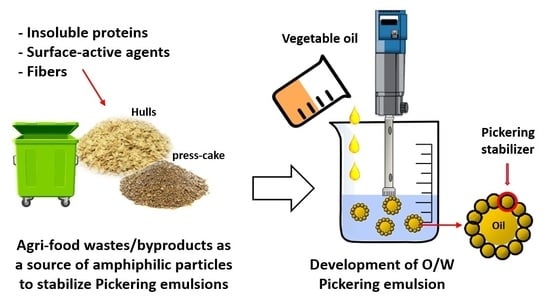
Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Agri-Food Byproducts
2.3. Powder Wastes/Byproducts Characterization
2.3.1. Proximate Composition of Powder Wastes/Byproducts
2.3.2. Determination of Soluble and Insoluble Fraction
2.3.3. Size and Zeta Potential Measurements
2.3.4. Total Polyphenols Content
2.3.5. DPPH Radical Scavenging Ability
2.3.6. Water Contact Angle (WCA) Measurements
2.3.7. Microstructure Observation of Pickering Particles
2.4. Pickering Emulsion Preparation
2.5. Pickering Emulsion Characterization
2.5.1. Microstructure Observation of Pickering Emulsions
2.5.2. Droplet Size Measurements
2.5.3. Creaming Index of Emulsions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Agri-Food Byproducts Particle Characterization
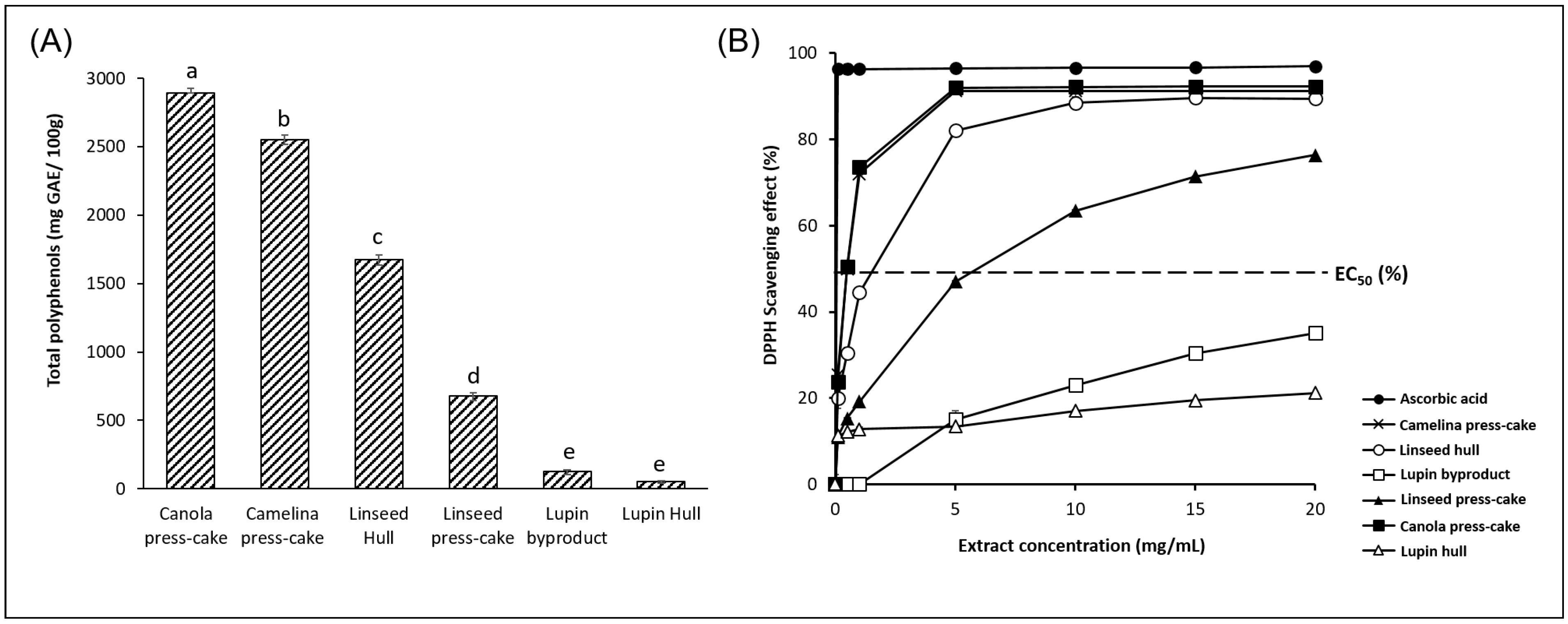
3.2. Antioxidant Activity of Agri-Food Byproduct Particles
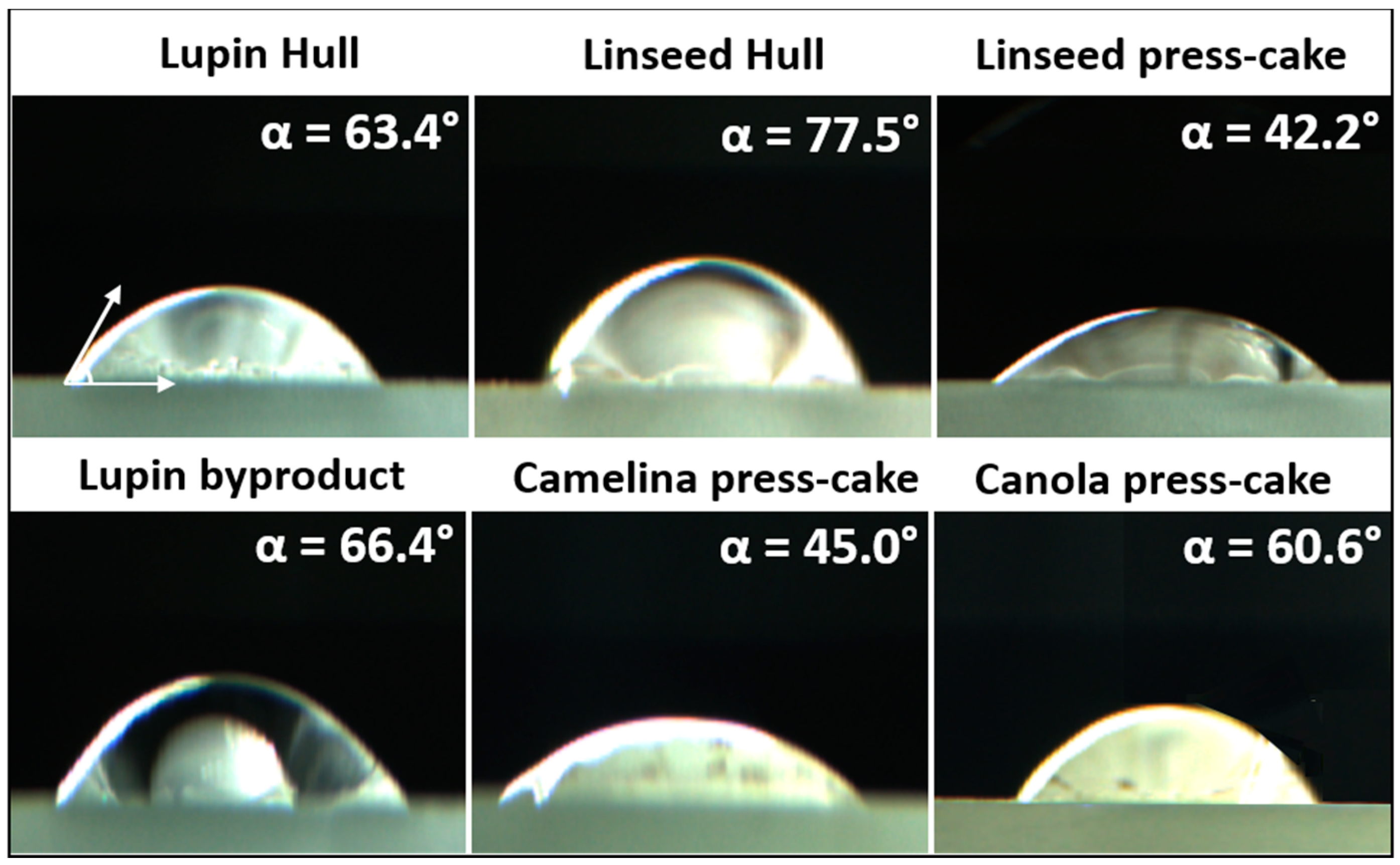
3.3. Wetting Properties of Pickering Stabilizers
3.4. Pickering Emulsions Characterization
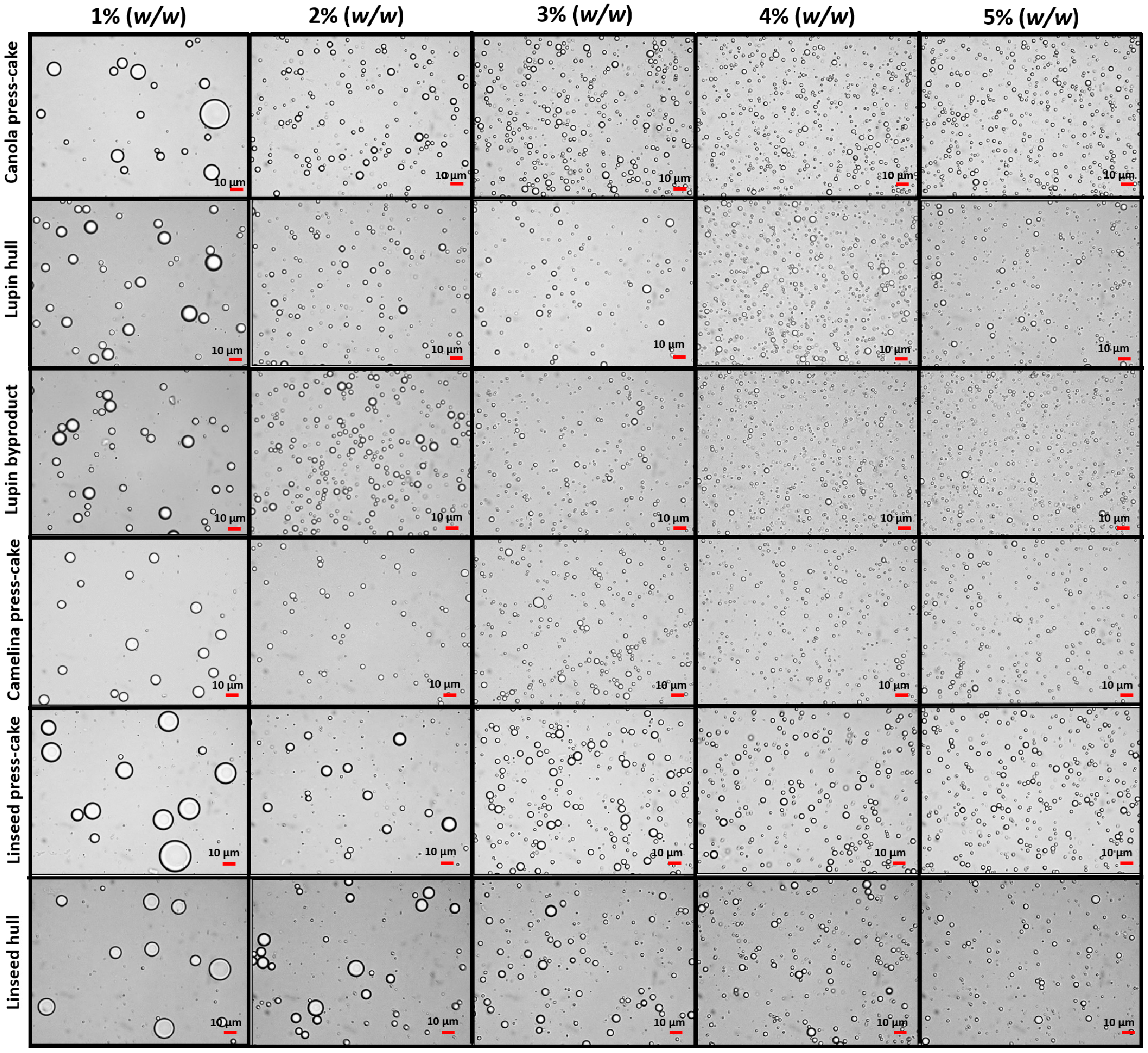
3.4.1. Influence of Agri-Food Byproduct Concentration on Emulsion Droplet Size
3.4.2. Emulsion Microstructure Characterization
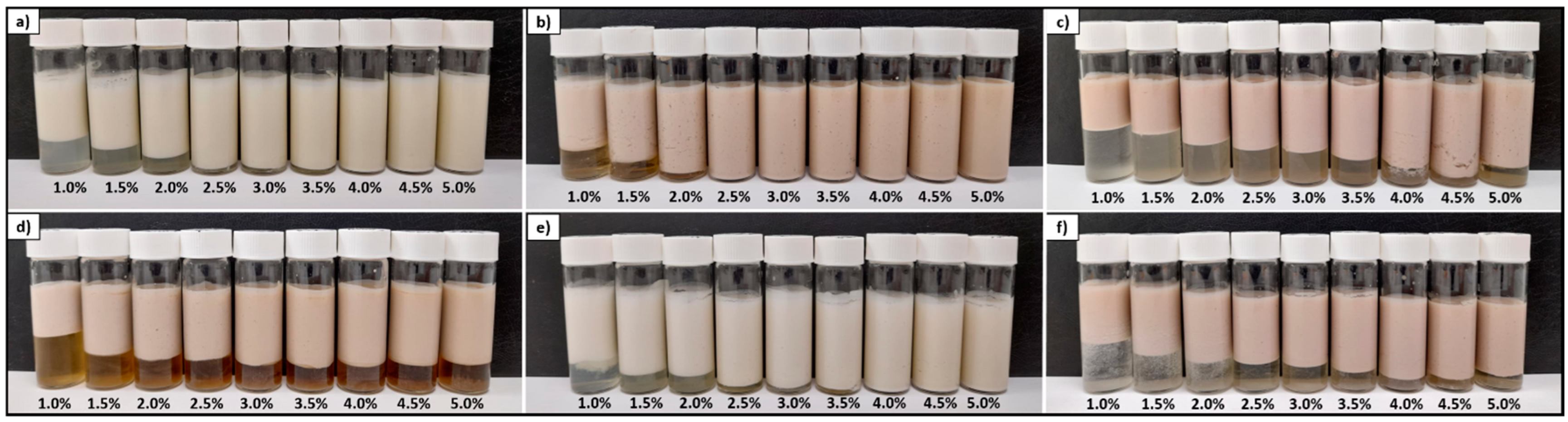
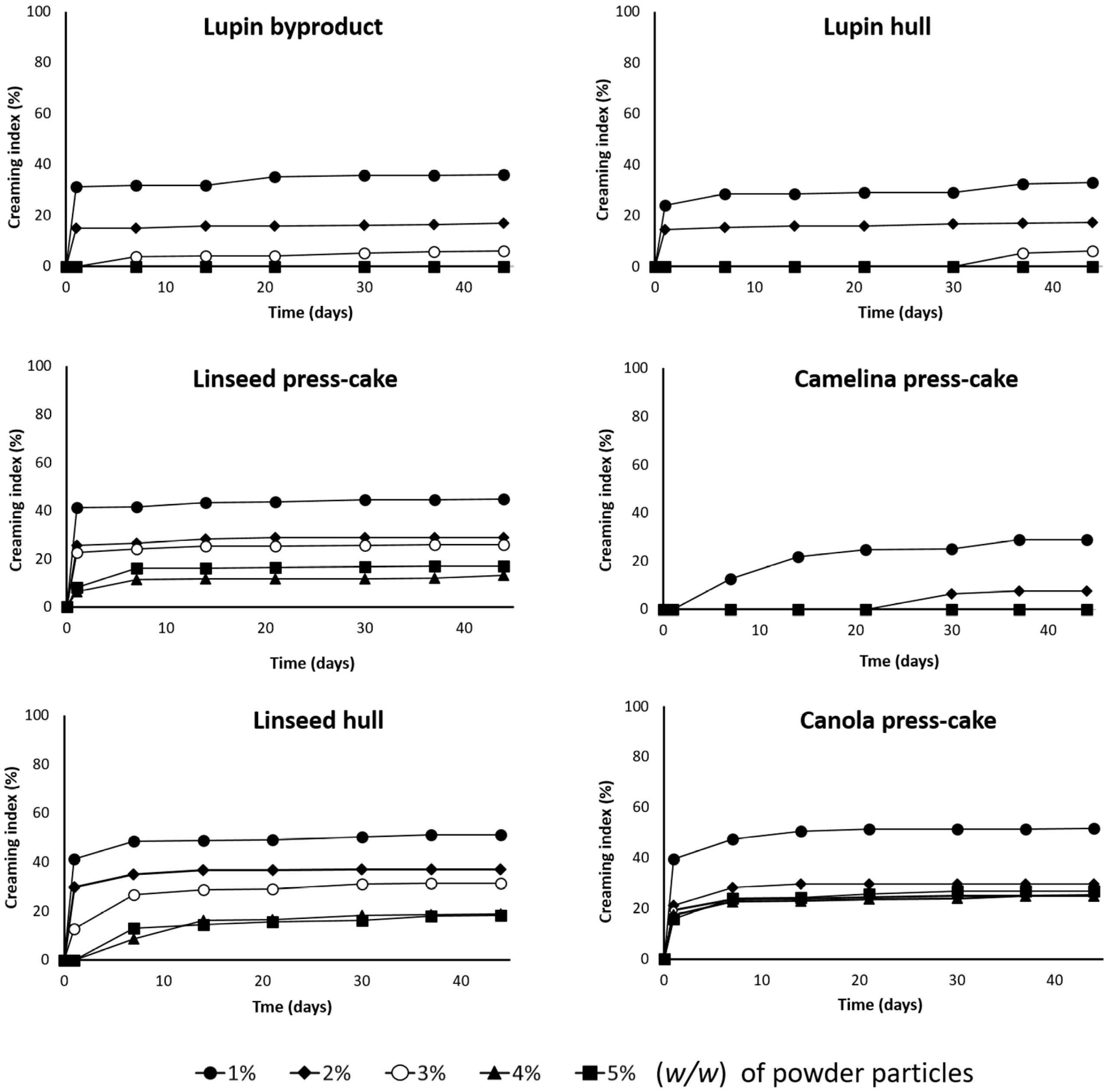
3.4.3. Evolution of Emulsion Stability in the Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hafemann, E.; Battisti, R.; Marangoni, C.; Machado, R.A.F. Valorization of Royal Palm Tree Agroindustrial Waste by Isolating Cellulose Nanocrystals. Carbohydr. Polym. 2019, 218, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Bharat Helkar, P.; Sahoo, A.; Patil, N. Review: Food Industry By-Products Used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 1000248. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and Evaluation of a Novel High Internal Phase Pickering Emulsion Based on Whey Protein Isolate Nanofibrils Derived by Hydrothermal Method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Olivos, M.; Lichtin, N.; Bustamante, M.; Solans, C. Food-Grade Pickering Stabilizers Obtained from a Protein-Rich Lupin Cultivar (AluProt-CGNA®): Chemical Characterization and Emulsifying Properties. Food Hydrocoll. 2019, 87, 847–857. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Li, F.; Du, J.; Huang, Q.; Sun-Waterhouse, D.; Li, D. Antioxidative Pectin from Hawthorn Wine Pomace Stabilizes and Protects Pickering Emulsions via Forming Zein-Pectin Gel-like Shell Structure. Int. J. Biol. Macromol. 2020, 151, 193–203. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-Grade Pickering Emulsion as a Novel Astaxanthin Encapsulation System for Making Powder-Based Products: Evaluation of Astaxanthin Stability during Processing, Storage, and Its Bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 738. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-Grade Particles for Emulsion Stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Ooi, C.W.; Tey, B.T.; Chan, E.S. Facile Method for Forming Ionically Cross-Linked Chitosan Microcapsules from Pickering Emulsion Templates. Food Hydrocoll. 2016, 55, 26–33. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical Characteristics, Applications and Research Trends of Edible Pickering Emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- İlyasoğlu, H.; Nadzieja, M.; Guo, Z. Caffeic Acid Grafted Chitosan as a Novel Dual-Functional Stabilizer for Food-Grade Emulsions and Additive Antioxidant Property. Food Hydrocoll. 2019, 95, 168–176. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.Y.; Ji, C.M.; Lee, K.T.; Shin, J.A.; Lee, E.S.; Hong, S.T. Influence of Ginkgo biloba Extracts and of Their Flavonoid Glycosides Fraction on the in vitro Digestibility of Emulsion Systems. Food Hydrocoll. 2014, 42, 196–203. [Google Scholar] [CrossRef]
- Haj-shafiei, S.; Ghosh, S.; Rousseau, D. Kinetic Stability and Rheology of Wax-Stabilized Water-in-Oil Emulsions at Different Water Cuts. J. Colloid Interface Sci. 2013, 410, 11–20. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein- and Polysaccharide-Based Particles Used for Pickering Emulsion Stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, C.; Mu, C.; Ngai, T.; Lin, W. Advances in Pickering Emulsions Stabilized by Protein Particles: Toward Particle Fabrication, Interaction and Arrangement. Food Res. Int. 2022, 157, 111380. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef]
- Huc-Mathis, D.; Guilbaud, A.; Fayolle, N.; Bosc, V.; Blumenthal, D. Valorizing Apple By-Products as Emulsion Stabilizers: Experimental Design for Modeling the Structure-Texture Relationships. J. Food Eng. 2020, 287, 110115. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Redispersible Dry Emulsions Stabilized by Plant Material: Rapeseed Press-Cake or Cocoa Powder. LWT—Food Sci. Technol. 2019, 113, 108311. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. Micronized Apple Pomace as a Novel Emulsifier for Food O/W Pickering Emulsion. Food Chem. 2020, 330, 127325. [Google Scholar] [CrossRef] [PubMed]
- Wandersleben, T.; Morales, E.; Burgos-Díaz, C.; Barahona, T.; Labra, E.; Rubilar, M.; Salvo-Garrido, H. Enhancement of Functional and Nutritional Properties of Bread Using a Mix of Natural Ingredients from Novel Varieties of Flaxseed and Lupine. LWT—Food Sci. Technol. 2018, 91, 48–54. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Wandersleben, T.; Soto-Añual, M.; Barahona, T.; Bustamante, M. Chemical and Nutritional Evaluation of Protein-Rich Ingredients Obtained through a Technological Process from Yellow Lupin Seeds (Lupinus luteus). Plant Foods Hum. Nutr. 2019, 74, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Girish, V.; Keulen, D.; Wijngaard, H.; Lauteslager, X.; Ferreira, G.; Ottens, M. Recovery of Sinapic Acid from Canola/Rapeseed Meal Extracts by Adsorption. Food Bioprod. Process. 2020, 120, 69–79. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemist, 18th ed.; Gaithersburg, MD: Washington, DC, USA, 2005. [Google Scholar]
- Opazo-Navarrete, M.; Burgos-Díaz, C.; Soto-Cerda, B.; Barahona, T.; Anguita-Barrales, F.; Mosi-Roa, Y. Assessment of the Nutritional Value of Traditional Vegetables from Southern Chile as Potential Sources of Natural Ingredients. Plant Foods Hum. Nutr. 2021, 76, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Palacios, J.L.; Barahona, T.; Mosi-Roa, Y.; Anguita-Barrales, F.; Bustamante, M. Synthesis of New Chitosan from an Endemic Chilean Crayfish Exoskeleton (Parastacus pugnax): Physicochemical and Biological Properties. Polymers 2021, 13, 2304. [Google Scholar] [CrossRef]
- He, K.; Li, Q.; Li, Y.; Li, B.; Liu, S. Water-Insoluble Dietary Fibers from Bamboo Shoot Used as Plant Food Particles for the Stabilization of O/W Pickering Emulsion. Food Chem. 2020, 310, 125925. [Google Scholar] [CrossRef]
- Qi, J.-R.; Song, L.-W.; Zeng, W.-Q.; Liao, J.-S. Citrus Fiber for the Stabilization of O/W Emulsion through Combination of Pickering Effect and Fiber-Based Network. Food Chem. 2021, 343, 128523. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of Garlic Extract by Complex Coacervation Using Whey Protein Isolate/Chitosan and Gum Arabic/Chitosan as Wall Materials: Influence of Anionic Biopolymers on the Physicochemical and Structural Properties of Microparticles. Food Bioprocess Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Hughes, J.M.; Budd, P.M.; Grieve, A.; Dutta, P.; Tiede, K.; Lewis, J. Highly Monodisperse, Lanthanide-Containing Polystyrene Nanoparticles as Potential Standard Reference Materials for Environmental “Nano” Fate Analysis. J. Appl. Polym. Sci. 2015, 132, 42061. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-Industrial by-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef]
- Klojdová, I.; Stathopoulos, C. The Potential Application of Pickering Multiple Emulsions in Food. Foods 2022, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Wu, Z.L.; Zhu, J.Y.; Yin, S.W.; Tang, C.H.; Wu, L.Y.; Yang, X.Q. Development of Antioxidant Pickering High Internal Phase Emulsions (HIPEs) Stabilized by Protein/Polysaccharide Hybrid Particles as Potential Alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiao, J.; Huang, Q. Pickering Emulsions Stabilized by Media-Milled Starch Particles. Food Res. Int. 2018, 105, 140–149. [Google Scholar] [CrossRef]
- Schröder, A.; Sprakel, J.; Schroën, K.; Spaen, J.N.; Berton-Carabin, C.C. Coalescence Stability of Pickering Emulsions Produced with Lipid Particles: A Microfluidic Study. J. Food Eng. 2018, 234, 63–72. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.A.; Chevalier, Y. Effects of Solid Particle Content on Properties of o/w Pickering Emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Pickering Emulsions Stabilized by Various Plant Materials: Cocoa, Rapeseed Press Cake and Lupin Hulls. LWT—Food Sci. Technol. 2020, 130, 109621. [Google Scholar] [CrossRef]
- Schröder, A.; Laguerre, M.; Sprakel, J.; Schroën, K.; Berton-Carabin, C.C. Pickering Particles as Interfacial Reservoirs of Antioxidants. J. Colloid Interface Sci. 2020, 575, 489–498. [Google Scholar] [CrossRef]
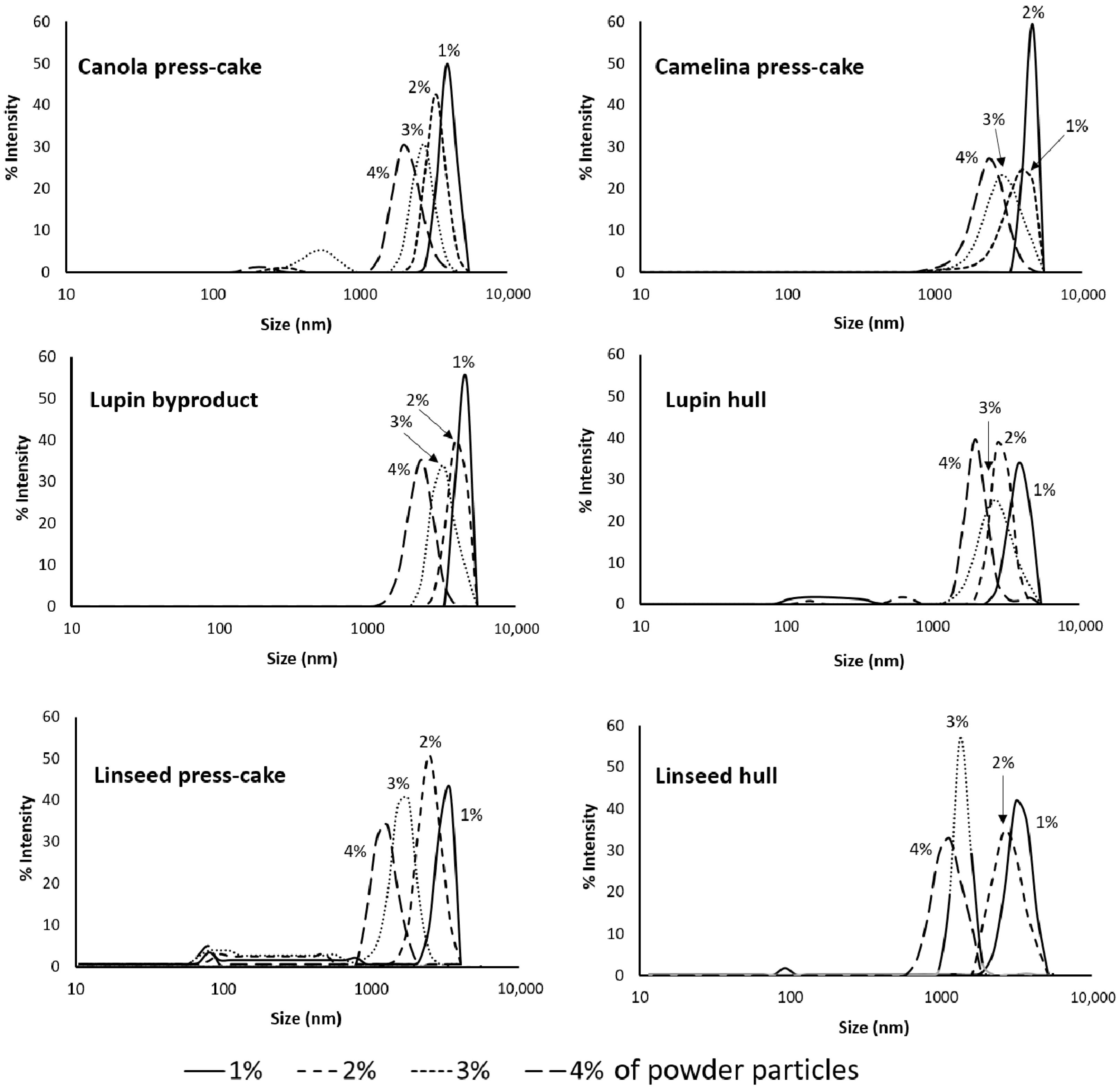
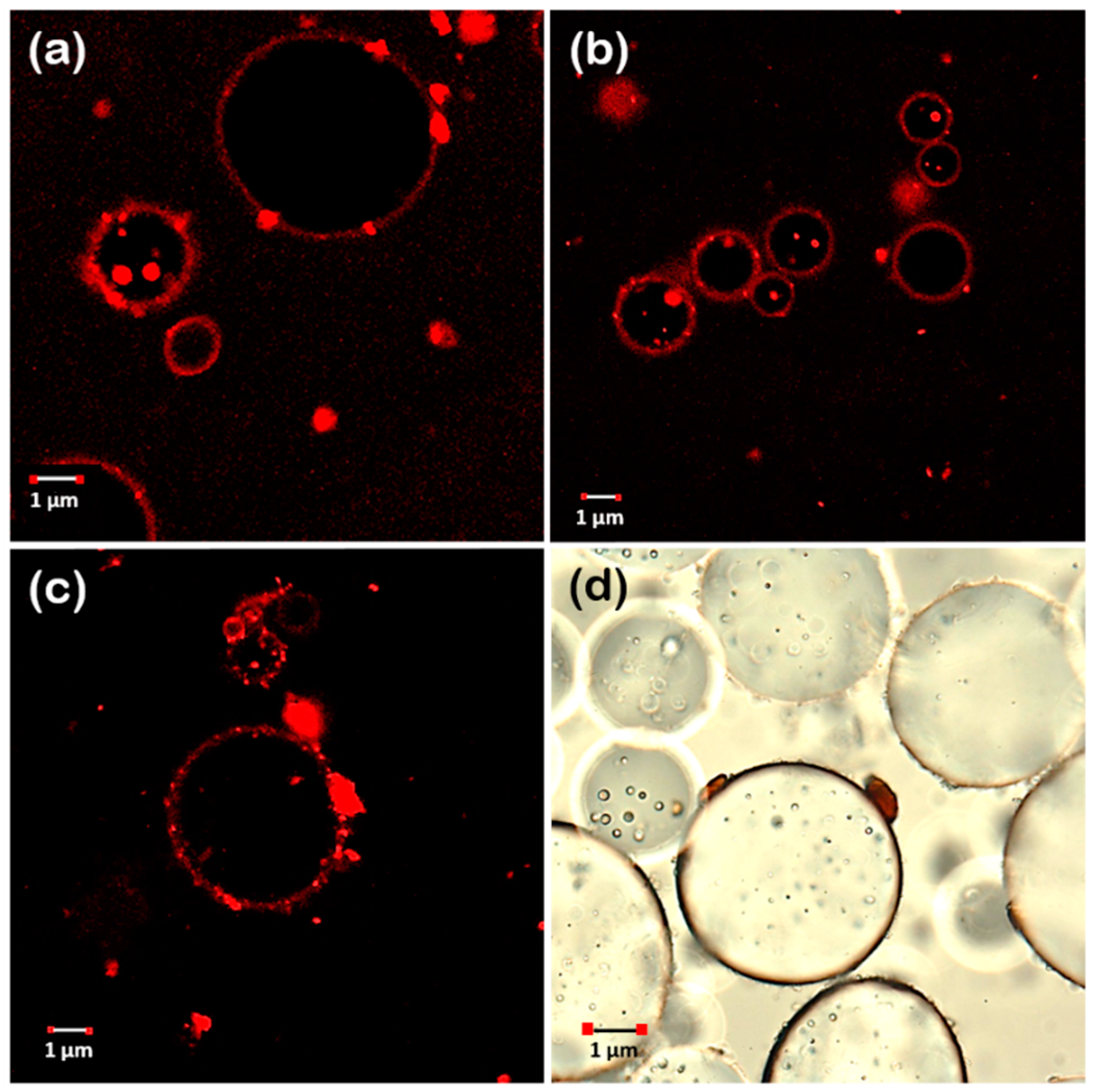
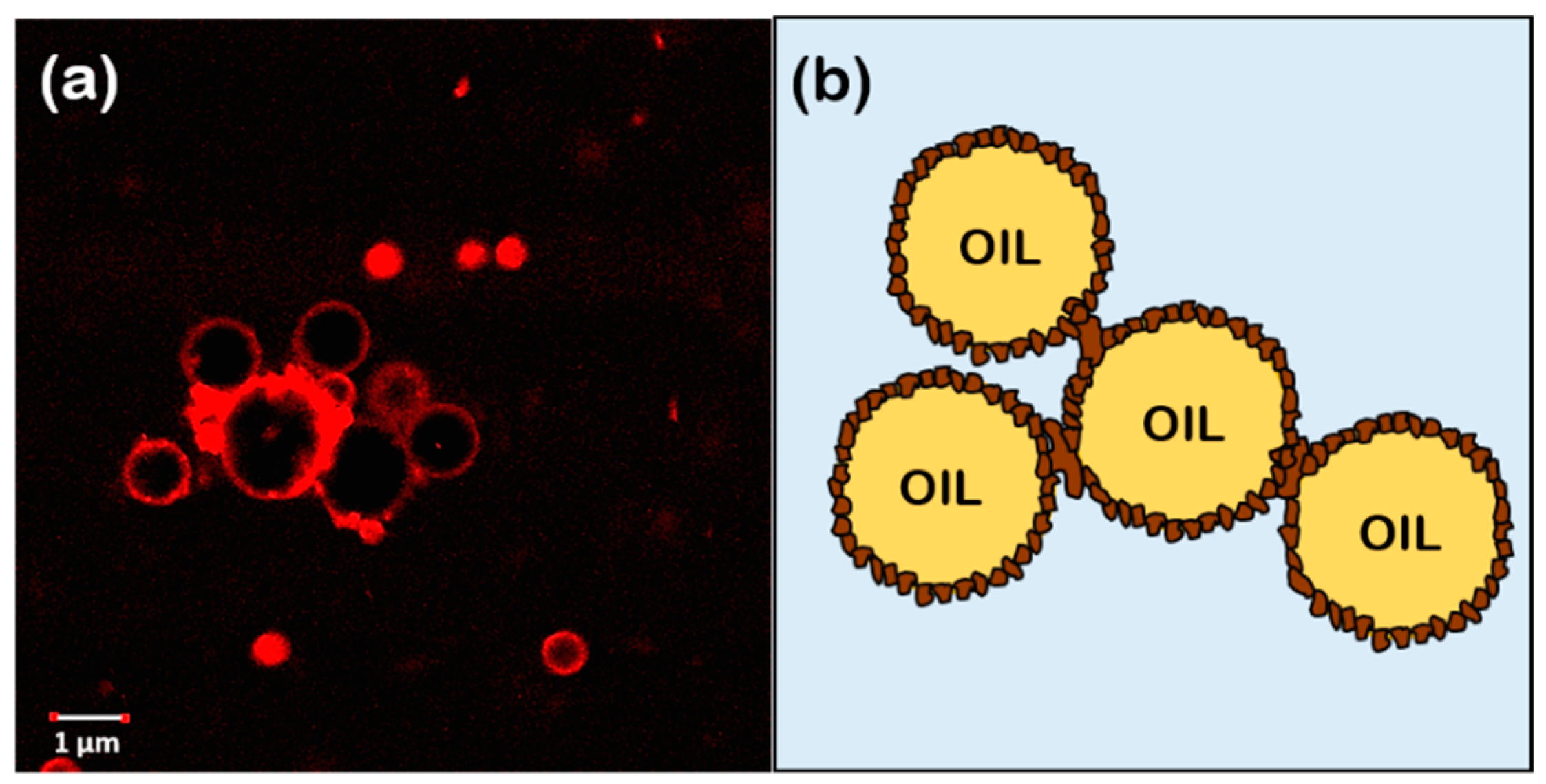
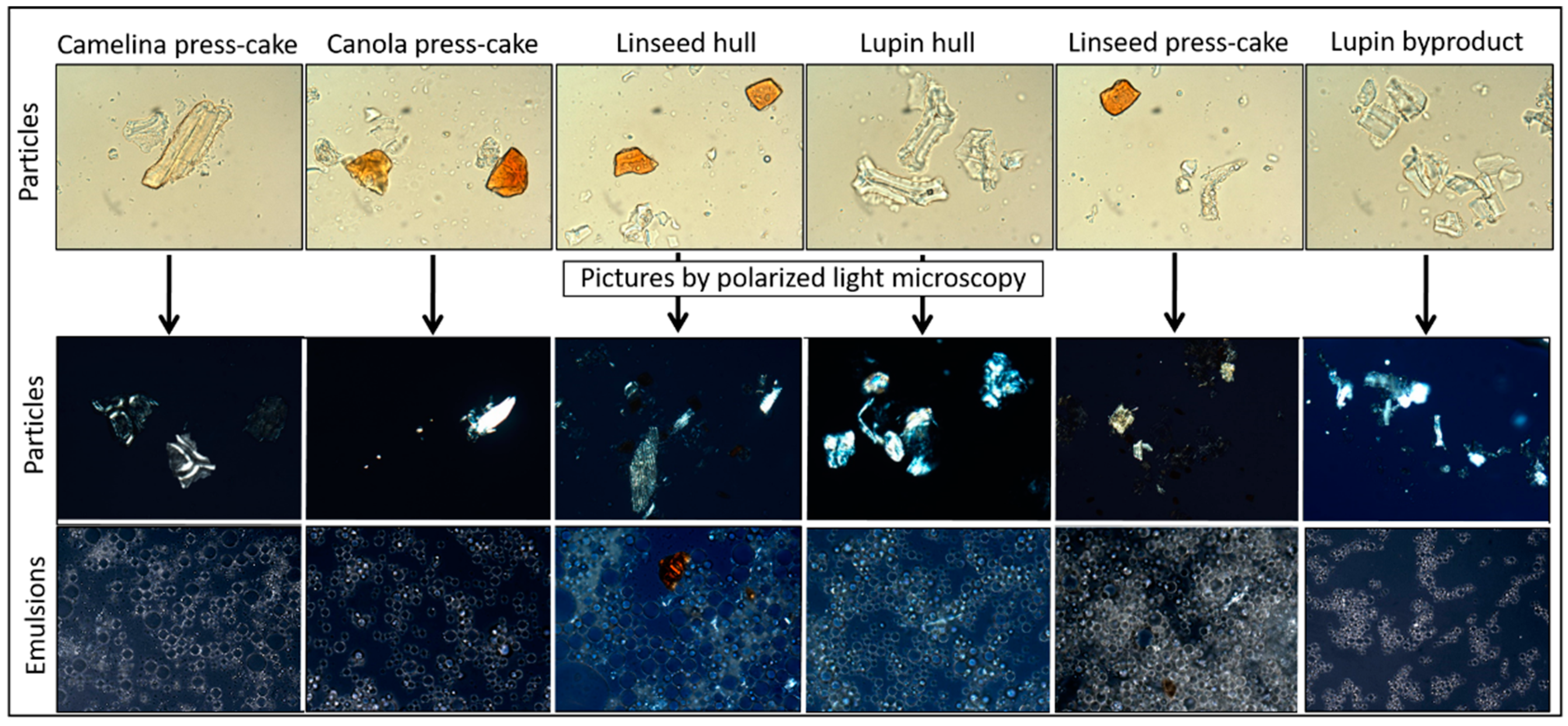
| Parameter | Lupin Hull | Lupin Byproduct | Linseed Hull | Linseed Press-Cake | Canola Press-Cake | Camelina Press-Cake |
|---|---|---|---|---|---|---|
| Protein | 17.90 ± 1.08 a | 30.80 ± 2.11 c | 22.90 ± 1.23 b | 40.97 ± 2.97 d | 39.80 ± 1.88 d | 46.71 ± 2.32 e |
| Fat | 2.07 ± 0.27 c | 4.78 ± 0.33 d | 0.34 ± 0.08 a | 8.71 ± 0.90 e | 0.88 ± 0.04 b | 0.32 ± 0.02 a |
| Ash | 2.39 ± 0.23 a | 5.07 ± 0.47 bc | 6.53 ± 0.53 d | 5.64 ± 0.61 cd | 5.51 ± 0.39 c | 4.67 ± 0.42 b |
| Dietary fiber | 67.10 ± 2.19 b | 60.59 ± 2.37 b | 61.10 ± 2.09 b | 40.02 ± 1.21 a | 41.60 ± 1.99 a | 38.58 ± 1.55 a |
| Carbohydrates available | 10.53 ± 0.82 c | 0.11 ± 0.01 a | 10.10 ± 0.88 c | 41.54 ± 1.91 d | 11.20 ± 0.78 c | 5.42 ± 0.76 b |
| Parameter | Lupin Hull | Lupin Byproduct | Linseed Hull | Linseed Press-Cake | Canola Press-Cake | Camelina Press-Cake |
|---|---|---|---|---|---|---|
| Soluble fraction (%) | 19.19 ± 0.91 b | 27.92 ± 1.11 c | 27.75 ± 2.83 c | 16.63 ± 0.95 a | 33.56 ± 0.25 d | 28.80 ± 0.43 c |
| Insoluble fraction (%) | 80.80 ± 0.91 c | 72.08 ± 1.11 b | 72.25 ± 2.83 b | 83.37 ± 0.95 d | 66.44 ± 0.25 a | 71.20 ± 0.43 b |
| Mean size (µm) | 1.52 ± 0.28 a | 1.06 ± 0.33 a | 2.48 ± 0.13 b | 3.70 ± 0.93 c | 1.26 ± 0.07 a | 4.51 ± 0.58 c |
| Polydispersity index | 0.68 ± 0.01 d | 0.99 ± 0.39 c | 0.72 ± 0.22 c | 0.43 ± 0.11 b | 0.84 ± 0.38 c | 0.08 ± 0.08 a |
| Form | Irregular | Irregular | Irregular | Irregular | Irregular | Irregular |
| Zeta potential (mV) | −28.60 ± 2.40 b | −28.15 ± 2.47 b | −25.13 ± 1.56 a | −26.60 ± 4.81 a | −29.10 ± 3.96 bc | −37.53 ± 3.55 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-Díaz, C.; Mosi-Roa, Y.; Opazo-Navarrete, M.; Bustamante, M.; Garrido-Miranda, K. Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity. Foods 2022, 11, 2514. https://doi.org/10.3390/foods11162514
Burgos-Díaz C, Mosi-Roa Y, Opazo-Navarrete M, Bustamante M, Garrido-Miranda K. Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity. Foods. 2022; 11(16):2514. https://doi.org/10.3390/foods11162514
Chicago/Turabian StyleBurgos-Díaz, César, Yohanna Mosi-Roa, Mauricio Opazo-Navarrete, Mariela Bustamante, and Karla Garrido-Miranda. 2022. "Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity" Foods 11, no. 16: 2514. https://doi.org/10.3390/foods11162514
APA StyleBurgos-Díaz, C., Mosi-Roa, Y., Opazo-Navarrete, M., Bustamante, M., & Garrido-Miranda, K. (2022). Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity. Foods, 11(16), 2514. https://doi.org/10.3390/foods11162514