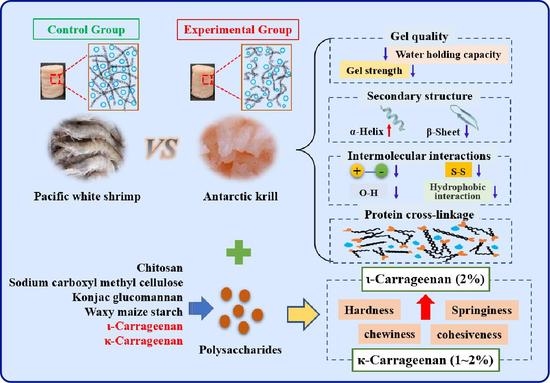
Insight into the Gel Properties of Antarctic Krill and Pacific White Shrimp Surimi Gels and the Feasibility of Polysaccharides as Texture Enhancers of Antarctic Krill Surimi Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Shrimp Surimi Gels
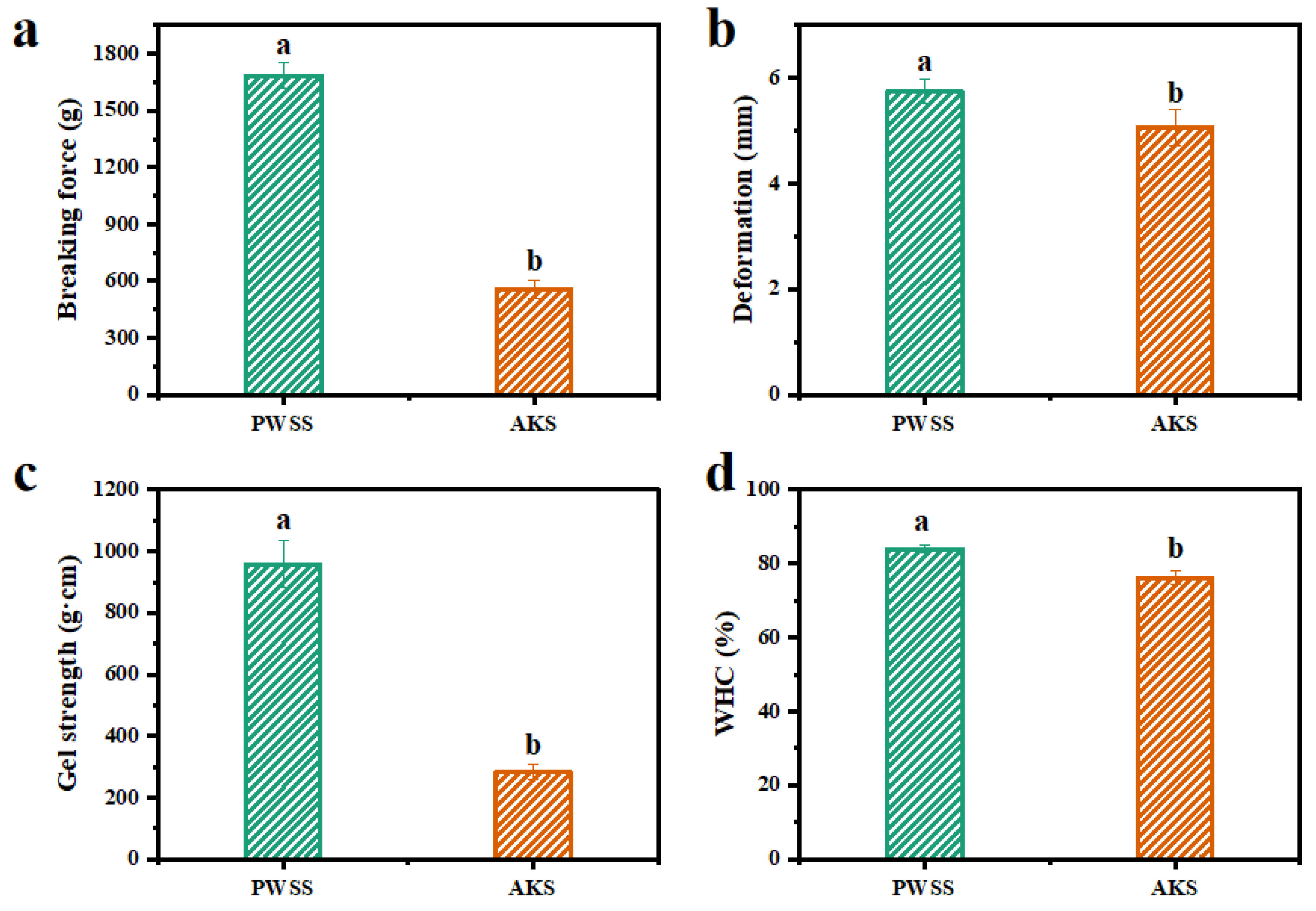
2.3. Texture Profile Analysis (TPA)
2.4. Gel Strength
2.5. Rheological Tests
2.6. Water-Holding Capacity
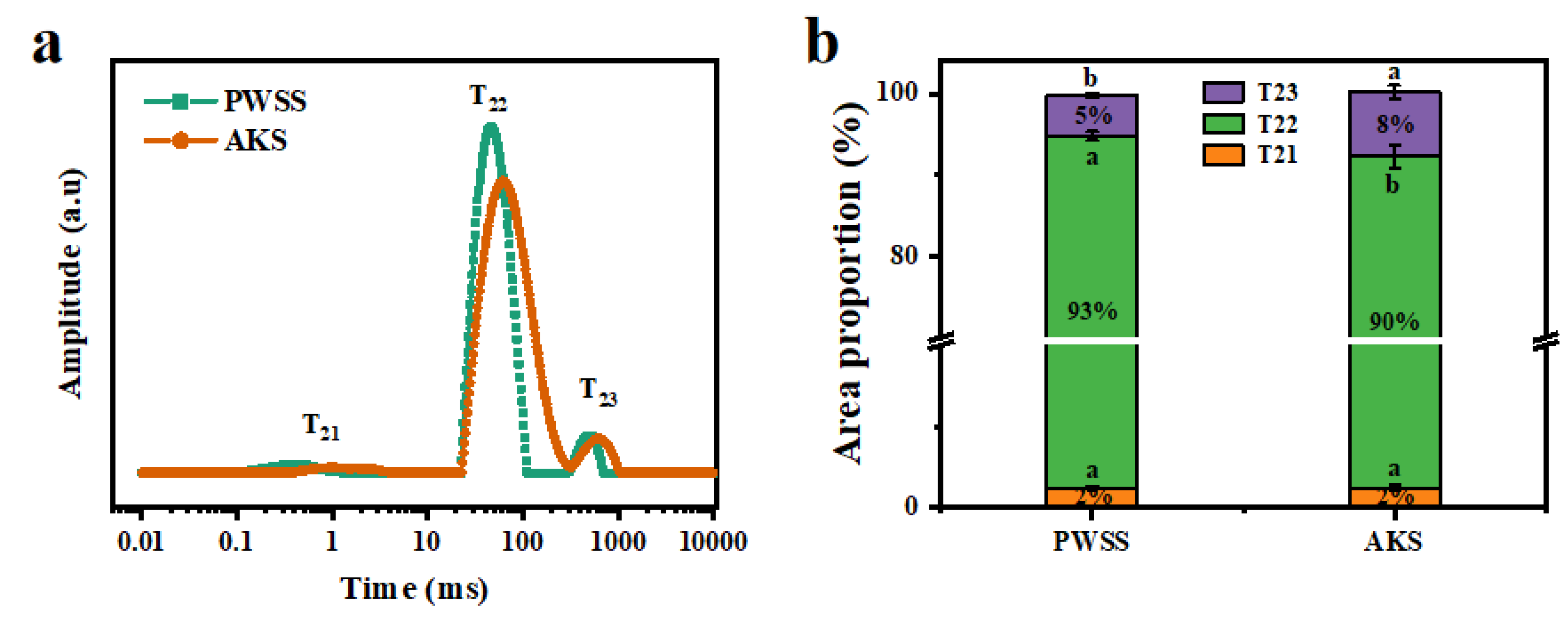
2.7. Low Field Nuclear Magnetic Resonance
2.8. SDS-PAGE
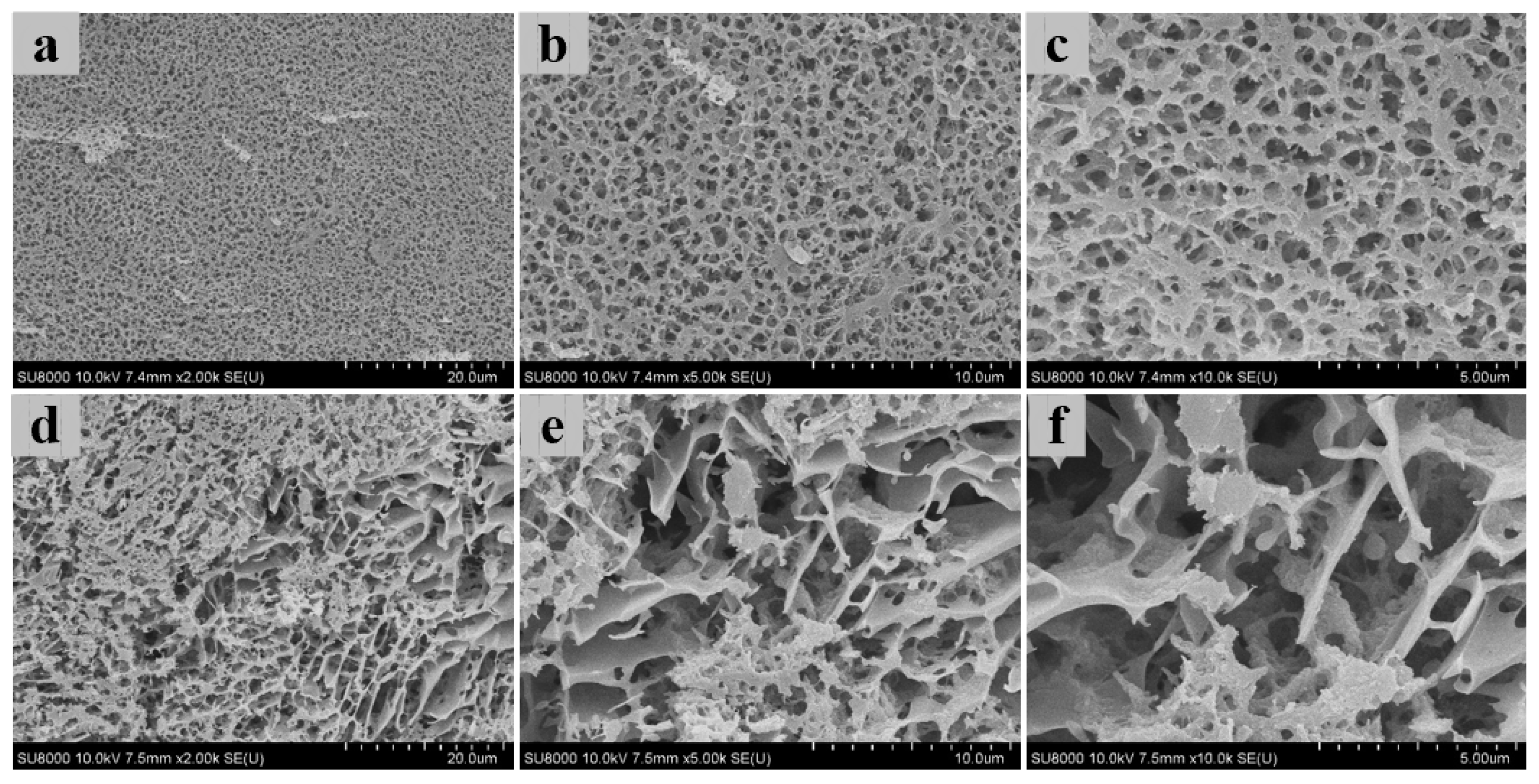
2.9. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.10. Fourier-Transform Infrared (FTIR) Spectroscopy
2.11. Molecular Forces
2.12. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Gel Quality of Heat-Induced Antarctic Krill Surimi
3.2. Changes in the Rheological Properties of Antarctic Krill Surimi during the Heat-Induced Gelation Process
3.3. Analysis of Water State and Distribution of Antarctic Krill Surimi after Heat-Induced Gel Formation
3.4. Three-Dimensional Microstructure of Heat-Induced Antarctic Krill Surimi Gel
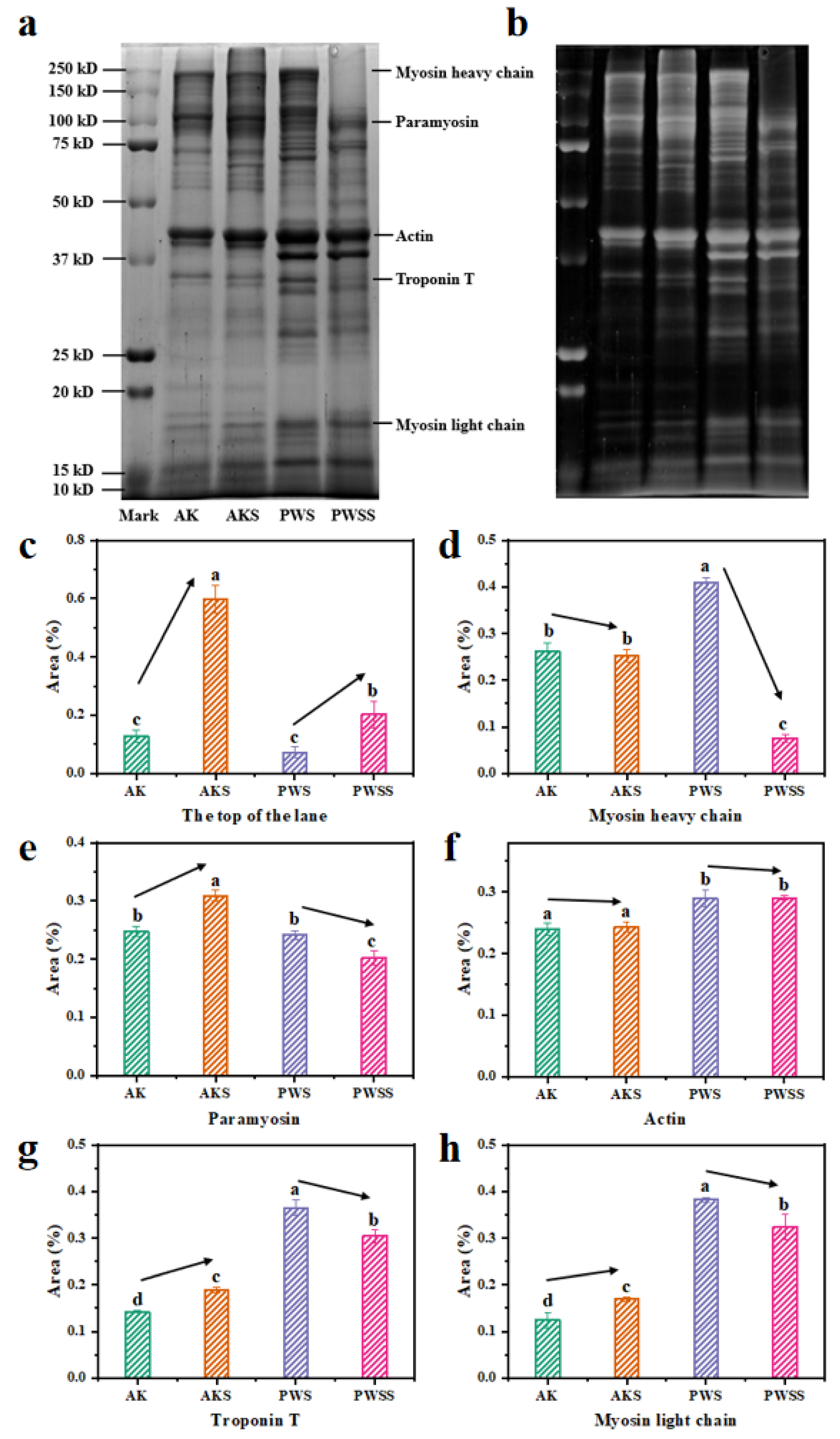
3.5. Protein Patterns of Antarctic Krill Surimi with and without Heat Treatment
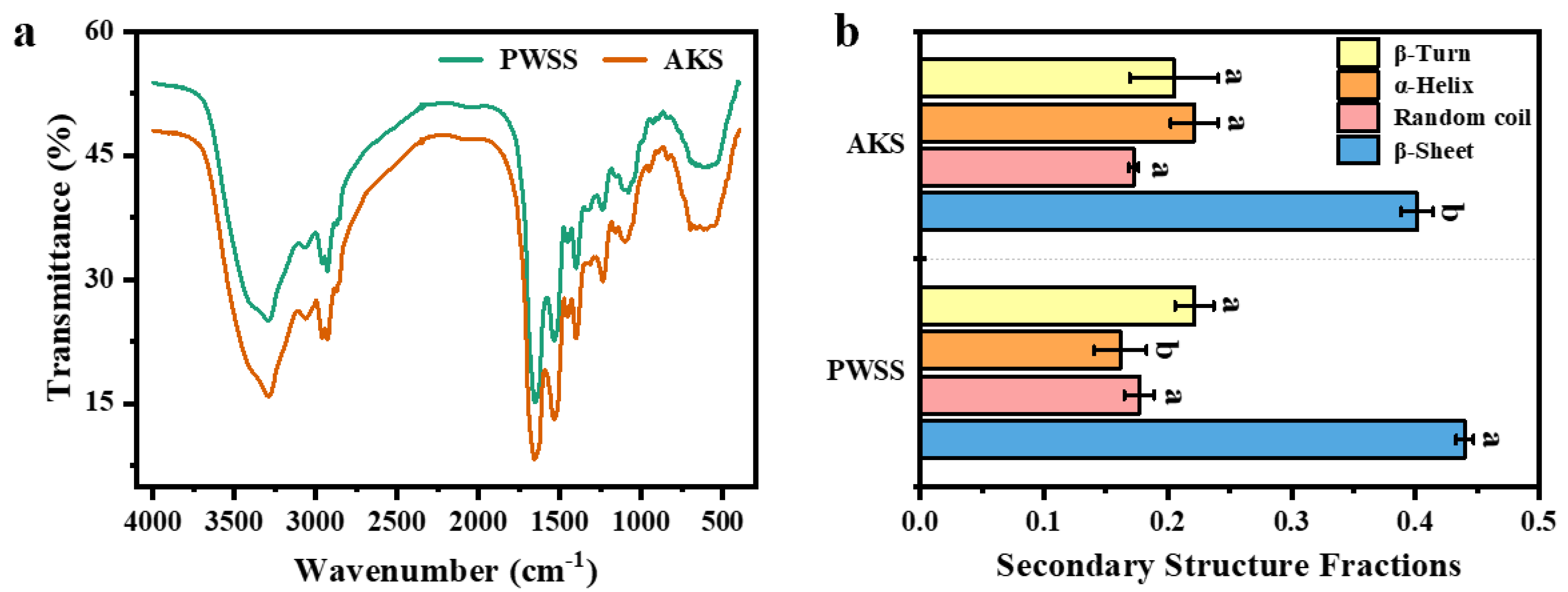
3.6. Analysis of Protein Secondary Structure in Antarctic Krill Surimi Gel
3.7. Molecular Forces Involved in Antarctic Krill Surimi Gel Formation
3.8. Correlation Analysis and Principal Component Analysis
3.9. Feasibility Exploration of Polysaccharide Addition to Improve the Texture Properties of Heat-Induced Antarctic Krill Surimi Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Suzuki, T.; Shibata, N. The utilization of Antarctic krill for human food. Food Rev. Int. 1990, 6, 119–147. [Google Scholar] [CrossRef]
- Zheng, H.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of kappa-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Sjödahl, J.; Emmer, A.; Vincent, J.; Roeraade, J. Characterization of proteinases from Antarctic krill (Euphausia superba). Protein Expr. Purif. 2002, 26, 153–161. [Google Scholar] [CrossRef]
- Grantham, G.J. The Southern Ocean: The Utilization of Krill; FAO: Rome, Italy, 1977; pp. 1–61. [Google Scholar]
- Nakamura, Y.; Takahashi, S.; Takahashi, K. Long-term suppression of suwari phenomenon for improvement in the manufacturing process of surimi gel product. LWT 2021, 150, 111934. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Hu, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef]
- Li, D.Y.; Tan, Z.F.; Liu, Z.Q.; Wu, C.; Liu, H.L.; Guo, C.; Zhou, D.Y. Effect of hydroxyl radical induced oxidation on the physicochemical and gelling properties of shrimp myofibrillar protein and its mechanism. Food Chem. 2021, 351, 129344. [Google Scholar] [CrossRef]
- Song, L.L.; Yu, J.; Xie, H.L.; Zhang, R.F.; Xue, Y.; Xue, C.H. Physical properties and conformational changes of shrimp surimi from Litopenaeus vannamei during cold gelation. LWT 2022, 153, 112516. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xue, Y.; Xue, C.; Zhao, Y. The process of heat-induced gelation in Litopenaeus vannamei. Food Hydrocoll. 2020, 98, 105260. [Google Scholar] [CrossRef]
- Shi, L.; Beamer, S.K.; Yang, H.; Jaczynski, J. Micro-emulsification/encapsulation of krill oil by complex coacervation with krill protein isolated using isoelectric solubilization/precipitation. Food Chem. 2018, 244, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.J.; Wang, Y.M.; Xue, Y.; Xue, C.H. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhou, A.; Xiao, J.; Benjakul, S. Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration. Foods 2021, 341, 1815. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guo, J.; Xie, Y.; Zhou, K.; Li, P.J.; Xu, B.C. Modulating the aggregation of myofibrillar protein to alleviate the textural deterioration of protein gels at high temperature: The effect of hydrophobic interactions. Food Chem. 2021, 10, 128274. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S. Effect of formaldehyde on protein cross-linking and gel forming ability of surimi from lizardfish induced by microbial transglutaminase. Food Hydrocoll. 2013, 30, 704–711. [Google Scholar] [CrossRef]
- Wei, Y.N.; Zhang, T.; Yu, F.; Xue, Y.; Li, Z.J.; Wang, Y.M.; Xue, C.H. Effects of curdlan on the texture and structure of Alaska pollock surimi gels treated at 120 °C. Int. J. Food Prop. 2017, 21, 1778–1788. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S. Property of fish gelatin gel as affected by the inorporation of gellan and calcium chloride. Food Biophys. 2017, 12, 339–347. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, H.; Zhu, S.; Xu, X.; Ding, Y. Textural, rheological and chemical properties of surimi nutritionally-enhanced with lecithin. LWT 2020, 122, 108984. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S. Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolates. Food Chem. 2013, 136, 929–937. [Google Scholar] [CrossRef]
- Bi, C.; Li, D.; Wang, L.; Gao, F.; Adhikari, B. Effect of high shear homogenization on rheology, microstructure and fractal dimension of acid-induced SPI gels. J. Food Eng. 2014, 126, 48–55. [Google Scholar] [CrossRef]
- Hunt, A.; Park, J.W. Alaska pollock fish protein gels as affected by refined carrageenan and various salts. J. Food Qual. 2013, 36, 51–58. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S.; Konno, K. Improvement of gel quality of sardine surimi with low setting phenomenon by ethanolic coconut husk extract. J. Texture Stud. 2017, 48, 47–56. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Mleko, S.; Foegeding, E.A. pH induced aggregation and weak gel formation of whey protein polymers. J. Food Sci. 2000, 65, 139–143. [Google Scholar] [CrossRef]
- Liang, R.; Lin, S.Y.; Chen, D.; Sun, N. Differentiation of Penaeus vannamei from different thermal processing methods in physico-chemical, flavor and sensory characteristics. Food Chem. 2022, 378, 132092. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, M.P. Effect of different protein extracts from Dosidicus gigas muscle co-products on edible films development. Food Hydrocoll. 2013, 33, 118–131. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Effect of serine protease inhibitor from squid ovary on gel properties of surimi from Indian mackerel. J. Texture Stud. 2017, 48, 541–549. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, 215–221. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, R.; Xu, X.; Zhou, G.; Liu, D. Structural modification by high- pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Lin, S.Y.; Liu, Y.; He, X.Q.; Zhang, S.M.; Sun, N. Exploration of iron-binding mode, digestion Kinetics, and iron absorption behavior of Antarctic Krill–derived heptapeptide–iron complex. Food Res. Int. 2022, 154, 110996. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green preparation and characterization of waxy maize starch nanoparticles through enzymolysis and recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Elmi, F.; Movaghar, A.F.; Elmi, M.M.; Alinezhad, H.; Nikbakhsh, N. Application of FT-IR spectroscopy on breast cancer serum analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.B.; Zhang, W.G.; Zou, Y.F.; Xing, L.J.; Zheng, H.B.; Xu, X.L.; Zhou, G.H. Influence of RosA-protein adducts formation on myofibrillar protein gelation properties under oxidative stress. Food Hydrocoll. 2017, 67, 197–205. [Google Scholar] [CrossRef]
- Su, Y.; Dong, Y.; Niu, F.; Wang, C.; Liu, Y.; Yang, Y. Study on the gel properties and secondary structure of soybean protein isolate/egg white composite gels. Eur. Food Res. Technol. 2015, 240, 367–378. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Wang, B.; Kong, B.H.; Li, F.F.; Liu, Q.; Zhang, H.W.; Xia, X.F. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020, 316, 126375. [Google Scholar] [CrossRef]
- Ni, N.; Wang, Z.; He, F.; Wang, L.; Pan, H.; Li, X.; Wang, Q.; Zhang, D. Gel properties and molecular forces of lamb myofibrillar protein during heat induction at different pH values. Process Biochem. 2014, 49, 631–636. [Google Scholar] [CrossRef]
- Gong, J.; Shen, H.; Zheng, J.Y.; Tao, N.; Gu, S.Q.; Huang, Y.W.; Wang, M. Identification of key umami-related compounds in Yangtze Coilia ectenes by combining electronic tongue analysis with sensory evaluation. RSC Adv. 2016, 6, 45689–45695. [Google Scholar] [CrossRef]
- Chang, L.L.; Lin, S.Y.; Zou, B.W.; Zheng, X.H.; Zhang, S.M.; Tang, Y. Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics. Foods. 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C.; Comstock, S.H. Effect of degree of compression on texture profile paremeters. J. Texture Stud. 1981, 12, 201–216. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M.; Ishizaki, S.; Suthidham, R.; Sungpech, O. Effect of chitin and chitosan on gelling properties of surimi from barred garfish (Hemiramphus far). J. Sci. Food Agric. 2001, 81, 102–108. [Google Scholar] [CrossRef]



| Samples | Molecular Forces in the Gel (mg/L) | |||
|---|---|---|---|---|
| Ionic Bonds | Hydrogen Bonds | Hydrophobic Interactions | Disulfide Bonds | |
| PWSS | 47.87 ± 2.45 a | 150.29 ± 1.18 a | 667.29 ± 17.16 a | 1677.41 ± 29.26 a |
| AKS | 39.66 ± 1.33 b | 144.69 ± 4.17 b | 536.17 ± 9.15 b | 1496.92 ± 23.75 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Lin, S.; Jiang, P.; Bao, Z.; Li, S.; Sun, N. Insight into the Gel Properties of Antarctic Krill and Pacific White Shrimp Surimi Gels and the Feasibility of Polysaccharides as Texture Enhancers of Antarctic Krill Surimi Gels. Foods 2022, 11, 2517. https://doi.org/10.3390/foods11162517
Li S, Lin S, Jiang P, Bao Z, Li S, Sun N. Insight into the Gel Properties of Antarctic Krill and Pacific White Shrimp Surimi Gels and the Feasibility of Polysaccharides as Texture Enhancers of Antarctic Krill Surimi Gels. Foods. 2022; 11(16):2517. https://doi.org/10.3390/foods11162517
Chicago/Turabian StyleLi, Shuang, Songyi Lin, Pengfei Jiang, Zhijie Bao, Sibo Li, and Na Sun. 2022. "Insight into the Gel Properties of Antarctic Krill and Pacific White Shrimp Surimi Gels and the Feasibility of Polysaccharides as Texture Enhancers of Antarctic Krill Surimi Gels" Foods 11, no. 16: 2517. https://doi.org/10.3390/foods11162517
APA StyleLi, S., Lin, S., Jiang, P., Bao, Z., Li, S., & Sun, N. (2022). Insight into the Gel Properties of Antarctic Krill and Pacific White Shrimp Surimi Gels and the Feasibility of Polysaccharides as Texture Enhancers of Antarctic Krill Surimi Gels. Foods, 11(16), 2517. https://doi.org/10.3390/foods11162517