Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
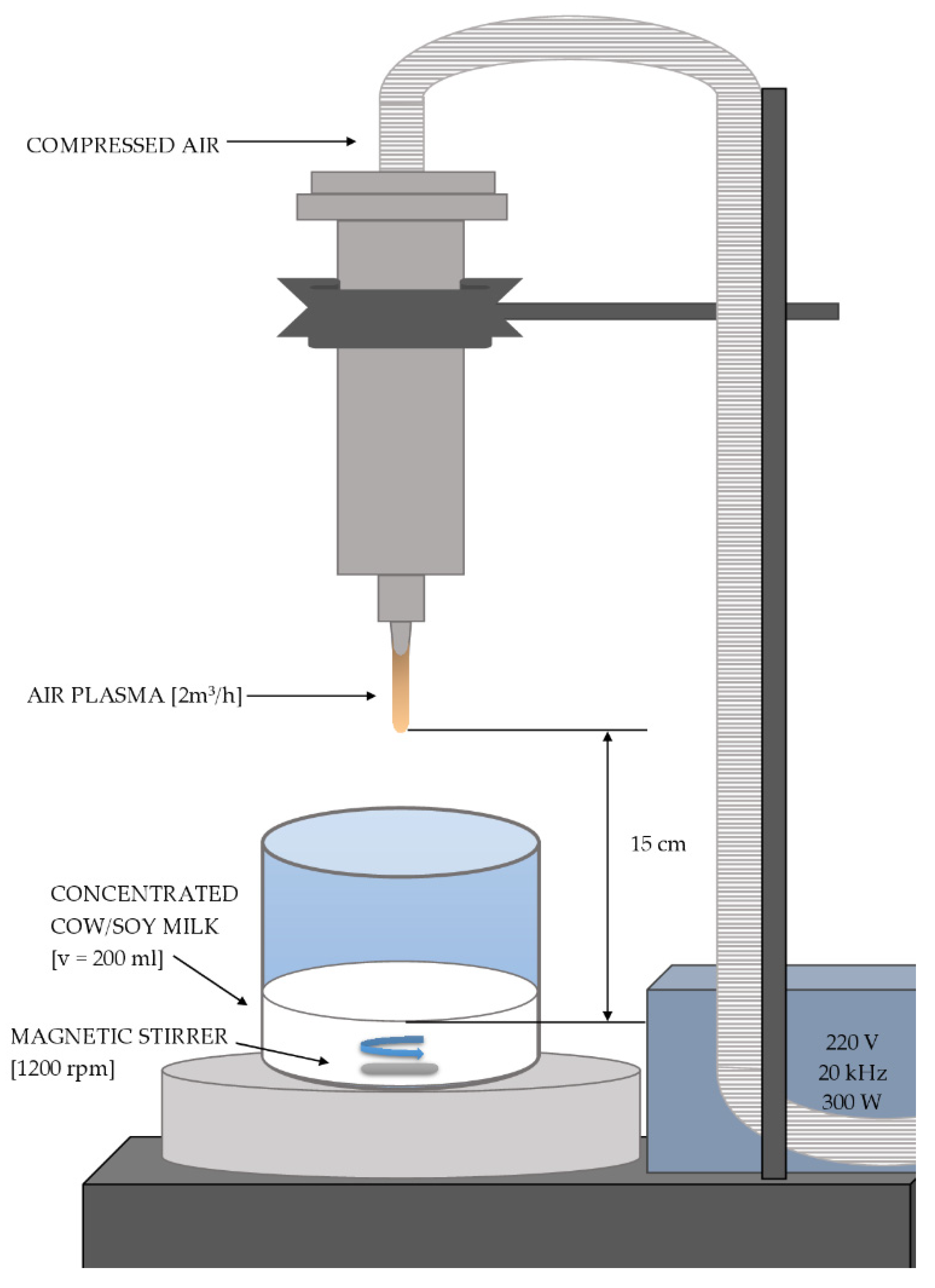
2.2. Preparation of Curing Agents
2.3. Processing of Cured Pork Loins
2.4. Physicochemical Analyzes
2.5. Aroma Profile
2.6. Statistical Analysis
3. Results and Discussion
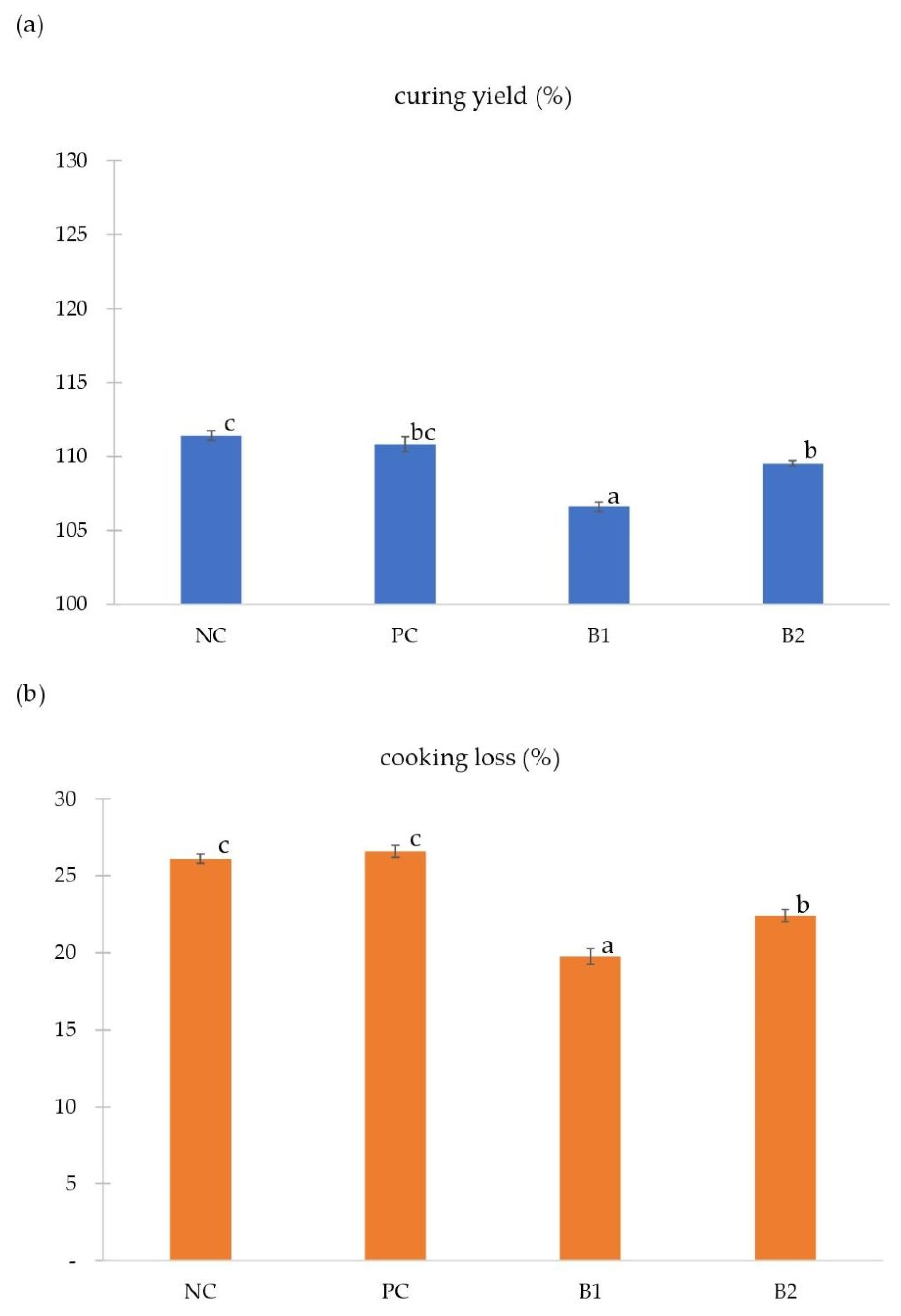
3.1. Curing Yield and Cooking Loss
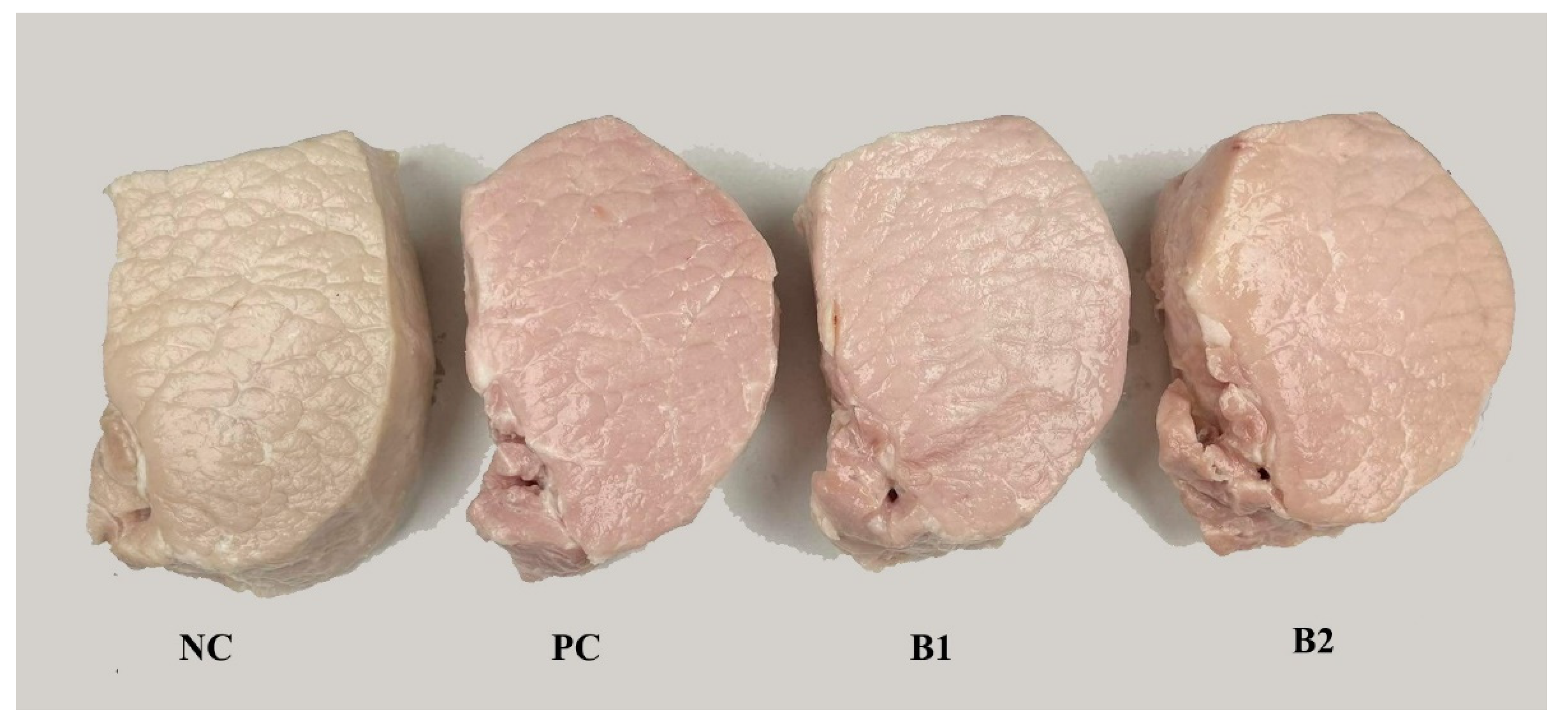
3.2. pH and Color Parameters
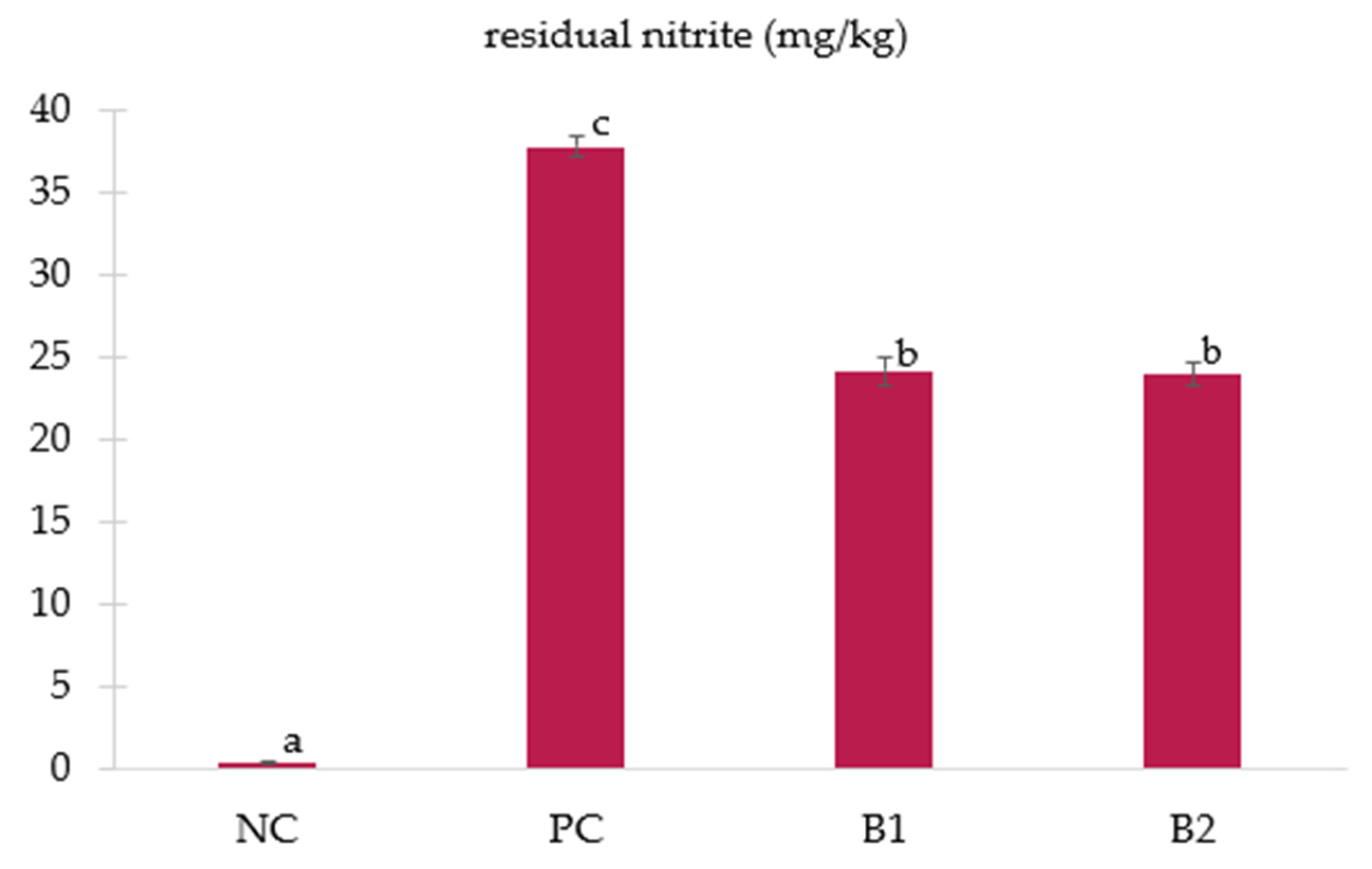
3.3. Residual Nitrite and Nitrosylhemochrome Content
3.4. Thiobarbituric Acid Reactive Substances and Warner-Bratzler Shear Force
3.5. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramkumar, M.C.; Cools, P.; Arunkumar, A.; De Geyter, N.; Morent, R.; Kumar, V.; Udaykumar, S.; Gopinath, P.; Jaganathan, S.K.; Pandiyaraj, K.N. Polymer Coatings for Biocompatibility and Reduced Nonspecific Adsorption; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081004968. [Google Scholar]
- Domonkos, M.; Tich, P.; Trejbal, J. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Wiktor, A.; Śledź, M.; Nowacka, M.; Witrowa-Rajchert, D. Możliwości zastosowania niskotemperaturowej plazmy w technologii żywności. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2013, 20, 5–14. [Google Scholar]
- Stryczewska, H.D. Technlogie zimnej plazmy. Wytwarzanie, modelowanie, zastosowania. Elektryka 2011, 1, 41–60. [Google Scholar]
- Dasan, B.G.; Onal-Ulusoy, B.; Pawlat, J.; Diatczyk, J.; Sen, Y.; Mutlu, M. A New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma. Food Bioprocess Technol. 2017, 10, 650–661. [Google Scholar] [CrossRef]
- Xu, L.; Garner, A.L.; Tao, B.; Keener, K.M. Microbial Inactivation and Quality Changes in Orange Juice Treated by High Voltage Atmospheric Cold Plasma. Food Bioprocess Technol. 2017, 10, 1778–1791. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- Thirumdas, R.; Saragapani, C.; Ajinkya, M.T.; Deshmukh, R.R.; Annapure, U.S. Influence of low pressure cold plasma on cooking and textural properties of brown rice. Innov. Food Sci. Emerg. Technol. 2016, 37, 53–60. [Google Scholar] [CrossRef]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum “Salamanca”. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Warne, G.R.; Williams, P.M.; Pho, H.Q.; Tran, N.N.; Hessel, V.; Fisk, I.D. Impact of cold plasma on the biomolecules and organoleptic properties of foods: A review. J. Food Sci. 2021, 86, 3762–3777. [Google Scholar] [CrossRef]
- Sakudo, A.; Misawa, T.; Yagyu, Y. Equipment Design for Cold Plasma Disinfection of Food Products; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128149218. [Google Scholar]
- Siekmann, L.; Plötz, M.; Krischek, C. Alternative Curing Methods. Curr. Clin. Microbiol. Rep. 2021, 8, 40–48. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T. Potential alternative to nitrite in roasted lamb for sensory attributes: Atmospheric nonthermal plasma treatment. Foods 2021, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Govari, M.; Pexara, A. Nitrates and nitrites in meat products. J. Hell. Vet. Med. Soc. 2015, 66, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Braida, W.; Ong, S.K. Decomposition of nitrite under various pH and aeration conditions. Water Air Soil Pollut. 2000, 118, 13–26. [Google Scholar] [CrossRef]
- Yong, H.I.; Park, J.; Kim, H.J.; Jung, S.; Park, S.; Lee, H.J.; Choe, W.; Jo, C. An innovative curing process with plasma-treated water for production of loin ham and for its quality and safety. Plasma Process. Polym. 2018, 15, 1700050. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Oliveira, M.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Plasma-activated water as an alternative nitrite source for the curing of beef jerky: Influence on quality and inactivation of Listeria innocua. Innov. Food Sci. Emerg. Technol. 2020, 59, 102276. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; In Yong, H.; Choe, J.H.; Jeon, H.J.; Choe, W.; Jo, C. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Poltorak, A. Application of atmospheric pressure cold plasma activated plant protein preparations solutions as an alternative curing method for pork sausages. Meat Sci. 2022, 187, 108751. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.J.; Shin, D.J.; Baek, K.H.; Yong, H.I.; Jung, S.; Jo, C. Enrichment of nitrite in onion powder using atmospheric pressure plasma and egg whites for meat curing. LWT 2021, 135, 110050. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Jo, C.; Jeon, H.J.; Choe, J.H.; Choi, Y.S.; Jung, S. Utility of winter mushroom treated by atmospheric non-thermal plasma as an alternative for synthetic nitrite and phosphate in ground ham. Meat Sci. 2020, 166, 108151. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Półtorak, A. Plasma-activated milk powder as a sodium nitrite alternative in pork sausages. Meat Sci. 2022, 192, 108880. [Google Scholar] [CrossRef] [PubMed]
- Visy, A.; Hidas, K.I.; Barkó, A.; Nguyen, L.L.P.; Friedrich, L.; Jónás, G. Effect of wet-curing on physical properties and proteins of cured ham. Prog. Agric. Eng. Sci. 2021, 17, 145–155. [Google Scholar] [CrossRef]
- Ku, S.K.; Kim, H.J.; Yu, S.C.; Jeon, K.H.; Kim, Y.B. Effects of injection and tumbling methods on the meat properties of marinated beef. Korean J. Food Sci. Anim. Resour. 2013, 33, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Dimakopoulou-Papazoglou, D.; Katsanidis, E. Osmotic Processing of Meat: Mathematical Modeling and Quality Parameters. Food Eng. Rev. 2020, 12, 32–47. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Katsanidis, E. Mass transfer kinetics during osmotic processing of beef meat using ternary solutions. Food Bioprod. Process. 2016, 100, 560–569. [Google Scholar] [CrossRef]
- Filipović, V.; Lončar, B.; Nićetin, M.; Knežević, V.; Filipović, I.; Pezo, L. Modeling counter-current osmotic dehydration process of pork meat in molasses. J. Food Process Eng. 2014, 37, 533–542. [Google Scholar] [CrossRef]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of Three Modified TBA Methods for Measuring Lipid Oxidation in Chicken Meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głabska, D.; Brodowska, M.; Sun, D.W.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Kim, T.K.; Hwang, K.E.; Song, D.H.; Ham, Y.K.; Kim, Y.B.; Paik, H.D.; Choi, Y.S. Effects of natural nitrite source from Swiss chard on quality characteristics of cured pork loin. Asian-Australas J. Anim. Sci. 2019, 32, 1933–1941. [Google Scholar] [CrossRef]
- Kim, T.K.; Hwang, K.E.; Lee, M.A.; Paik, H.D.; Kim, Y.B.; Choi, Y.S. Quality characteristics of pork loin cured with green nitrite source and some organic acids. Meat Sci. 2019, 152, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.Y.; Ghanimah, M. Studies on Fluid Milk Viscosity as Affected by Some Factors. J. Biol. Chem. Environ. Sci. 2013, 8, 57–66. [Google Scholar]
- Phisut, N. Factors affecting mass transfer during osmotic dehydration of fruits. Int. Food Res. J. 2012, 19, 7–18. [Google Scholar]
- Pang, B.; Yu, X.; Bowker, B.; Zhang, J.; Yang, Y.; Zhuang, H. Effect of meat temperature on moisture loss, water properties, and protein profiles of broiler pectoralis major with the woody breast condition. Poult. Sci. 2021, 100, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef]
- Luo, J.; Yan, W.; Nasiru, M.M.; Zhuang, H.; Zhou, G.; Zhang, J. Evaluation of physicochemical properties and volatile compounds of Chinese dried pork loin curing with plasma-treated water brine. Sci. Rep. 2019, 9, 13793. [Google Scholar] [CrossRef] [Green Version]
- Patarata, L.; Carvalho, F.; Fraqueza, M.J. Nitrite-Free Implications on Consumer Acceptance and the Behavior of Pathogens in Cured Pork Loins. Foods 2022, 11, 796. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Vital, A.C.P.; Guerrero, A.; Monteschio, J.D.O.; Valero, M.V.; Carvalho, C.B.; De Abreu Filho, B.A.; Madrona, G.S.; Do Prado, I.N. Effect of edible and active coating (with rosemary and oregano essential oils) on beef characteristics and consumer acceptability. PLoS ONE 2016, 11, e0160535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, N.; Bunning, M.; Martin, J. Uncured-Labeled Meat Products Produced Using Plant-Derived Nitrates and Nitrites: Chemistry, Safety, and Regulatory Considerations. J. Agric. Food Chem. 2019, 67, 8074–8084. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO. Codex Alimentarius Commission Codex Alimentarius: Food Additives. J. Chem. Inf. Model. 2014, 53, 1689–1699. [Google Scholar]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of sodium nitrite reduction on lipid oxidation and antioxidant properties of cooked meat products. Antioxidants 2020, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Cullere, M.; Kovitvadhi, A.; Chundang, P.; Dalle Zotte, A. Effect of different killing methods on physicochemical traits, nutritional characteristics, in vitro human digestibility and oxidative stability during storage of the house cricket (Acheta domesticus L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102444. [Google Scholar] [CrossRef]
- Choe, J.H.; Choi, M.H.; Rhee, M.S.; Kim, B.C. Estimation of sensory pork loin tenderness using Warner-Bratzler shear force and texture profile analysis measurements. Asian-Australas J. Anim. Sci. 2016, 29, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
| GROUP 1,2 | pH | L* (−) | a* (−) | b* (−) | C* | ΔE |
|---|---|---|---|---|---|---|
| raw | ||||||
| NC | 5.54 ± 0.02 | 53.10 b ± 0.26 | 1.99 a ± 0.11 | −0.21 a ± 0.08 | 2.04 a ± 0.11 | - |
| PC | 5.53 ± 0.02 | 52.15 b ± 0.33 | 3.27 b ± 0.10 | −0.52 a ± 0.11 | 3.37 b ± 0.10 | 2.50 a ± 0.16 |
| B1 | 5.55 ± 0.01 | 48.96 a ± 0.31 | 3.81 c ± 0.11 | 1.40 b ± 0.14 | 4.11 c ± 0.12 | 5.09 c ± 0.31 |
| B2 | 5.56 ± 0.01 | 52.55 b ± 0.31 | 3.99 c ± 0.10 | 2.42 b ± 0.15 | 4.72 d ± 0.12 | 3.84 b ± 0.17 |
| cooked | ||||||
| NC | 5.78 ± 0.01 | 76.98 d ± 0.09 | 6.51 a ± 0.04 | 5.70 b ± 0.06 | 8.66 a ± 0.04 | - |
| PC | 5.82 ± 0.01 | 70.43 a ± 0.28 | 11.57 c ± 0.14 | 5.10 a ± 0.05 | 12.24 b ± 0.14 | 6.30 a ± 0.26 |
| B1 | 5.79 ± 0.01 | 72.79 c ± 0.23 | 11.12 b ± 0.15 | 5.73 b ± 0.08 | 12.53 b ± 0.13 | 8.33 b ± 0.31 |
| B2 | 5.81 ± 0.02 | 71.93 b ± 0.23 | 11.14 b ± 0.12 | 5.87 b ± 0.07 | 12.98 c ± 0.12 | 6.92 a ± 0.23 |
| GROUP 1,2 | Nitrosylhemochrome Content (%) | TBARS 3 (mg MDA/kg) | WBSF 4 (N) |
|---|---|---|---|
| NC | 13.36 a ± 0.72 | 1.19 c ± 0.04 | 20.79 ± 0.84 |
| PC | 42.59 d ± 0.75 | 0.24 a ± 0.01 | 20.69 ± 0.56 |
| B1 | 34.85 b ± 0.83 | 0.34 b ± 0.01 | 19.58 ± 0.27 |
| B2 | 38.67 c ± 0.79 | 0.32 b ± 0.01 | 20.12 ± 0.26 |
| Compounds | DB5 1 | Sensory Descriptors | NC 2 | PC | B1 | B2 |
|---|---|---|---|---|---|---|
| alcohols | ||||||
| Ethanol | 450 | alcoholic | + 3 | + | + | |
| Methanol | 451 | cabbage | + | |||
| 1-propanol | 519 | alcoholic | + | + | ||
| 2-mercaptoethanol | 562 | strong; sulfurous | + | |||
| 1-propanol, 2-methyl | 636 | alcoholic | + | |||
| Propylenglycol | 733 | alcoholic | + | |||
| 2-furanmethanol | 843 | alcoholic; bread | + | + | ||
| (Z)-2-hexene-1-ol | 869 | + | ||||
| Trans-Carveol | 1211 | caraway | + | |||
| aldehydes | ||||||
| Acetaldehyde | 420 | aldehydic | + | + | + | + |
| Propanal | 491 | acetaldehydic | + | + | + | |
| 1-methylpropanal | 517 | aldehydic | + | |||
| 2-methylpropanal | 519 | aldehydic | + | + | ||
| Butanal | 565 | chocolate | + | + | + | |
| But-(E)-2-enal | 665 | floral | + | + | + | |
| 2-methylpentanal | 763 | cheeese | + | |||
| Furfural | 844 | almond | + | |||
| 5-methylfurfural | 952 | acidic | + | |||
| Benzaldehyde | 968 | almond | + | |||
| terpenes | ||||||
| Alpha-Pinene | 950 | camphor | + | |||
| Beta-pinene | 958 | dry | + | |||
| Alpha-Phalladrene | 1002 | citrus | + | |||
| p-Cymene | 1029 | aromatic | + | |||
| 1,8-cineole | 1048 | camphor | + | |||
| Gamma-Terpinene | 1081 | citrus; etheral | + | + | + | |
| esters | ||||||
| Methyl formate | 387 | agereeable; fruity | + | + | + | + |
| Isopropyl acetate | 652 | banana | + | + | ||
| Methyl isobutyrate | 655 | apple | + | |||
| Methyl butanoate | 735 | apple | + | + | ||
| Methyl hexanoate | 933 | acetone | + | + | ||
| hydrocarbons | ||||||
| Pentane | 478 | alkane | + | + | ||
| Heptane | 705 | alkane | + | + | ||
| 1-hydroxy-2(methylthio)-ethane | 848 | meaty | + | |||
| N-compounds | ||||||
| Pyrolle | 754 | chloroform | + | + | + | |
| Pyridine | 755 | cold meat fat | + | + | ||
| 2-acetyl-1-pyrroline | 926 | meaty | + | |||
| acids | ||||||
| pentanoic acid | 905 | acidic | + | + | ||
| Hexanoic acid | 1004 | cheese | + | |||
| C-compounds | ||||||
| Dihydro-2(3H)-Furanone | 811 | aromatic | + | |||
| S-compounds | ||||||
| Dimethyl trisulfide | 970 | aliaceous | + | + | ||
| pyrazines | ||||||
| Pyrazine | 725 | bitter | + | + | ||
| thiols | ||||||
| Heptyl mercaptan | 1027 | onion | + | + | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Poltorak, A. Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma. Foods 2022, 11, 2523. https://doi.org/10.3390/foods11162523
Marcinkowska-Lesiak M, Wojtasik-Kalinowska I, Onopiuk A, Stelmasiak A, Wierzbicka A, Poltorak A. Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma. Foods. 2022; 11(16):2523. https://doi.org/10.3390/foods11162523
Chicago/Turabian StyleMarcinkowska-Lesiak, Monika, Iwona Wojtasik-Kalinowska, Anna Onopiuk, Adrian Stelmasiak, Agnieszka Wierzbicka, and Andrzej Poltorak. 2022. "Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma" Foods 11, no. 16: 2523. https://doi.org/10.3390/foods11162523
APA StyleMarcinkowska-Lesiak, M., Wojtasik-Kalinowska, I., Onopiuk, A., Stelmasiak, A., Wierzbicka, A., & Poltorak, A. (2022). Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma. Foods, 11(16), 2523. https://doi.org/10.3390/foods11162523










