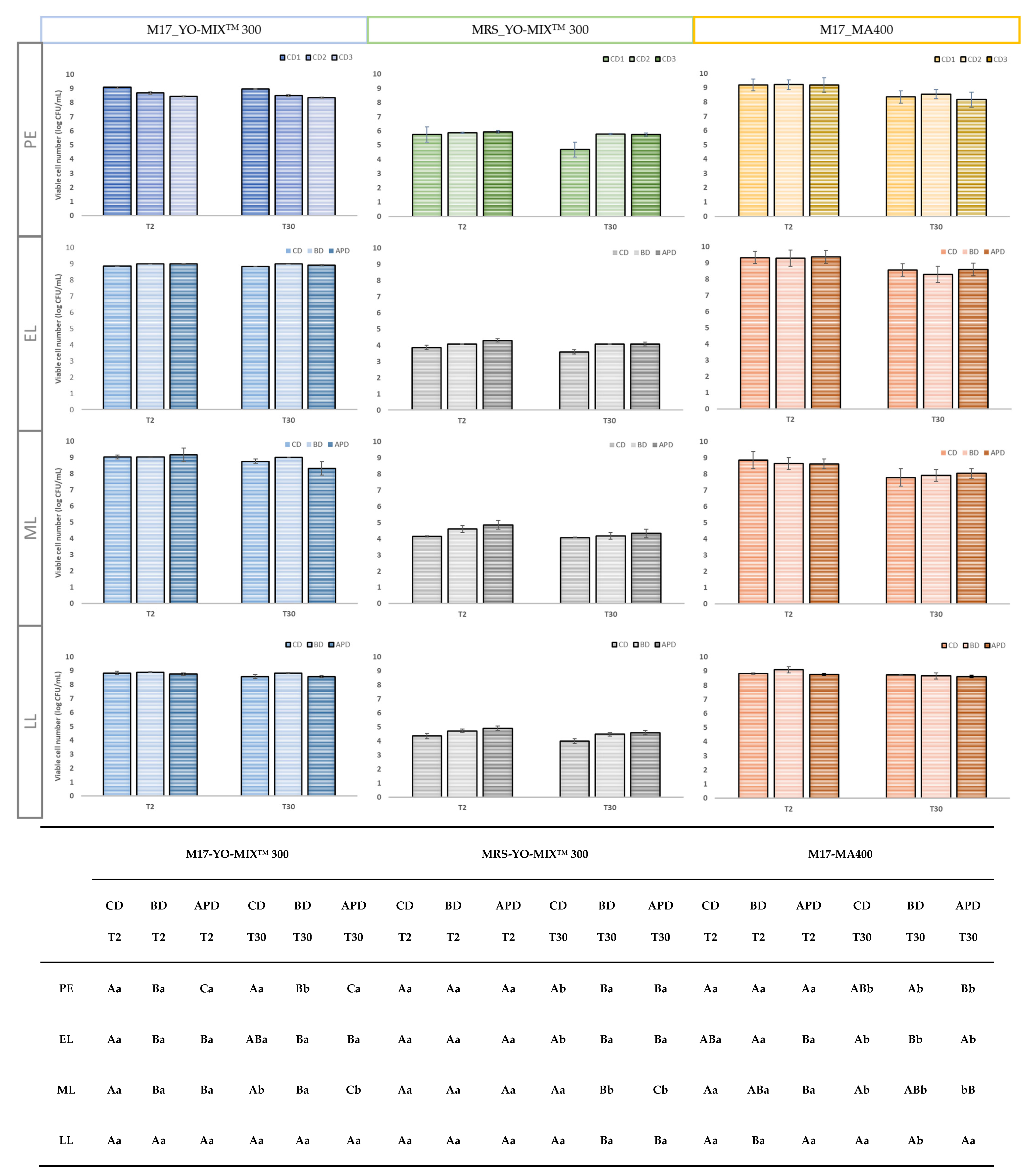
Figure 1, the type of diet, storage time and lactation stage did not significantly affect the number of viable microorganisms of all milk fermented by YO-MIX
TM 300 starter culture. The sum of the number of viable cells of
Streptococcus thermophilus and
Lactobacillus delbrueckii spp.
bulgaricus was >10
7 CFU/mL (colony forming units CFU) and the ratio 2:1 (
S. thermophilus: L. bulgaricus) was maintained during refrigerated storage, being the established requirement for yogurt fermented milk by CODEX ALIMENTARIUS [
25,
40,
41,
42]. The symbiosis between these microorganisms has been extensively studied, the protocooperation. Markakiou et al. (2020) [
26] explained the sum of the acid production in a mixed culture is greater than in a single culture.
Streptococcus thermophillus produce several components (formic acid, pyruvic acid, carbon dioxide, long chain fatty acids, among others) which stimulate the growth of
L. delbrueckii spp.
bulgaricus, which is highly proteolytic. This causes a pH reduction due to the transformation of lactose into lactic acid, and then, the denaturation of milk proteins and the release of aminoacids that are essential for the growth of
Streptococcus. According to Liu et al. (2016) [
13] the fermentation time decreases when symbiotic cultures are used. Previously, Dimitrellou et al. (2019) [
25] found differences in the bacterial count of
S. thermophilus and
L. delbrueckii spp.
bulgaricus in fermented goat milk, increasing the viability of
S. thermophilus even under low temperature conditions and after 28 days of storage, while the viability of
L. delbrueckii spp.
bulgaricus decreased. In our study, a general reduction of viable cells was detected with the advance of the lactation stage; counts on FMs at PE and EL productions (three diets) were higher than those on FM from LL stage.
As to FMs by MA400 starter culture, a general decrease in the number of viable cells was observed due to the storage time from T2 to T30 of refrigeration (
p < 0.05) for the three studied diets and at the four lactation stages (PE; EL; ML and LL). Considering lactation stage and diet, although some significant differences were detected they were considered irrelevant as they were minor in quantitative terms. No scientific studies on fermented milks by MA400 culture were found in the scientific literature as it is especially used for the manufacture of cheese, and also butter and buttermilk. The decrease in viability during storage cannot be directly attributed to any of the microorganisms that are part of this starter culture, but it could be potentially linked to
Lactococcus lactis. L. lactis has been used together with other microorganisms in cheese making and it has been observed after six days of storage at 6 °C, that the decrease in the number of viable cells was due to
L. Lactis reduction (from 2.3 × 10
9 CFU/g to 1.7 × 10
9 CFU/g) [
43]. This decrease was attributed to the fact that while LAB grows, lactate is increasing which causes a reduction in pH, inhibiting the growth of
L. lactis [
44]
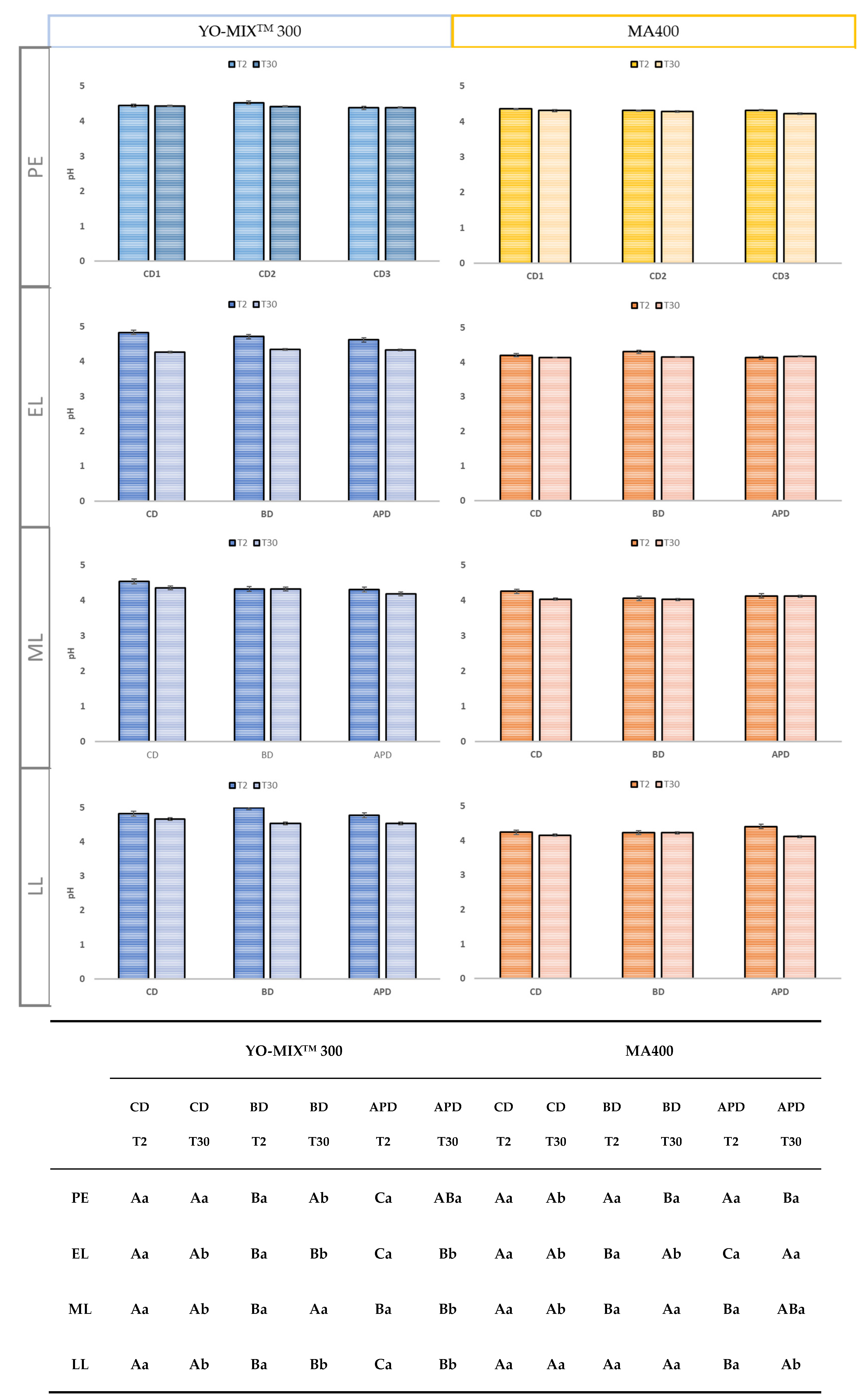
One of the key parameters in FMs production is pH decrease in milk; it is the direct consequence of the activity of the inoculated microorganisms. Fast acidification ensures food safety and pH is used as an indicator of the end point of fermentation. No significant differences were found among diets and lactation stage (
Figure 2). FMs by YO-MIX
TM 300 (range 4.6 and 4.18) had higher pH values than FMs by MA400 (range 4.36 and 4.02). A slight decrease of pH was noticed between T2 and T30 for both cultures. These results agree to what was previously found by other authors, were no significant variations of pH during storage for FMs was noticed. It is observed that the slight reduction of pH, especially FMs by YO-MIX
TM 300 and an increase in acidity could be explained by the improvement of microbial growth and the peptidase activity of
L. delbrueckii subsp. bulgaricus, which is greatly favoured in goat milk [
41,
45]. Moreover, the urease activity of
S. thermophilus present a significant correlation with the use of lactose and the production of lactic acid [
24,
27].
3.2. Composition of Milk and Fermented Milk
Composition of raw bulk tank milk is shown in
Table 1. Regarding fat content, a 10% increase was observed at the EL stage sampling as compared to PE sampling for diets BD (5.30% vs. 4.80%) and APD (5.07% vs. 4.56%), the increase was only slight for group on CD diet (4.91% vs. 4.73%). Fat content increased till the end of lactation reaching values of 5.39% in CD and 5.61% in BD. However, fat content in APD remained constant throughout the experiment. BD diet yielded the milk with the highest fat content throughout the study. Protein content was slightly lower in BD and APD with respect to CD at EL and ML stages (0.1 units
p < 0.05). An increase in protein was observed at the end of lactation (LL) for all diets, reaching contents of 3.62%, 3.56% and 3.42% for CD, BD and APD, respectively. Protein content was always higher in milk from the CD diet. A parallel study conducted by Monllor et al. [
8] with an inclusion of 40% broccoli and artichoke plant by-products throughout lactation, observed similar results with an increase of 0.5% in absolute terms of fat in milk from of diets with broccoli silage and a higher protein content for milk from a conventional diet. They also observed an increase in fat and protein throughout lactation. Other parallel studies [
6,
7,
8,
29] but with a short-term inclusion showed that 40% broccoli silage by-products and 40% and 12.5% artichoke plant silage also increased the percentage of fat with respect to the conventional diet. However, the percentage of protein was hardly modified by the differentiated diets.
The average macrocomposition of the milk fermented by YOMIX
TM 300 starter culture is presented in
Table 2. As to fat percentage, an increase throughout lactation was detected, being more pronounced in fermented milks from BD diet with an interval of 4.46–5.50% between PE and LL production, with an increase (
p < 0.05) of 1.04 percentage units. Fermented milk derived from CD also showed the same tendency but slightly less pronounced with an increase of 0.52 percentage units between PE and LL production, followed by fermented milks made from APD (an increase of 0.43 percentage units). As to protein percentage, a similar trend was observed, increasing throughout lactation being LL production significantly higher than PE in all batches (0.38, 0.32 and 0.19 percentage units for CD, APD and BD, respectively). This increase was also observed ML stage in the CD batch. The tendency above described for protein content in milk with the same proportion in the goat diet (40% of by-product) was maintained when milk is fermented by YO-MIX
TM 300: CD (3.75%) presented higher values than APD (3.67%), followed by BD (3.54%).
Regarding the composition of milk fermented by MA400 starter culture (
Table 3) the same tendency was observed, increasing fat and protein content during lactation. A fat percentage increase (
p < 0.05) of 0.24 percentage units was observed in the BD diet (5.63%) compared to the APD (5.41%) and CD (5.39%) diets between PE and LL. These differences were also found in milk from goat fed with the incorporation of 40% of by-product. Milk from animals fed broccoli by-product silage had a higher average fat content compared to the conventional diet and with artichoke plant by-product silage [
6,
8,
29]. Nevertheless, when milk was fermented by MA400 no differences in protein percentage were found between APD and BD (3.45%).
Fermented milks macrocomposition (from both starter cultures) were not affected by the studied storage time. In some cases, significant differences were detected but these results presented no clear trend, being the differences between T2 and T30 very small (in percentage units). Average macrocomposition of the fermented milks with the different starter cultures and in the different productions and diets did not quantitatively differ much. Consequently, when evaluating gel stability (syneresis and whey composition) the behavior was similar, as will be discussed later.
Table 4 and
Table 5 show the sugars (lactose, glucose and galactose) and lactic acid present in the FMs. These components contribute to the characteristic taste of this type of product. Lactose content in raw milk ranged from 4.75 to 4.29%, and was reduced by fermentation with YO-MIX
TM 300 and MA400 to values up to 1.78 and 1.70%, and 1.72 and 1.56% after T2 and T30 during refrigerated storage, respectively. Significant differences were observed between T2 and T30 of storage in some elaborations (
p < 0.05) but with no clear trend, and could be considered caused by the elaboration process. It can be said that the highest content of lactose was observed in PE and EL FMs and the lowest content in LL FMs. In previous studies [
46] the residual lactose was 1.90% in FMs by
L. bulgaricus, which is one of the YOMIX
TM 300 microorganisms in the present study. Data from Muelas et al., 2018 [
46] agree with the observation that only traces of glucose were detected in EL in T2 with MA400 culture, but it was observed that glucose values increased (0.24 and 0.34% for T2 and T30 of refrigeration, respectively) in later lactations (EL and ML) and T30 of storage. A decrease of glucose in FMs at the end of lactation was noticed.
It is noteworthy that the highest values of galactose were reached in YO-MIX
TM 300 FMs, and regarding lactation stage PE had the highest values which were reduced throughout lactation. As to MA400 fermented milk, a slight increase of galactose values was observed during lactation, however residual levels of glucose and galactose were both in similar range and not clearly influenced by the factors under study storage time and feeding [
46]. Although statistically differences were detected (
p < 0.05), these may not be considered relevant.
The lactic acid content was not affected by storage time, whereas due to the diet quantitatively slight differences (
p < 0.05) were detected but with no clear trend. Lactic acid content was consistent with pH values. YO-MIX
TM 300 FMs had less lactic acid and higher pH than those from MA400. Muelas et al., 2018 [
46] reported a lactic acid content of 0.59% in FMs with MA400, similar to the present study in PE. It can be seen that it increases to higher than 0.70% from week 12 of lactation and it is maintained until the end of the experiment with slight variations. It was also observed that lactic acid content was higher in fermented milk from broccoli by-product feeding, which was also the one with the highest dry matter content.
3.3. Syneresis and Whey Composition
Syneresis in FMs is the separation of the liquid phase from the gel. According to Domagała (2009) [
47] it can occur spontaneously or when the gel is mechanically disrupted during cutting, shaking or freezing, and is undesirable in firm and stirred fermented milk because it can negatively influence consumer acceptance of the food product. In previous studies, it has been observed that when the protein content and total solids are increased, an increase of whey retention capacity is observed, and then spontaneous syneresis process disappear, obtaining products with greater apparent viscosity and firmness [
47,
48].
Table 6 and
Table 7 show the percentage of syneresis and composition of the expelled whey. It is observed that the syneresis values are slightly higher in FMs with YO-MIX
TM 300 compared to those obtained with MA400. Similar values (average 64%) were found in previous studies [
46]. Other authors [
48] compared the percentage of syneresis 14 days after fermentation in yogurts based on different types of milk, obtaining considerably higher values in goat milk (39%) compared to cow milk (25%) or sheep (17%). Martín-Diana et al. (2003) [
41] explained that when goat milk is used for FMs, it is required the inclusion of a fortification to improve the coagulum characteristics because goat milk presents slightly lower casein content (α-s1-casein) than cow’s milk. The content of αs1-casein in goat milk depends on the genetic polymorphisms, whereas goats with alleles A, B or C have contents of αs1-casein up to 25% of the total milk protein, goats with O or N alleles have no αs1-casein. The lower the content of αs1-casein the larger the casein micelles and the number of hydrated pores yielding a less dense gel structure than cow milk [
49]. It is important to mention that no significant differences on syneresis were observed between storage times, as opposed to observations by Domagała, (2009) [
47] who did appreciate a decrease due to storage. Other authors also reported increased percentage of syneresis with storage time in cow’s milk yogurt [
22]. In a previous study, whey composition was as follows: 0.32% protein, 0.24% fat, 4.4% lactose, and 3.5% of total solids [
46] In the present study, lactose was much lower, presenting values <2.5%. It should be noted that the protein values were around 0.30% and decreased considerably in all fermented milks regardless of the feeding, at late lactation. It can be observed that FMs cultured with MA400 lost more fat with the whey than those cultured with YO-MIX
TM 300.
3.5. Volatile Profile of Fermented Milks
Main volatile compounds in fermented milks were analyzed in samples from PE (before introducing silages in the diet) and LL (after four months in different diets), results are presented in
Table 10 as prevalence of each volatile compound (% of area). Fifty-one compounds were identified in milks fermented by the mesophilic culture MA400 being 11 aldehydes, 8 hydrocarbons, 8 terpenes, 7 ketones, 6 esters, 5 alcohols, 5 acids and 1 sulfur compound. Forty-five compounds were identified in milks fermented by the thermophilic culture YO-MIX
TM300: 10 aldehydes, 7 hydrocarbons, 6 terpenes (some of them in the limit below 0.2 prevalence), 7 ketones, 6 esters, 4 alcohols, 4 acids and 1 sulfur compound. The prevalence of the chemical families is similar to the previously reported in other fermented goat milk products [
19]. Most of the compounds were isolated in both type of fermented milks, although with different prevalence. Major compounds in milk cultured with both cultures were hexanal, 2-heptanone, heptanal, hexanoic acid, octanal, 2-ethylhexanol, benzyl alcohol, 2-nonanone, nonanal and nonanedienol.
Five of the identified compounds are considered key compounds in the flavor of fermented milks: acetoin, hexanal, 2-heptanone, 2-nonanone and nonanal [
53]. Regarding the compounds mainly responsible for the goaty flavor, hexanoic acid was the most prevalent and showed a tendency to increase at late lactation and also in fermented milk from broccoli BD and artichoke plant silages APD fed groups. Hexanoic acid is also recognized as a major source of flavor in fermented milks [
54]. Octanoic and decenoic acids had a lower prevalence and were not affected by lactation stage.
The prevalence of volatile compounds in fermented milks by mesophilic culture was modified due to lactation stage for the following compounds: hexanal, heptanal, dodecane and tridecane decreased at late lactation, whereas butanoic acid, 2-heptanone, 2-nonanone increased. Regarding the diet, it significantly modified the prevalence of several compounds. Butanoic acid, hexanoic acid, octanal and octanoic acid were more prevalent in those FMs from diets including silages (BD and ADP), whereas heptanal, benzyl alcohol, nonanal and methyl salicylate contents were lower than in CD. The inclusion of broccoli significantly (
p < 0.05) increased 2-heptanone prevalence in fermented milk. In the case of the thermophilic culture, the lactation stage affected acetoin, hexanal, heptanal, octanal, nonadienal and decenal that decreased, whereas 2-heptanone, hexanoic acid, ethyl-hexenol, benzyl alcohol, 2-nonanone, octanoic acid and decanal increased at late lactation. The diet caused significant differences on hexanal, nonanal and decanal that increased due to the inclusion of silages (BD and APD) and 2-heptanone and octanoic acid that decreased as compared to CD at late lactation. The inclusion of broccoli silage yielded milk with reduced prevalence of heptanal and cubebene. The inclusion of artichoke plant silage caused a decreased prevalence of 2-nonanone. Overall, aldehydes decreased at late lactation in both fermented milks regardless the diet. Aldehyde content in fermented milks is mainly dependent on the enzymatic activity of the substrate as they are produced from the degradation of milkfat or from the catabolism of aminoacids, and they are degraded by oxidation to carboxylic acids or by reduction to alcohols [
55]. In milk cultured with MA400 the prevalence of acids increased when silages were included in the diet, so expecting those products as having higher goaty flavor, whereas in milk fermented with yogurt culture aldehydes prevalence increased when silages were included, so expecting green and fresh flavors. When handling the samples, authors perceived them all as having mild odor and no evident odor differences were detected among groups, in the future, sensory analysis would be of interest to evaluate flavor perception in detail.
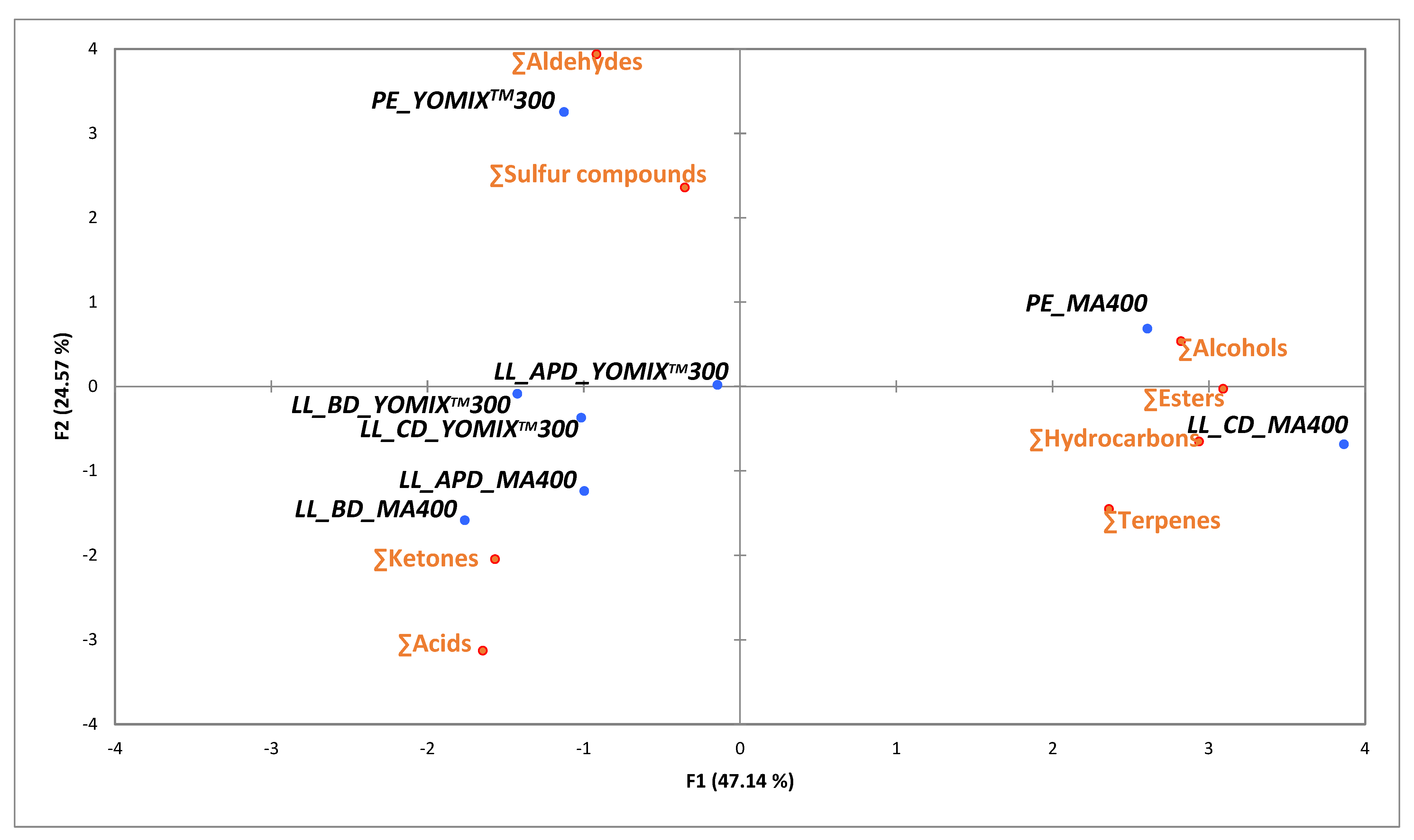
For an easy visualization of the relationships among volatile compounds, a PCA was run, including only significantly different chemical families of volatile compounds.
Figure 3 shows the two principal components which explained 71.71% of the samples variation. Milks fermented by YO-MIX
TM300 were closely located and positively correlated with aldehydes prevalence, whereas in milk fermented by MA400 the inclusion of silages positively correlate with the prevalence of acids and were clearly separated from CD and PE fermented milks.
Although diets are expected to greatly affect the aroma of milk, the fact that so little significant differences in volatiles could be linked to the different diets may be related to the previously reported observation that flavor complexity is highest when animals are fed a mixed ration including concentrates [
56] as all diets included concentrates, besides, lactic acid culture is considered the main factor affecting the volatile profile of fermented milks [
54].
From all the identified families of compounds terpenes have been proposed as indicators of the diet mainly when animals were grazing different pastures and their content may by five times higher when in pasture as compared to in-door feeding [
57]. In fact, they have been proposed as indicators of mountain pasture milk [
58], some others reported that even in semi-pasture combined with in-door feeding terpenes do not allow the differentiation with in-door feeding [
59]. Regarding goat milk, it has been pointed out that terpenes may not be a good indicator of the diet [
60]. In the present study the feeding system was in-doors and included silages. Fan et al. (2020) reported that terpenoid from artichoke were well preserved in silages preserved for 60 days, and main changes in their profile occurred during the first days of silage and were highly dependent on the microbiota in the silage [
61]. In the present study silages had been stored for much longer times and no presence of the terpenoids reported in artichoke [
61] were found in fermented milks. Terpenes may suffer further transformations, they may be directly transferred from the diet (on inhaled) to milk or may be further modified in the rumen (biohydrogenated and isomerized) [
62]. Even during milk fermentation terpenes may undergo further modifications [
57].