An Evaluation Model for the Quality of Frying Oil Using Key Aldehyde Detected by HS-GC/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Frying Oil Samples
2.2. Chemicals
2.3. Determination of Oil Composition and Quality Index
2.4. Determination of Aldehyde
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Frying on Aldehydes Detected in Oil
3.2. The Change in the Common Aldehydes during Frying
3.3. Selecting Aldehydes to Evaluate the Quality of Frying Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durmaz, A.; Yuksel, F. Deep fried wheat chips added with potato peel flour—Effect on quality parameters. Qual. Assur. Saf. Crop. 2021, 13, 115–124. [Google Scholar] [CrossRef]
- Choe, E.; Min, D. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Chang, C.; Wu, G.C.; Zhang, H.; Jin, Q.Z.; Wang, X.G. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α, β unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.; Shen, Q.; Saleh, A.S. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015, 52, 7683–7696. [Google Scholar] [CrossRef] [Green Version]
- Takhar, M.; Li, Y.; Ditto, J.C.; Chan, A.W.H. Formation pathways of aldehydes from heated cooking oils. Environ. Sci. Process. Impacts 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, X.; Qu, Z.; Gao, Y.; Li, Q.; Yu, X. Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.D.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Chemical and sensory assessment of deep-frying oil alternatives for the processing of French fries. Eur. J. Lipid Sci. Technol. 2013, 115, 935–945. [Google Scholar] [CrossRef]
- Romano, R.; Giordano, A.; Le Grottaglie, L.; Manzo, N.; Paduano, A.; Sacchi, R.; Santini, A. Volatile compounds in intermittent frying by gas chromatography and nuclear magnetic resonance. Eur. J. Lipid Sci. Technol. 2013, 115, 764–773. [Google Scholar] [CrossRef]
- Liu, X.; Hoshino, N.; Wang, S.; Masui, E.; Chen, J.; Zhang, H. A novel evaluation index for predicting the degradation rate of frying oils based on their fatty acid composition. Eur. J. Lipid Sci. Technol. 2018, 120, 1700528. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Analysis of the Dynamic Decomposition of Unsaturated Fatty Acids and Tocopherols in Commercial Oils During Deep Frying. Anal. Lett. 2019, 52, 1991–2005. [Google Scholar] [CrossRef]
- Tentative 13-2003; Standard Methods for the Analysis of Fats, Oils and Related Materials. JOCS: Tokyo, Japan, 2003.
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R.J.F.C. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef]
- Battaloglu, R. Calculation of retention indices of essential oils with the aid of the Van den Dool and Kratz equation and Bézier curves. Math. Meth. Appl. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Fujisaki, M.; Endo, Y.; Fujimoto, K. Retardation of volatile aldehyde formation in the exhaust of frying oil by heating under low oxygen atmospheres. J. Am. Oil Chem. Soc. 2002, 79, 909–914. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Model for prediction of the carbonyl value of frying oil from the initial composition. LWT-Food Sci. Technol. 2020, 117, 108660. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Takenami, S.; Imagi, J.; Shiramasa, H. Comparison of odor compounds analyzed by automatic dynamic headspace GC/MS system and sensory score from thermal-deteriorated edible oil. Jpn. J. Food Eng. 2014, 15, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Zribi, A.; Jabeur, H.; Flamini, G.; Bouaziz, M. Quality assessment of refined oil blends during repeated deep frying monitored by SPME–GC–EIMS, GC and chemometrics. Int. J. Food Sci. Technol. 2016, 51, 1594–1603. [Google Scholar] [CrossRef]
- Morales, M.; Rios, J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.W.; Cheng, Y.J.; Liu, Y.F. Effect of frying oils’ fatty acid profile on quality, free radical and volatiles over deep-frying process: A comparative study using chemometrics. LWT-Food Sci. Technol. 2019, 101, 331–341. [Google Scholar] [CrossRef]
- Wang, L.; Csallany, A.S.; Kerr, B.J.; Shurson, G.C.; Chen, C. Kinetics of forming aldehydes in frying oils and their distribution in French fries revealed by LC–MS-based Chemometrics. J. Agric. Food Chem. 2016, 64, 3881–3889. [Google Scholar] [CrossRef]
- Peng, C.Y.; Lang, C.H.; Lin, P.C.; Kuo, Y.C. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard. Mater. 2017, 324, 160–167. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef] [Green Version]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, A.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- Ben Hammouda, I.; Freitas, F.; Ammar, S.; Da Silva, M.G.; Bouaziz, M. Comparison and characterization of volatile compounds as markers of oils stability during frying by HS-SPME-GC/MS and Chemometric analysis. J. Chromatogr. B 2017, 1068, 322–334. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M.D. Effect of supplementing ewes’ diet with thyme (Thymus zygis ssp. gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem. 2011, 125, 1147–1152. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, C.; Wang, C.; Chen, H.; Liu, Y.; Li, S.; Lin, D.; Wu, D.; Qin, W. Evaluation of the non-aldehyde volatile compounds formed during deep-fat frying process. Food Chem. 2018, 243, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.; Orr, P.; Glynn, M. Effect of fatty acid composition of oils on flavor and stability of fried foods. J. Am. Oil Chem. Soc. 1997, 74, 347–356. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000, 68, 339–345. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Parisini, C.; Battimo, I.; Falco, S.; Sacchi, R. Frying performance of a sunflower/palm oil blend in comparison with pure palm oil. Eur. J. Lipid Sci. Technol. 2007, 109, 237–246. [Google Scholar] [CrossRef]
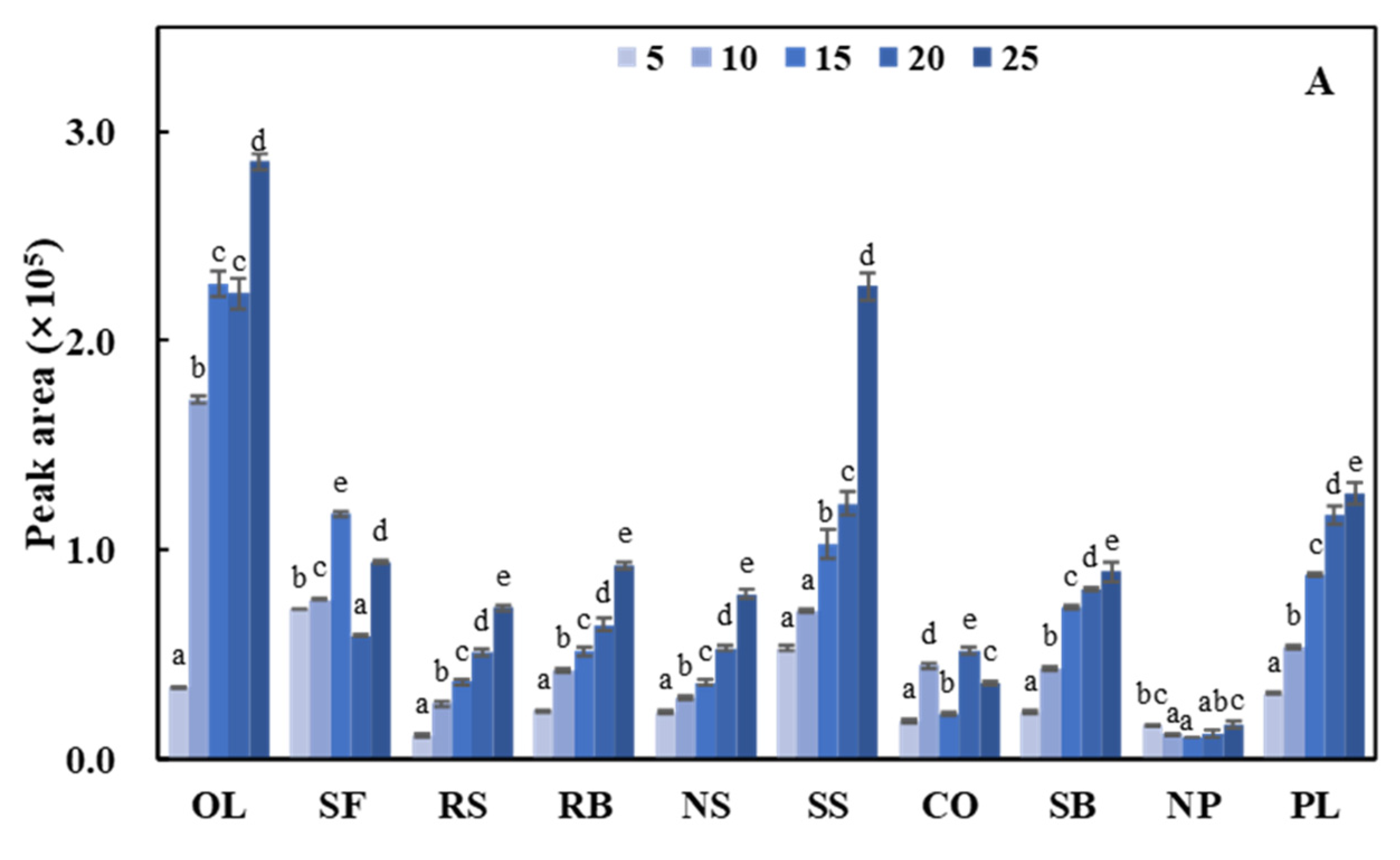
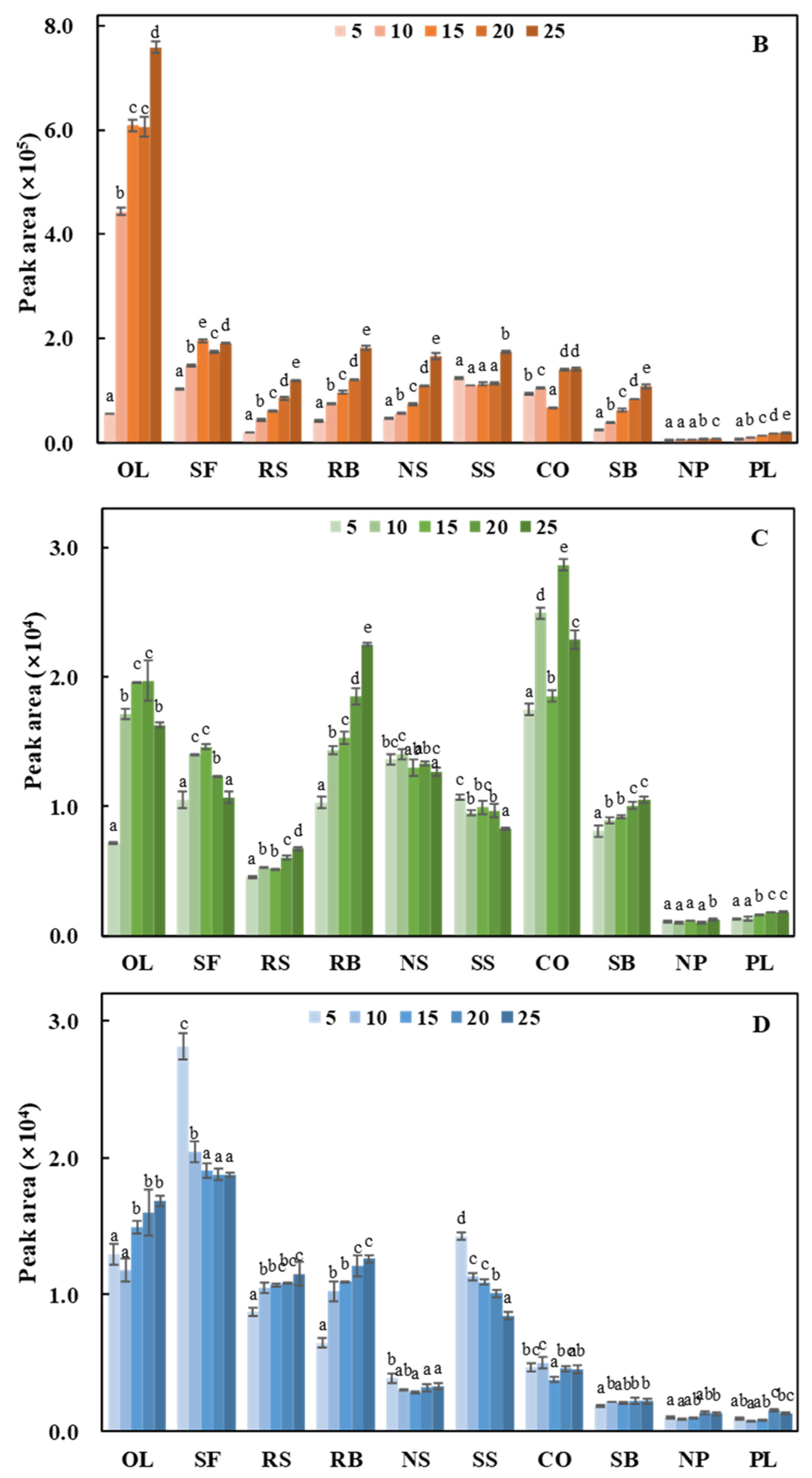
| Name | Fatty Acid (%) | Tocopherol (mg/100 g of Oil) | Carbonyl Value (μmol/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | PUFA | C18:1 + C18:2 | α | β | γ | δ | TToc | ||
| OL | 8.58 ± 0.14 | 2.44 ± 0.03 | 81.51 ± 0.15 | 6.27 ± 0.13 | 0.35 ± 0.02 | 6.62 | 87.78 | 14.44 ± 0.94 | 0.57 ± 0.03 | 1.40 ± 0.04 | - | 16.40 | 3.41 ± 0.21 |
| SF | 4.31 ± 0.13 | 1.71 ± 0.17 | 79.69 ± 0.80 | 13.58 ± 0.60 | 0.33 ± 0.23 | 13.91 | 93.27 | 38.67 ± 0.63 | 0.70 ± 0.01 | 0.90 ± 0.01 | - | 40.27 | 4.67 ± 0.32 |
| RS | 3.22 ± 0.08 | 1.28 ± 0.02 | 67.71 ± 0.77 | 18.31 ± 0.30 | 9.26 ± 0.58 | 27.57 | 86.02 | 46.30 ± 0.39 | 0.50 ± 0.03 | 74.66 ± 1.56 | 5.46 ± 0.08 | 126.92 | 4.32 ± 0.29 |
| RB | 14.98 ± 0.10 | 1.53 ± 0.14 | 46.68 ± 0.14 | 35.34 ± 0.55 | 1.47 ± 0.31 | 36.81 | 82.02 | 38.37 ± 1.08 | 2.10 ± 0.15 | 6.13 ± 0.16 | 0.35 ± 0.02 | 46.95 | 5.92 ± 0.38 |
| NS | 8.12 ± 0.08 | 4.71 ± 0.02 | 41.25 ± 0.14 | 45.67 ± 0.25 | 0.25 ± 0.07 | 45.92 | 86.92 | - | - | 47.32 ± 0.92 | - | 47.32 | 2.36 ± 0.11 |
| SS | 8.20 ± 0.16 | 4.57 ± 0.06 | 38.88 ± 0.20 | 47.74 ± 0.37 | 0.21 ± 0.02 | 47.95 | 86.62 | - | - | 64.55 ± 4.15 | - | 64.55 | 3.02 ± 0.24 |
| CO | 10.67 ± 0.21 | 1.47 ± 0.01 | 28.49 ± 0.22 | 58.35 ± 0.56 | - | 58.35 | 86.84 | 17.00 ± 0.24 | 0.47 ± 0.00 | 44.17 ± 1.06 | 1.18 ± 0.01 | 62.81 | 4.48 ± 0.40 |
| SB | 10.09 ± 0.11 | 3.24 ± 0.15 | 19.21 ± 0.32 | 57.83 ± 0.35 | 9.63 ± 0.21 | 67.46 | 77.04 | 27.20 ± 0.56 | 1.97 ± 0.27 | 123.31 ± 4.25 | 18.89 ± 0.51 | 171.37 | 6.30 ± 0.13 |
| NP | 5.16 ± 0.01 | 1.31 ± 0.03 | 16.68 ± 0.07 | 12.59 ± 0.04 | 64.26 ± 0.04 | 76.85 | 29.27 | 1.86 ± 0.11 | - | 74.75 ± 0.84 | 159.44 ± 0.80 | 236.05 | 2.46 ± 0.17 |
| PL | 5.10 ± 0.02 | 1.54 ± 0.03 | 15.18 ± 0.03 | 12.34 ± 0.03 | 65.83 ± 0.03 | 78.17 | 27.52 | 2.37 ± 0.13 | 1.32 ± 0.04 | 73.78 ± 1.15 | 1.04 ± 0.07 | 78.51 | 3.12 ± 0.11 |
| No. | Volatile Compound | RI | RIr | Difference between the Peak Areas of Volatile Aldehydes before and after Heating for 25 h (×103) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OL | SF | RS | RB | NS | SS | CO | SB | NP | PL | ||||
| 1 | Butanal | 619 | 601 | * 219.41 ± 6.65 | - | 0.57 ± 0.01 | 425.03 ± 2.36 | 2.53 ± 0.13 | 473.79 ± 16.34 | - | −12.92 ± 0.77 | - | −71.43 ± 4.04 |
| # (16.08) | - | (0.28) | (56.28) | (1.07) | (58.62) | - | (7.56) | - | (50.73) | ||||
| 2 | (E)-But-2-enal | 692 | 657 | 8.65 ± 0.38 | - | 7.55 ± 0.52 | - | - | - | - | - | 11.54 ± 0.70 | 52.06 ± 0.86 |
| (0.63) | - | (3.68) | - | - | - | - | - | (17.45) | (36.98) | ||||
| 3 | 2-Methylbutanal | 704 | 664 | - | 4.21 ± 0.29 | - | 6.84 ± 0.25 | 10.03 ± 0.42 | 8.35 ± 0.33 | −1.45 ± 0.07 | −8.87 ± 0.30 | - | 6.29 ± 0.30 |
| - | (1.28) | - | (0.91) | (4.24) | (1.03) | (0.72) | (5.19) | - | (4.46) | ||||
| 4 | Pentanal | 724 | 701 | 275.20 ± 4.18 | 86.27 ± 1.79 | 63.73 ± 1.53 | 84.66 ± 1.61 | 68.03 ± 1.25 | 202.76 ± 6.15 | 31.13 ± 0.78 | 72.38 ± 3.96 | 14.85 ± 1.71 | 126.90 ± 5.45 |
| (20.16) | (26.19) | (31.03) | (11.21) | (28.80) | (25.09) | (15.38) | (42.34) | (22.44) | (90.13) | ||||
| 5 | (E)-2-Methylbut-2-enal | 766 | 745 | - | - | - | - | - | - | - | - | - | 4.43 ± 0.15 |
| - | - | - | - | - | - | - | - | - | (3.14) | ||||
| 6 | (E)-Pent-2-enal | 779 | 759 | 7.19 ± 0.21 | - | 3.87 ± 0.30 | - | - | - | - | - | 6.03 ± 0.08 | 14.94 ± 0.46 |
| (0.53) | - | (1.88) | - | - | - | - | - | (9.11) | (10.61) | ||||
| 7 | Hexanal | 824 | 802 | 754.02 ± 11.37 | 182.71 ± 2.28 | 95.47 ± 0.95 | 170.96 ± 5.27 | 138.54 ± 6.35 | 136.15 ± 1.43 | 116.24 ± 2.97 | 89.24 ± 4.69 | −3.43 ± 0.42 | −5.27 ± 0.31 |
| (55.25) | (55.47) | (46.49) | (22.64) | (58.64) | (16.84) | (57.41) | (52.20) | (5.18) | (3.74) | ||||
| 8 | Furan-2-carbaldehyde | 854 | 830 | - | - | - | - | - | −20.35 ± 0.27 | - | - | - | - |
| - | - | - | - | - | (2.52) | - | - | - | - | ||||
| 9 | (E)-Hex-2-enal | 877 | 864 | 2.31 ± 0.09 | 2.17 ± 0.15 | - | 2.83 ± 0.20 | 0.71 ± 0.01 | −6.04 ± 0.19 | 3.76 ± 0.25 | 0.67 ± 0.02 | - | −10.78 ± 0.55 |
| (0.17) | (0.66) | - | (0.37) | (0.30) | (0.75) | (1.85) | (0.39) | - | (7.66) | ||||
| 10 | Heptanal | 927 | 903 | 27.14 ± 1.02 | 7.57 ± 0.26 | 6.86 ± 0.46 | 5.82 ± 0.28 | 1.75 ± 0.05 | 4.72 ± 0.06 | 3.41 ± 0.07 | 1.99 ± 0.12 | - | −5.96 ± 0.29 |
| (1.99) | (2.30) | (3.34) | (0.77) | (0.74) | (0.58) | (1.68) | (1.16) | - | (4.23) | ||||
| 11 | (E)-Hept-2-enal | 981 | 956 | 16.29 ± 0.18 | 10.42 ± 0.57 | 4.5 ± 0.07 | 20.51 ± 0.45 | 6.38 ± 0.03 | 7.03 ± 0.23 | 22.86 ± 0.73 | 7.75 ± 0.13 | 1.27 ± 0.07 | 1.82 ± 0.05 |
| (1.19) | (3.16) | (2.19) | (2.72) | (2.70) | (0.87) | (11.29) | (4.53) | (1.92) | (1.29) | ||||
| 12 | 5-Methylfuran-2-carbaldehyde | 984 | 980 | - | - | - | - | - | −13.4 ± 0.62 | - | - | - | −8.23 ± 0.43 |
| - | - | - | - | - | (1.66) | - | - | - | (5.84) | ||||
| 13 | Octanal | 1029 | 1003 | 24.15 ± 0.92 | 6.53 ± 0.45 | 3.09 ± 0.17 | 2.66 ± 0.12 | 1.59 ± 0.10 | 3.95 ± 0.09 | 1.11 ± 0.01 | - | - | - |
| (1.77) | (1.98) | (1.50) | (0.35) | (0.67) | (0.49) | (0.55) | - | - | - | ||||
| 14 | (2E,4E)-Hepta-2,4-dienal | 1036 | 1015 | 2.03 ± 0.08 | - | 5.64 ± 0.38 | 3.20 ± 0.16 | - | - | - | 8.95 ± 0.13 | 34.62 ± 0.85 | 35.25 ± 1.51 |
| (0.15) | - | (2.75) | (0.42) | - | - | - | (5.23) | (52.33) | (25.03) | ||||
| 15 | (E)-Oct-2-enal | 1083 | 1064 | 4.77 ± 0.30 | 1.39 ± 0.03 | 1.07 ± 0.00 | 3.36 ± 0.18 | 0.62 ± 0.02 | 2.03 ± 0.07 | 2.29 ± 0.09 | 1.68 ± 0.06 | - | - |
| (0.35) | (0.42) | (0.52) | (0.45) | (0.26) | (0.25) | (1.13) | (0.98) | - | - | ||||
| 16 | Nonanal | 1132 | 1104 | 16.85 ± 0.39 | 18.73 ± 0.15 | 9.31 ± 0.70 | 11.54 ± 0.53 | 0.56 ± 0.12 | 6.94 ± 0.06 | 4.56 ± 0.30 | 1.60 ± 0.04 | 1.28 ± 0.09 | 0.77 ± 0.00 |
| (1.23) | (5.69) | (4.53) | (1.53) | (0.24) | (0.86) | (2.25) | (0.94) | (1.94) | (0.55) | ||||
| 17 | (E)-Non-2-enal | 1187 | 1165 | 1.49 ± 0.05 | 0.80 ± 0.02 | - | - | - | 2.34 ± 0.12 | - | - | - | - |
| (0.11) | (0.24) | - | - | - | (0.29) | - | - | - | - | ||||
| 18 | (E)-Dec-2-enal | 1294 | 1263 | 3.55 ± 0.19 | 3.46 ± 0.26 | 1.38 ± 0.05 | 2.16 ± 0.09 | 0.92 ± 0.03 | - | - | - | - | - |
| (0.26) | (1.05) | (0.67) | (0.29) | (0.39) | - | - | - | - | - | ||||
| 19 | (2E,4E)-Deca-2,4-dienal | 1327 | 1316 | - | - | - | 2.63 ± 0.04 | - | - | 3.45 ± 0.01 | 1.66 ± 0.04 | - | - |
| - | - | - | (0.35) | - | - | (1.70) | (0.97) | - | - | ||||
| 20 | (E)-Undec-2-enal | 1350 | 1360 | - | 3.20 ± 0.02 | 2.34 ± 0.15 | 11.87 ± 0.50 | 4.60 ± 0.25 | - | 15.12 ± 1.45 | 6.85 ± 0.26 | - | - |
| - | (0.97) | (1.14) | (1.57) | (1.95) | - | (7.47) | (4.00) | - | - | ||||
| 21 | (2E,4E)-Undeca-2,4-dienal | 1396 | 1420 | 1.72 ± 0.12 | 1.91 ± 0.10 | - | 1.08 ± 0.06 | - | - | - | - | - | - |
| (0.13) | (0.58) | - | (0.14) | - | - | - | - | - | - | ||||
| Total | 1364.76 | 329.37 | 205.37 | 755.15 | 236.25 | 808.27 | 202.48 | 170.97 | 66.16 | 140.81 | |||
| Name | Correlation Coefficient with CV | ||||
|---|---|---|---|---|---|
| Total Aldehydes | Pentanal | Hexanal | (E)-Hept-2-enal | Nonanal | |
| OL | 1.000 ** | 0.943 ** | 0.943 ** | 0.657 | 0.943 ** |
| SF | 0.829 * | 0.543 | 0.829 * | 0.486 | −0.143 |
| RS | 1.000 ** | 1.000 ** | 0.943 ** | 0.943 ** | 1.000 ** |
| RB | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** |
| NS | 1.000 ** | 1.000 ** | 1.000 ** | −0.029 | 0.371 |
| SS | 0.371 | 1.000 ** | 0.657 | 0.029 | −0.143 |
| CO | 0.771 | 0.714 | 0.829 * | 0.714 | 0.086 |
| SB | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 0.943 ** |
| NP | 0.029 | 0.600 | −0.029 | 0.714 | 0.771 |
| PL | 1.000 ** | 1.000 ** | 0.143 | 0.943 ** | 0.771 |
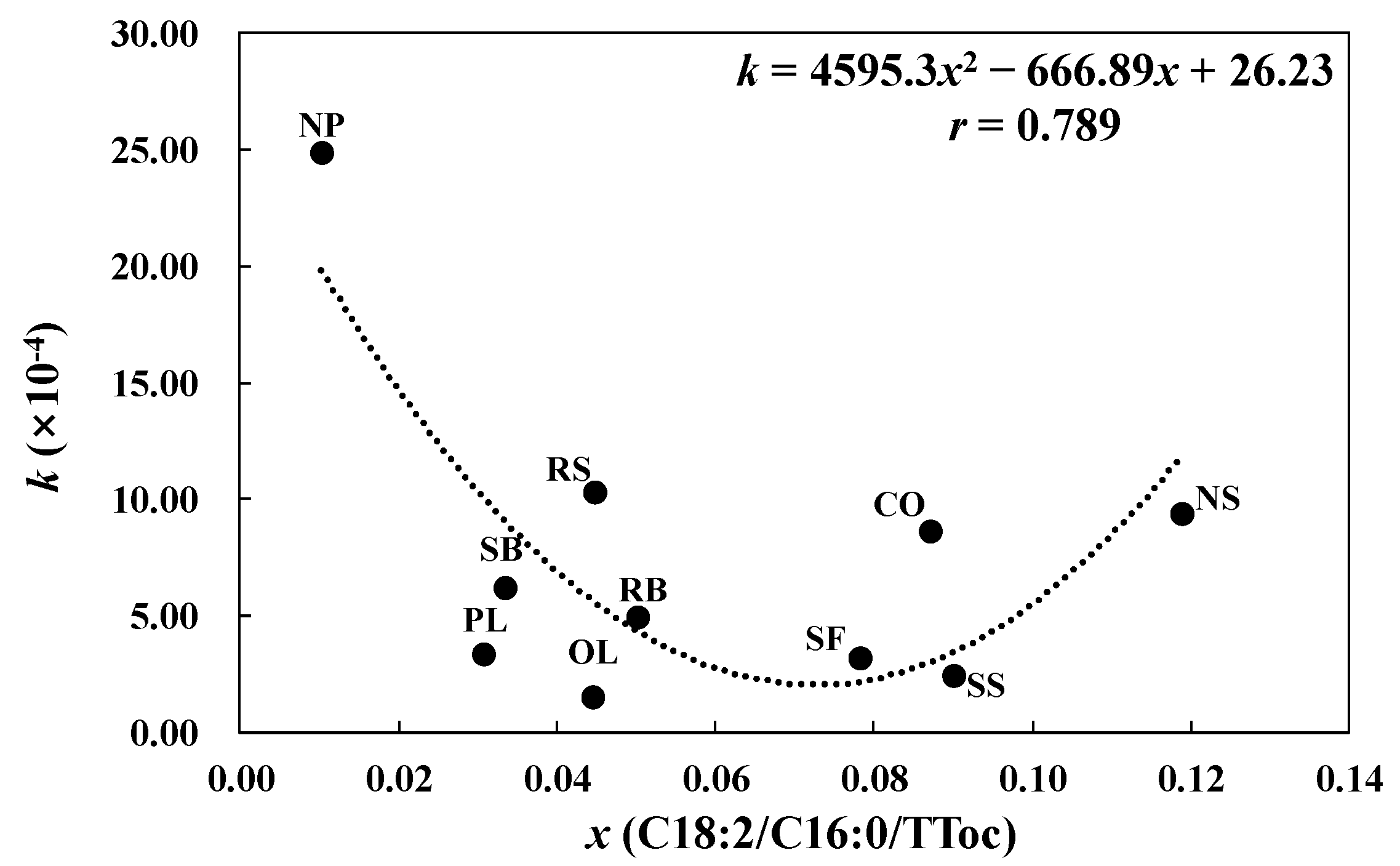
| Name | k (×10−4) | R2 |
|---|---|---|
| NP | 24.91 | 0.316 |
| RS | 10.32 | 0.803 |
| NS | 9.42 | 0.884 |
| CO | 8.63 | 0.388 |
| SB | 6.20 | 0.927 |
| RB | 4.98 | 0.977 |
| PL | 3.33 | 0.989 |
| SF | 3.23 | 0.444 |
| SS | 2.44 | 0.844 |
| OL | 1.51 | 0.937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, S.; Tamogami, S.; Chen, J.; Zhang, H. An Evaluation Model for the Quality of Frying Oil Using Key Aldehyde Detected by HS-GC/MS. Foods 2022, 11, 2413. https://doi.org/10.3390/foods11162413
Liu X, Wang S, Tamogami S, Chen J, Zhang H. An Evaluation Model for the Quality of Frying Oil Using Key Aldehyde Detected by HS-GC/MS. Foods. 2022; 11(16):2413. https://doi.org/10.3390/foods11162413
Chicago/Turabian StyleLiu, Xiaofang, Shuo Wang, Shigeru Tamogami, Jieyu Chen, and Han Zhang. 2022. "An Evaluation Model for the Quality of Frying Oil Using Key Aldehyde Detected by HS-GC/MS" Foods 11, no. 16: 2413. https://doi.org/10.3390/foods11162413
APA StyleLiu, X., Wang, S., Tamogami, S., Chen, J., & Zhang, H. (2022). An Evaluation Model for the Quality of Frying Oil Using Key Aldehyde Detected by HS-GC/MS. Foods, 11(16), 2413. https://doi.org/10.3390/foods11162413






