Enzymatic Glycerolysis of Palm Kernel Olein-Stearin Blend for Monolaurin Synthesis as an Emulsifier and Antibacterial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fat Blends
2.3. Enzymatic Glycerolysis of PKOo-PKS Blend
2.4. Production and Isolation of Monolaurin
2.5. Determination of Emulsion Capacity and Stability
2.6. Antibacterial Assay with the Paper Disc Diffusion Method
2.7. Statistical Analysis
3. Results and Discussion
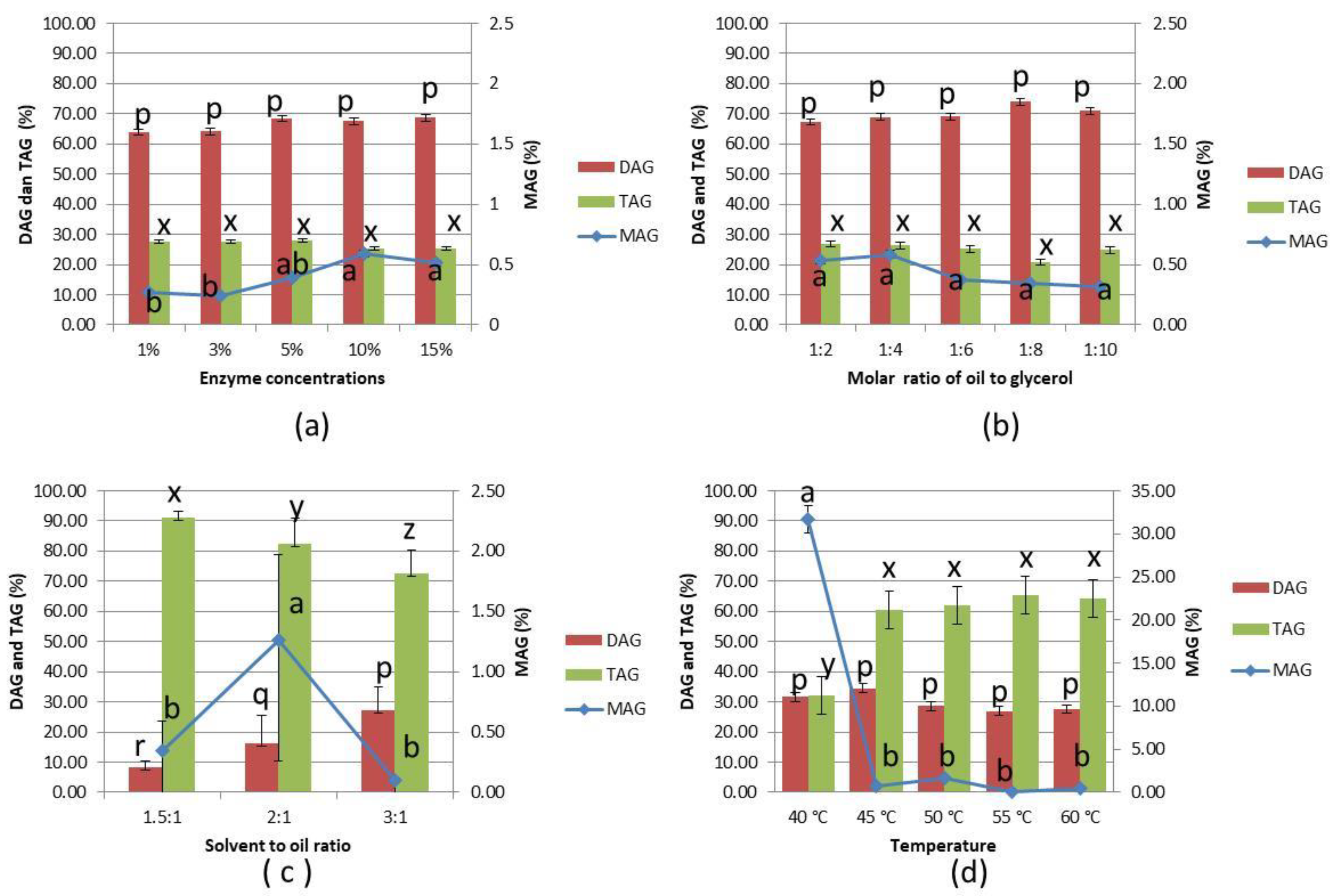
3.1. Effect of Reaction Conditions on Glycerolysis of PKOo-PKS Blend
3.2. Characterization of Monolaurin Isolated from Glycerolysis Product
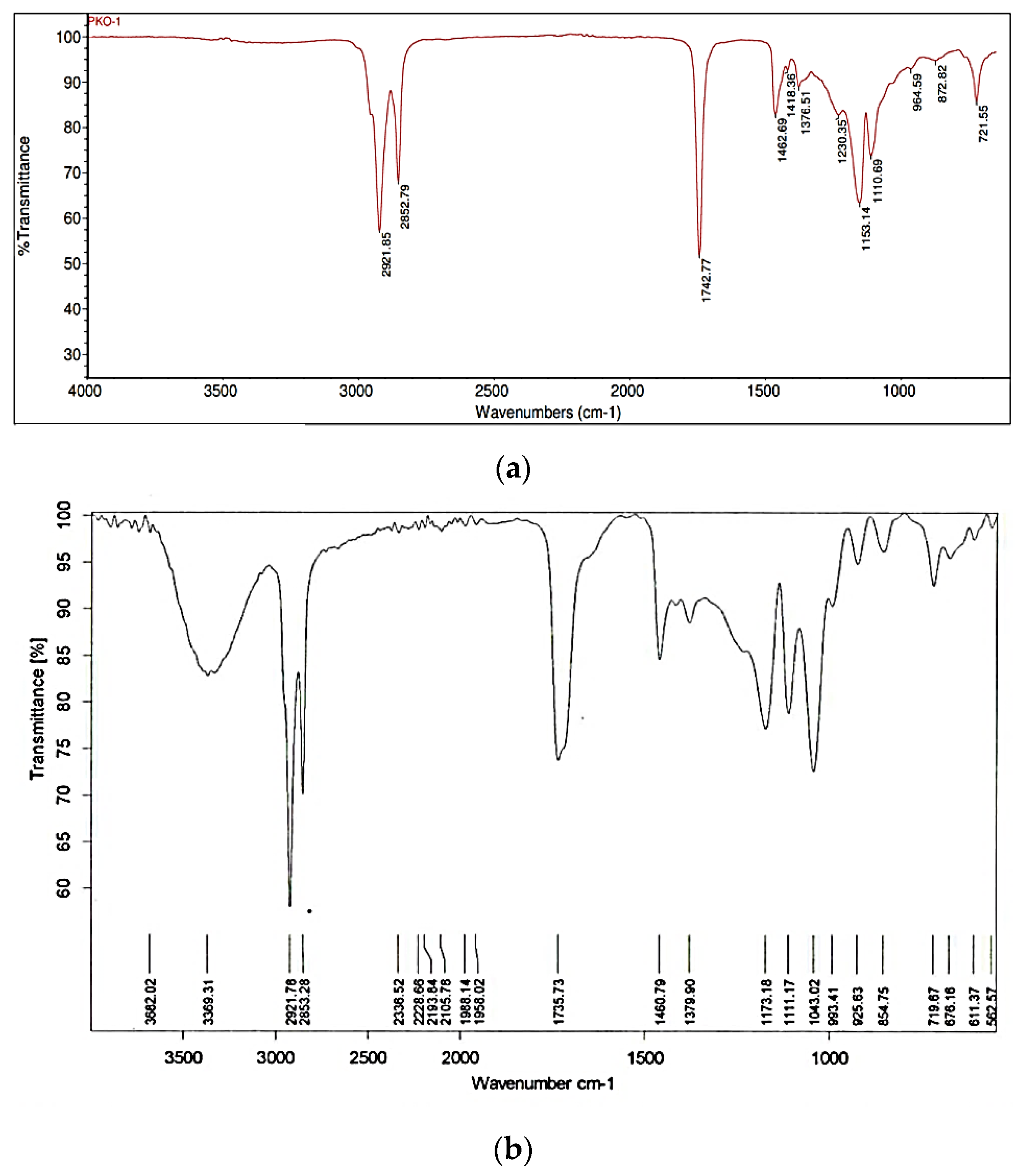
3.2.1. Analysis of FTIR (Fourier Transform Infra-Red) Monolaurin
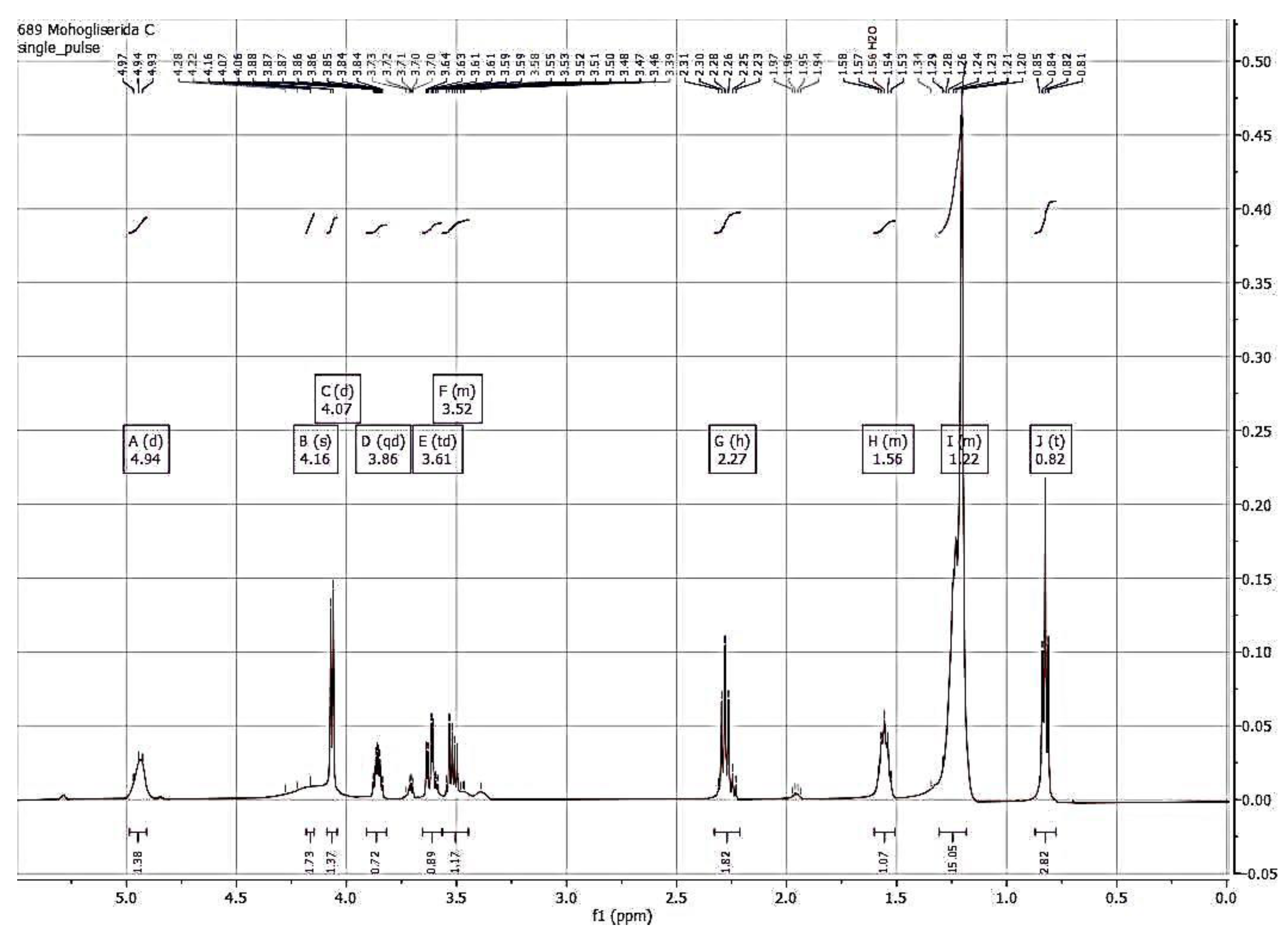
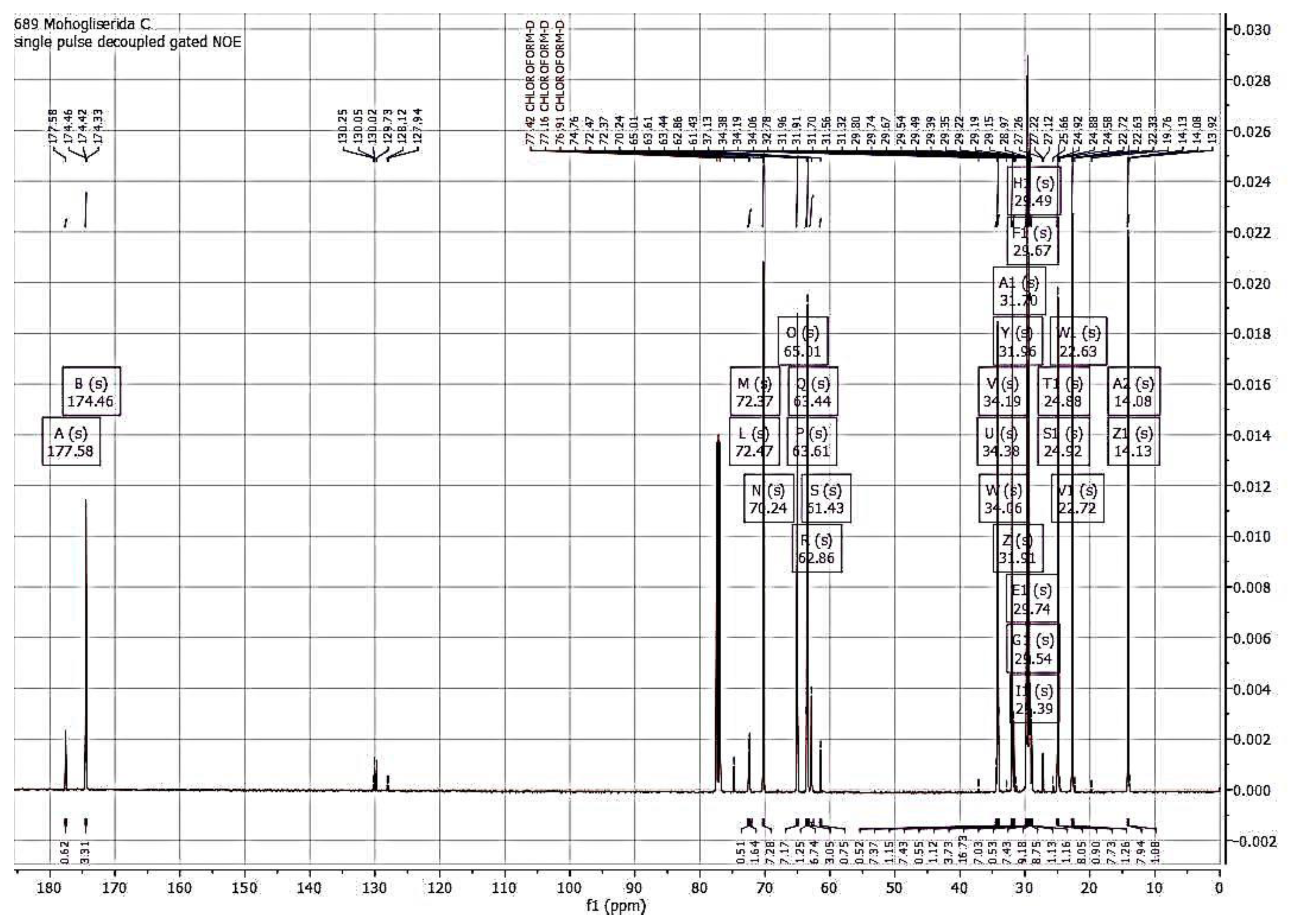
3.2.2. Analysis of NMR Monolaurin
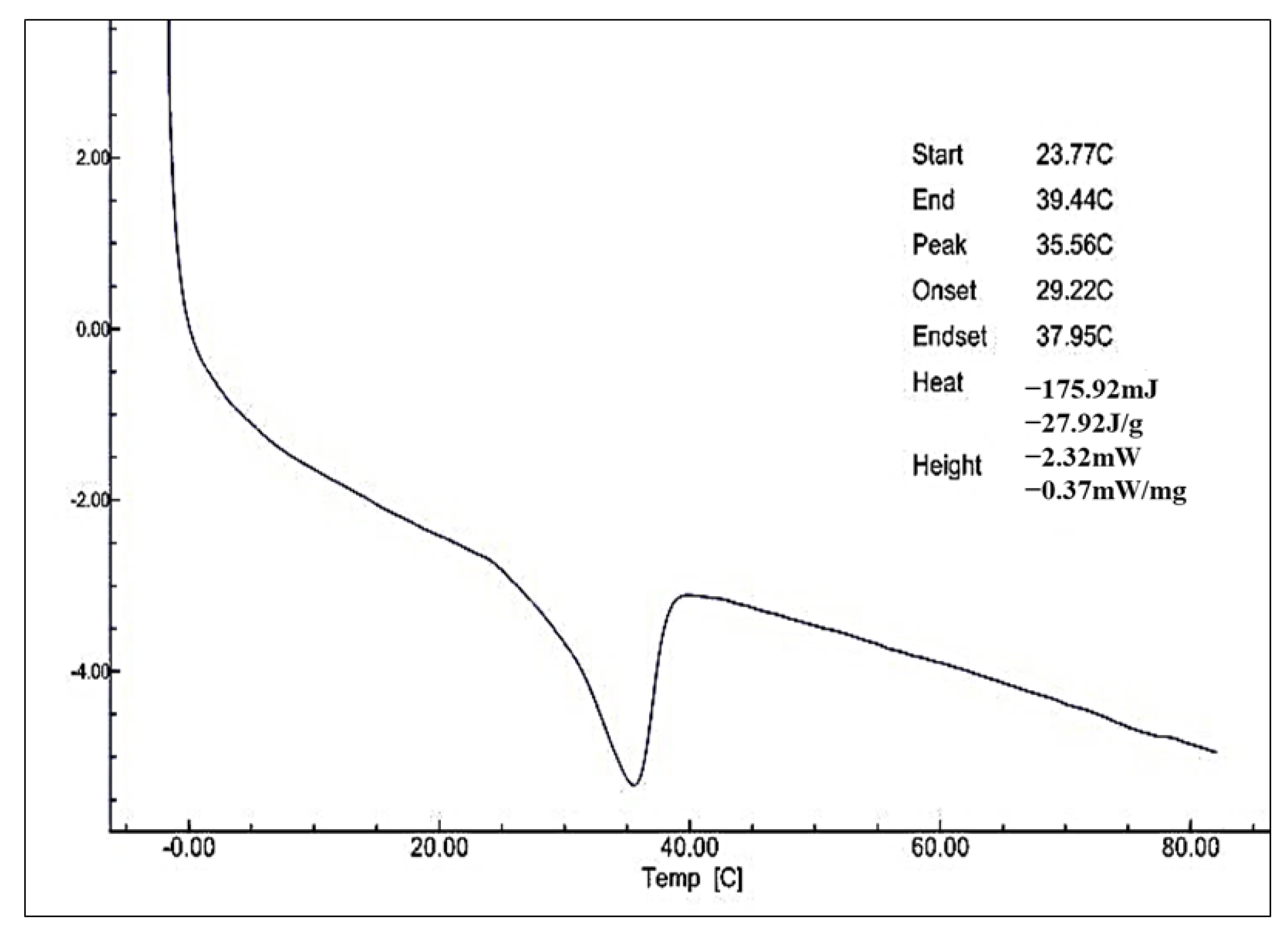
3.2.3. DSC Analysis of Monolaurin
3.3. Characterization of Monolaurin as an Emulsifier and Antibacterial
3.3.1. Physicochemical and Emulsifying Properties
3.3.2. Antibacterial Activity of Monolaurin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieberman, S.; Enig, M.G.; Preuss, H.G. A Review of Monolaurin and Lauric Acid:Natural Virucidal and Bactericidal Agents. Altern. Complement. Ther. 2006, 12, 310–314. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Jumina, J.; Siswanta, D.; Solikhah, E.N. Reaction path synthesis of monoacylglycerol from fat and oils. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 126–136. [Google Scholar]
- Jumina, J.; Lavendi, W.; Singgih, T.; Triono, S.; Kurniawan, Y.S.; Koketsu, M. Preparation of Monoacylglycerol Derivatives from Indonesian Edible Oil and Their Antimicrobial Assay against Staphylococcus aureus and Escherichia coli. Sci. Rep. 2019, 9, 10941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, H.; Cui, Y.; Zhao, G.; Feng, F. Antibacterial Interactions of Monolaurin with Commonly Used Antimicrobials and Food Components. J. Food Sci. 2009, 74, M418–M421. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Indiarto, R.; Wulandari, E.; Azimah, H.N. Oil to Glycerol Ratio in Enzymatic and Chemical Glycerolysis for the Production of Mono- and Diacylglycerol. Int. J. Eng. Trends Technol. 2021, 69, 117–125. [Google Scholar] [CrossRef]
- Ibrahim, N.A. Characteristics of malaysian palm kernel and its products. J. Oil Palm Res. 2013, 25, 245–252. [Google Scholar]
- Ngatirah, N.; Hidayat, C.; Rahayu, E.S.; Utami, T. The Role of Fat Blends in Improving the Physicochemical Properties of Palm Kernel Oil for Monolaurin Synthesis. Trends Sci. 2022, 19, 4214. [Google Scholar] [CrossRef]
- Rarokar, N.; Menghani, S.; Kerzare, D.; Khedekar, P.B. Progress in Synthesis of Monoglycerides for Use in Food and Pharmaceuticals. J. Exp. Food Chem. 2017, 3, 128. [Google Scholar] [CrossRef]
- Pinyaphong, P.; Sriburi, P.; Phutrakul, S. Synthesis of Monoacylglycerol from Glycerolysis of Crude Glycerol with Coconut Oil Catalyzed by Carica Papaya Lipase. Int. J. Chem. Mol. Eng. 2012, 6, 938–943. [Google Scholar]
- Zha, B.; Chen, Z.; Wang, L.; Wang, R.; Chen, Z.; Zheng, L. Production of glycerol monolaurate-enriched monoacylglycerols by lipase-catalyzed glycerolysis from coconut oil. Eur. J. Lipid Sci. Technol. 2014, 116, 328–335. [Google Scholar] [CrossRef]
- Su’I, M.; Sumaryati, E.; Anggraeni, F.D.; Utomo, Y.; Anggraini, N.; Rofiqoh, N.L. Monoglyceride biosynthesis from coconut milk with lypase enzyme of sesame seed sprouts as biocatalyst. Food Sci. Technol. 2021, 41, 328–333. [Google Scholar] [CrossRef]
- Widiyarti, G.; Hanafi, M.; Soewarso, W.P. Study on the synthesis of monolaurin as antibacterial agent againts staphylococcus aureus. Indones. J. Chem. 2010, 9, 99–106. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Jumina; Siswanta, D.; Sholikhah, E.N.; Fitriastuti, D. Synthesis and Antibacterial Activity of 2-Monolaurin. Orient. J. Chem. 2016, 32, 3113–3120. [Google Scholar] [CrossRef]
- Ngatirah, N.; Hidayat, C.; Rahayu, E.; Utami, T. Enzymatic Glycerolysis of Palm Kernel Olein and Palm Kernel Stearin in different Ratios for Monolaurin Synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012018. [Google Scholar] [CrossRef]
- Cano-Medina, A.; Jiménez-Islas, H.; Dendooven, L.; Herrera, R.P.; González-Alatorre, G.; Escamilla-Silva, E.M. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 2011, 44, 684–692. [Google Scholar] [CrossRef]
- Priscilia, B.; Nugraha, M.F.I.; Novita, H.; Elya, B. Antioxidant and Antibacterial Assay Against Fish Pathogen Bacteria of Kjellbergiodendron celebicum (Koord.) Merr. Leaf Extract. Pharmacogn. J. 2020, 12, 173–179. [Google Scholar] [CrossRef]
- Diao, X.; Guan, H.; Kong, B.; Zhao, X. Preparation of Diacylglycerol from Lard by Enzymatic Glycerolysis and Its Compositional Characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 813–822. [Google Scholar] [CrossRef]
- Solaesa, G.; Sanz, M.T.; Falkeborg, M.; Beltrán, S.; Guo, Z. Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chem. 2016, 190, 960–967. [Google Scholar] [CrossRef]
- Satyawali, Y.; Cauwenberghs, L.; Maesen, M.; Dejonghe, W. Lipase catalyzed solvent free synthesis of monoacylglycerols in various reaction systems and coupling reaction with pervaporation for in situ water removal. Chem. Eng. Process. Process Intensif. 2021, 166, 108475. [Google Scholar] [CrossRef]
- Cheirsilp, B.; H-Kittikul, A. A mathematical model approach to a glycerolysis reaction for monoacylglycerol production. WIT Trans. Model. Simul. 2007, 46, 225–232. [Google Scholar] [CrossRef]
- Sanz, M.T.; Solaesa, G.; Melgosa, R.; Bucio, S.L.; Beltrán, S. Glycerolysis of sardine oil catalyzed by a water dependent lipase in different tert-alcohols as reaction medium. Grasas Aceites 2015, 66, e102. [Google Scholar] [CrossRef]
- Choong, T.S.Y.; Yeoh, C.M.; Phuah, E.-T.; Siew, W.-L.; Lee, Y.-Y.; Tang, T.-K.; Abdullah, L.C. Kinetic study of lipase-catalyzed glycerolysis of palm olein using Lipozyme TLIM in solvent-free system. PLoS ONE 2018, 13, e0192375. [Google Scholar] [CrossRef]
- Satriana; Arpi, N.; Lubis, Y.M.; Adisalamun; Supardan, M.D.; Mustapha, W.A.W. Diacylglycerol-enriched oil production using chemical glycerolysis. Eur. J. Lipid Sci. Technol. 2016, 118, 1880–1890. [Google Scholar] [CrossRef]
- Subroto, E. Monoacylglycerols and diacylglycerols for fat-based food products: A review. Food Res. 2020, 4, 932–943. [Google Scholar] [CrossRef]
- Subroto, E.; Supriyanto; Utami, T.; Hidayat, C. Enzymatic glycerolysis–interesterification of palm stearin–olein blend for synthesis structured lipid containing high mono- and diacylglycerol. Food Sci. Biotechnol. 2019, 28, 511–517. [Google Scholar] [CrossRef]
- Solaesa, A.G.; Sanz, M.T.; Beltran, S.; Melgosa, R. Kinetic study and kinetic parameters of lipase-catalyzed glycerolysis of sardine oil in a homogeneous medium. Chin. J. Catal. 2016, 37, 596–606. [Google Scholar] [CrossRef]
- Skoronski, E.; Padoin, N.; Soares, C.; Furigo, A., Jr. Stability of immobilized Rhizomucor miehei lipase for the synthesis of pentyl octanoate in a continuous packed bed bioreactor. Braz. J. Chem. Eng. 2014, 31, 633–641. [Google Scholar] [CrossRef]
- Shahrin, N.A.M.; Chin, P.W.; Serri, N.A. Crude glycerol utilisation in monolaurin production using immobilised rhizomucor miehei lipase: Optimisation and thermodynamics study. J. Oil Palm Res. 2019, 31, 615–623. [Google Scholar] [CrossRef]
- Sangadah, K.; Handayani, S.; Setiasih, S.; Hudiyono, S. Enzymatic synthesis of glycerol ester hydrolyzed coconut oil fatty acid and lauric acid as emulsifier and antimicrobial compound. AIP Conf. Proc. 2018, 2023, 020111. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Jumina, J.; Siswanta, D.; Sholikhah, E.N.; Fitriastuti, D. Synthesis and Antibacterial Activity 1-Monolaurin. Orient. J. Chem. 2018, 34, 863–867. [Google Scholar] [CrossRef]
- Yee, M.M. Preparation of An Effective Antimicrobial Agent from Virgin Coconut Oil. Dragon Univ. Res. J. 2011, 3, 107–113. [Google Scholar]
- Tough, M.; Mengesha, A. Photostability of nifedipine in monolaurin solid matrix and micellar solution. J. Appl. Pharm. Sci. 2015, 5, 066–073. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Limmatvapirat, C.; Nunthanid, J.; Luangtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S. Design and characterization of monolaurin loaded electrospun shellac nanofibers with antimicrobial activity. Asian J. Pharm. Sci. 2018, 13, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Indiarto, R. Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: A review. Food Res. 2020, 4, 2355–2365. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef]
- Park, K.-M.; Lee, S.J.; Yu, H.; Park, J.-Y.; Jung, H.-S.; Kim, K.; Lee, C.J.; Chang, P.-S. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: Significant factors affecting antibacterial activity of lauric acid esters. Food Sci. Biotechnol. 2018, 27, 401–409. [Google Scholar] [CrossRef]
- Tajik, H.; Raeisi, M.; Razavi Rohani, S.M.; Hashemi, M.; Aminzare, M.; Naghili, H.; Rozbani, D.; Ben Ammar, D. Effect of monolaurin alone and in combination with EDTA on viability of Escherichia coli and Staphylococcus aureus in culture media and Iranian white cheese. J. Food Qual. Hazards Control. 2014, 1, 108–112. [Google Scholar]
- Schlievert, P.M.; Kilgore, S.H.; Kaus, G.M.; Ho, T.D.; Ellermeier, C.D. Glycerol Monolaurate (GML) and a Nonaqueous Five-Percent GML Gel Kill Bacillus and Clostridium Spores. mSphere 2018, 3, e00597-18. [Google Scholar] [CrossRef]
- Zare, M.A.; Rohani, S.M.R.; Raeisi, M.; Hosseini, S.H.J.; Hashemi, M. Antibacterial effects of monolaurin, sorbic acid and potassium sorbate on Staphylococcus aureus and Escherichia coli. J. Food Qual. Hazards Control. 2014, 1, 52–55. [Google Scholar]
- Sadiq, S.; Imran, M.; Habib, H.; Shabbir, S.; Ihsan, A.; Zafar, Y.; Hafeez, F.Y. Potential of monolaurin based food-grade nano-micelles loaded with nisin Z for synergistic antimicrobial action against Staphylococcus aureus. LWT-Food Sci. Technol. 2016, 71, 227–233. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Zhang, G.; Chen, F.; Xu, B. In Vitro Antibacterial Activities and Mechanisms of Action of Fatty Acid Monoglycerides Against Four Foodborne Bacteria. J. Food Prot. 2020, 83, 331–337. [Google Scholar] [CrossRef]
- Buňková, L.; Buňka, F.; Janiš, R.; Krejčí, J.; Doležálková, I.; Pospíšil, Z.; Růžička, J.; Tremlová, B. Comparison of antibacterial effect of seven 1-monoglycerides on food-borne pathogens or spoilage bacteria. Acta Vet. Brno 2011, 80, 029–039. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Xiong, J.; Lou, W.; Ning, Z.; Zhang, D.; Yang, J. The key lethal effect existed in the antibacterial behavior of short, medium, and long chain fatty acid monoglycerides on Escherichia coli. bioRxiv 2018, 339309. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
| No | Parameter | Unit | Value |
|---|---|---|---|
| 1 | Saponification value | mg KOH/g | 193.6 ± 0.83 |
| 2 | Iodine value | wijs | 0.82 ± 0.14 |
| 3 | Melting point | °C | 35.56 |
| 4 | MAG | % | 83.89 ± 5.52 |
| 5 | Emulsion capacity (%) | % | 93.66 ± 1.85 |
| 6 | Emulsion stability | % | 89.54 ± 3.36 |
| 7 | HLB value | 5.97 ± 0.06 | |
| 8 | Emulsion type | w/o |
| Bacteria | Monolaurin Dose ** (ppm) | Solvent * | |
|---|---|---|---|
| Aquadest | Alcohol | ||
| E. coli FNCC 0091 | 100 | 0.00 ± 0.00 c | 6.00 ± 1.41c |
| 500 | 7.50 ± 0.00 b | 8.83 ± 1.44 bc | |
| 1000 | 8.00 ± 0.50 b | 10.50 ± 2.29 bc | |
| 2500 | 10.33 ± 0.76 ab | 12.08 ± 3.09 ab | |
| 5000 | 13.33 ± 2.36 a | 13.67 ± 3.88 ab | |
| B. subtilis FNCC 0060 | 100 | 7.15 ± 0.92 ab | 9.00 ± 2.12 cd |
| 500 | 7.15 ± 0.92 ab | 11.25 ± 1.06 c | |
| 1000 | 7.50 ± 1.41 ab | 12.25 ± 0.35 b | |
| 2500 | 9.17 ± 1.26 a | 12.50 ± 0.50 b | |
| 5000 | 10.75 ± 1.56 a | 16.75 ± 3.70 a | |
| S. aureus FNCC 0047 | 100 | 0.00 ± 0.00 d | 9.00 ± 1.41 c |
| 500 | 8.33 ± 2.36 c | 9.67 ± 1.44 c | |
| 1000 | 10.33 ± 2.75 c | 10.17 ± 2.47 c | |
| 2500 | 14.00 ± 1.50 b | 13.33 ± 3.33 b | |
| 5000 | 17.27 ± 1.54 a | 15.17 ± 2.75 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngatirah, N.; Hidayat, C.; Rahayu, E.S.; Utami, T. Enzymatic Glycerolysis of Palm Kernel Olein-Stearin Blend for Monolaurin Synthesis as an Emulsifier and Antibacterial. Foods 2022, 11, 2412. https://doi.org/10.3390/foods11162412
Ngatirah N, Hidayat C, Rahayu ES, Utami T. Enzymatic Glycerolysis of Palm Kernel Olein-Stearin Blend for Monolaurin Synthesis as an Emulsifier and Antibacterial. Foods. 2022; 11(16):2412. https://doi.org/10.3390/foods11162412
Chicago/Turabian StyleNgatirah, Ngatirah, Chusnul Hidayat, Endang S. Rahayu, and Tyas Utami. 2022. "Enzymatic Glycerolysis of Palm Kernel Olein-Stearin Blend for Monolaurin Synthesis as an Emulsifier and Antibacterial" Foods 11, no. 16: 2412. https://doi.org/10.3390/foods11162412
APA StyleNgatirah, N., Hidayat, C., Rahayu, E. S., & Utami, T. (2022). Enzymatic Glycerolysis of Palm Kernel Olein-Stearin Blend for Monolaurin Synthesis as an Emulsifier and Antibacterial. Foods, 11(16), 2412. https://doi.org/10.3390/foods11162412





