Predicting Mandarin Fruit Acceptability: From High-Field to Benchtop NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, R.; Robert, J.B. A Brief History of High Resolution NMR BT—NMR at Very High Field; Robert, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–16. [Google Scholar]
- Blümich, B. Low-field and benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef]
- Giberson, J.; Scicluna, J.; Legge, N.; Longstaffe, J. Chapter Three—Developments in benchtop NMR spectroscopy 2015–2020. Annu. Rep. NMR Spectrosc. 2021, 102, 153–246. [Google Scholar]
- Halse, M.E. Perspectives for hyperpolarisation in compact NMR. TrAC Trends Anal. Chem. 2016, 83, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Kunjir, S.; Rodriguez-Zubiri, M.; Coeffard, V.; Felpin, F.-X.; Giraudeau, P.; Farjon, J. Merging Gradient-Based Methods to Improve Benchtop NMR Spectroscopy: A New Tool for Flow Reaction Optimization. ChemPhysChem 2020, 21, 2311–2319. [Google Scholar] [CrossRef]
- van Beek, T.A. Low-field benchtop NMR spectroscopy: Status and prospects in natural product analysis. Phytochem. Anal. 2021, 32, 24–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, J.E.; Bell, N.G.A. Molecular Properties of Caffeine Explored by NMR: A Benchtop NMR Experiment for Undergraduate Physical-Chemistry Laboratories. J. Chem. Educ. 2019, 96, 786–791. [Google Scholar] [CrossRef]
- Lee, Y.; Matviychuk, Y.; Holland, D.J. Quantitative analysis using external standards with a benchtop NMR spectrometer. J. Magn. Reson. 2020, 320, 106826. [Google Scholar] [CrossRef] [PubMed]
- Araneda, J.F.; Mendonça Barbosa, T.; Hui, P.; Leclerc, M.C.; Ma, J.; Maier, A.F.G.; Riegel, S.D. Incorporating Benchtop NMR Spectrometers in the Undergraduate Lab: Understanding Resolution and Circumventing Second-Order Effects. J. Chem. Educ. 2021, 98, 1227–1232. [Google Scholar] [CrossRef]
- Araneda, J.F.; Chu, T.; Leclerc, M.C.; Riegel, S.D.; Spingarn, N. Quantitative analysis of cannabinoids using benchtop NMR instruments. Anal. Methods 2020, 12, 4853–4857. [Google Scholar] [CrossRef]
- Assemat, G.; Balayssac, S.; Gerdova, A.; Gilard, V.; Caillet, C.; Williamson, D.; Malet-Martino, M. Benchtop low-field 1H Nuclear Magnetic Resonance for detecting falsified medicines. Talanta 2019, 196, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Keizers, P.H.J.; Bakker, F.; Ferreira, J.; Wackers, P.F.K.; van Kollenburg, D.; van der Aa, E.; van Beers, A. Benchtop NMR spectroscopy in the analysis of substandard and falsified medicines as well as illegal drugs. J. Pharm. Biomed. Anal. 2020, 178, 112939. [Google Scholar] [CrossRef]
- Isaac-Lam, M.F. Determination of alcohol content in alcoholic beverages using 45 MHz benchtop NMR spectrometer. Int. J. Spectrosc. 2016, 2016, 2526946. [Google Scholar] [CrossRef] [PubMed]
- Defernez, M.; Wren, E.; Watson, A.D.; Gunning, Y.; Colquhoun, I.J.; Le Gall, G.; Williamson, D.; Kemsley, E.K. Low-field 1H NMR spectroscopy for distinguishing between arabica and robusta ground roast coffees. Food Chem. 2017, 216, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, P.A.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, M.A.; Antiochia, R.; Capitani, D. Untargeted NMR-Based Methodology in the Study of Fruit Metabolites. Molecules 2015, 20, 4088–4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunning, Y.; Jackson, A.J.; Colmer, J.; Taous, F.; Philo, M.; Brignall, R.M.; El Ghali, T.; Defernez, M.; Kemsley, E.K. High-throughput screening of argan oil composition and authenticity using benchtop 1H NMR. Magn. Reson. Chem. 2020, 58, 1177–1186. [Google Scholar] [PubMed] [Green Version]
- Gunning, Y.; Taous, F.; El Ghali, T.; Gibbon, J.D.; Wilson, E.; Brignall, R.M.; Kemsley, E.K. Mitigating instrument effects in 60 MHz 1H NMR spectroscopy for authenticity screening of edible oils. Food Chem. 2022, 370, 131333. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Marincola, F.C.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Antonelo, D.S.; Cônsolo, N.R.B.; Gómez, J.F.M.; Beline, M.; Pavan, B.; Souza, C.; Goulart, R.S.; Colnago, L.A.; Silva, S.L. NMR-based metabolomics to assess metabolites correlated with beef sensory properties. In Proceedings of the 65th International Congress of Meat Science and Technology, Berlin, Germany, 4–9 August 2019; pp. 801–803. [Google Scholar]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and meat metabolomics in domestic animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Swarup, S. Metabolomics for evaluating flavor-associated metabolites in plant-based products. Metabolites 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Salvino, R.A.; Colella, M.F.; De Luca, G. NMR-based metabolomics analysis of Calabrian citrus fruit juices and its application to industrial process quality control. Food Control 2021, 121, 107619. [Google Scholar] [CrossRef]
- Cirillo, A.; Magri, A.; Scognamiglio, M.; D’Abrosca, B.; Fiorentino, A.; Petriccione, M.; Di Vaio, C. Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.). Horticulturae 2022, 8, 384. [Google Scholar] [CrossRef]
- Migues, I.; Hodos, N.; Moltini, A.I.; Gámbaro, A.; Rivas, F.; Moyna, G.; Heinzen, H. 1H NMR metabolic profiles as selection tools of new mandarin cultivars based on fruit acceptability. Sci. Hortic. 2021, 287, 110262. [Google Scholar] [CrossRef]
- Castaing-Cordier, T.; Ladroue, V.; Besacier, F.; Bulete, A.; Jacquemin, D.; Giraudeau, P.; Farjon, J. High-field and benchtop NMR spectroscopy for the characterization of new psychoactive substances. Forensic Sci. Int. 2021, 321, 110718. [Google Scholar] [CrossRef] [PubMed]
- Alamprese, C. Food Authentication. In Spectroscopic Methods in Food Analysis; Franca, A.S., Nollet, L.M.L., Eds.; CRC Press: Miami, FL, USA, 2018; pp. 327–352. [Google Scholar]
- Schiffman, S.S.; Booth, B.J.; Losee, M.L.; Pecore, S.D.; Warwick, Z.S. Bitterness of sweeteners as a function of concentration. Brain Res. Bull. 1995, 36, 505–513. [Google Scholar] [CrossRef]
- Stampanoni, C.R. Influence of acid and sugar content on sweetness, sourness and the flavour profile of beverages and sherbets. Food Qual. Prefer. 1993, 4, 169–176. [Google Scholar] [CrossRef]
- Matviychuk, Y.; Yeo, J.; Holland, D.J. A field-invariant method for quantitative analysis with benchtop NMR. J. Magn. Reson. 2019, 298, 35–47. [Google Scholar] [CrossRef]
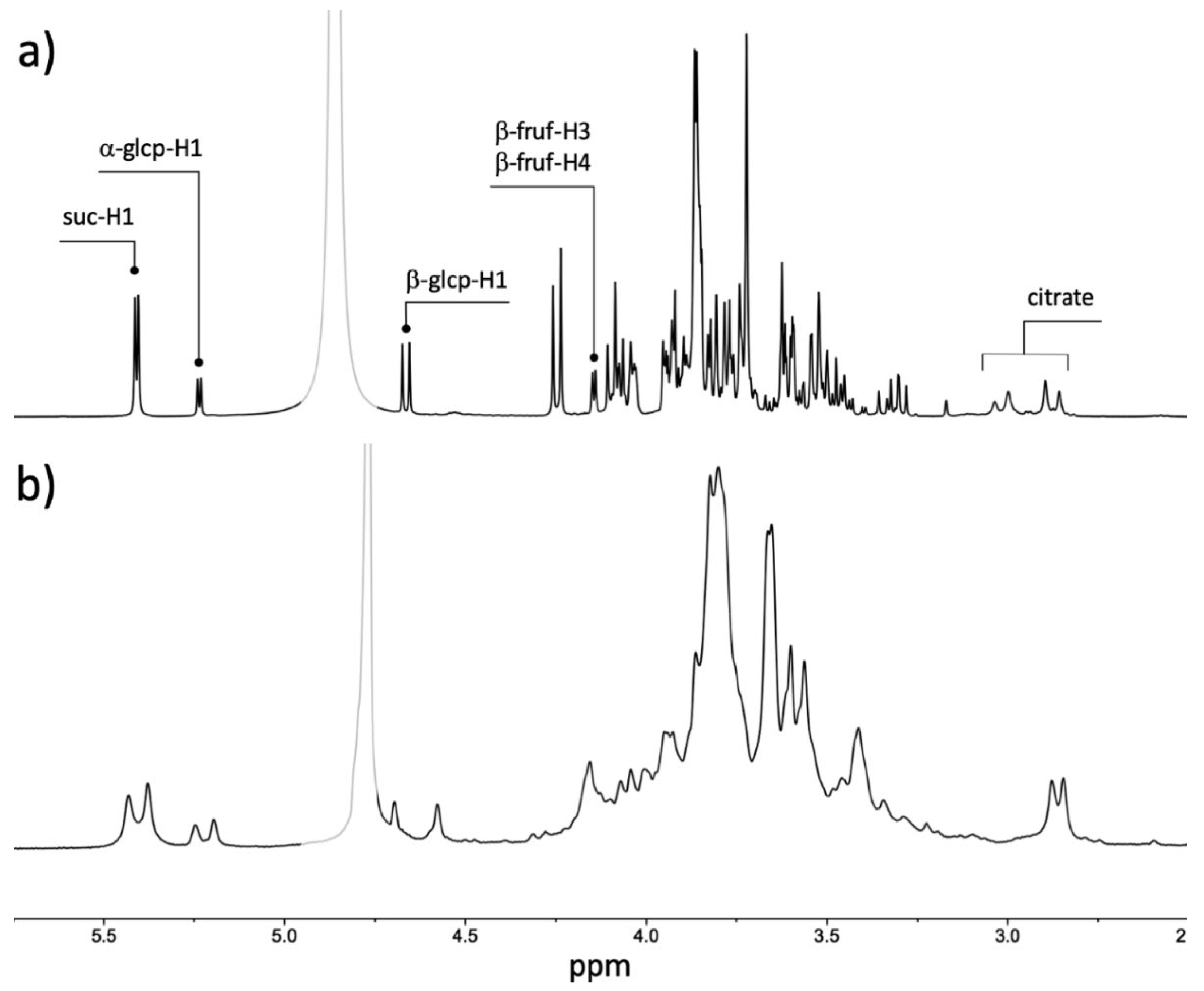
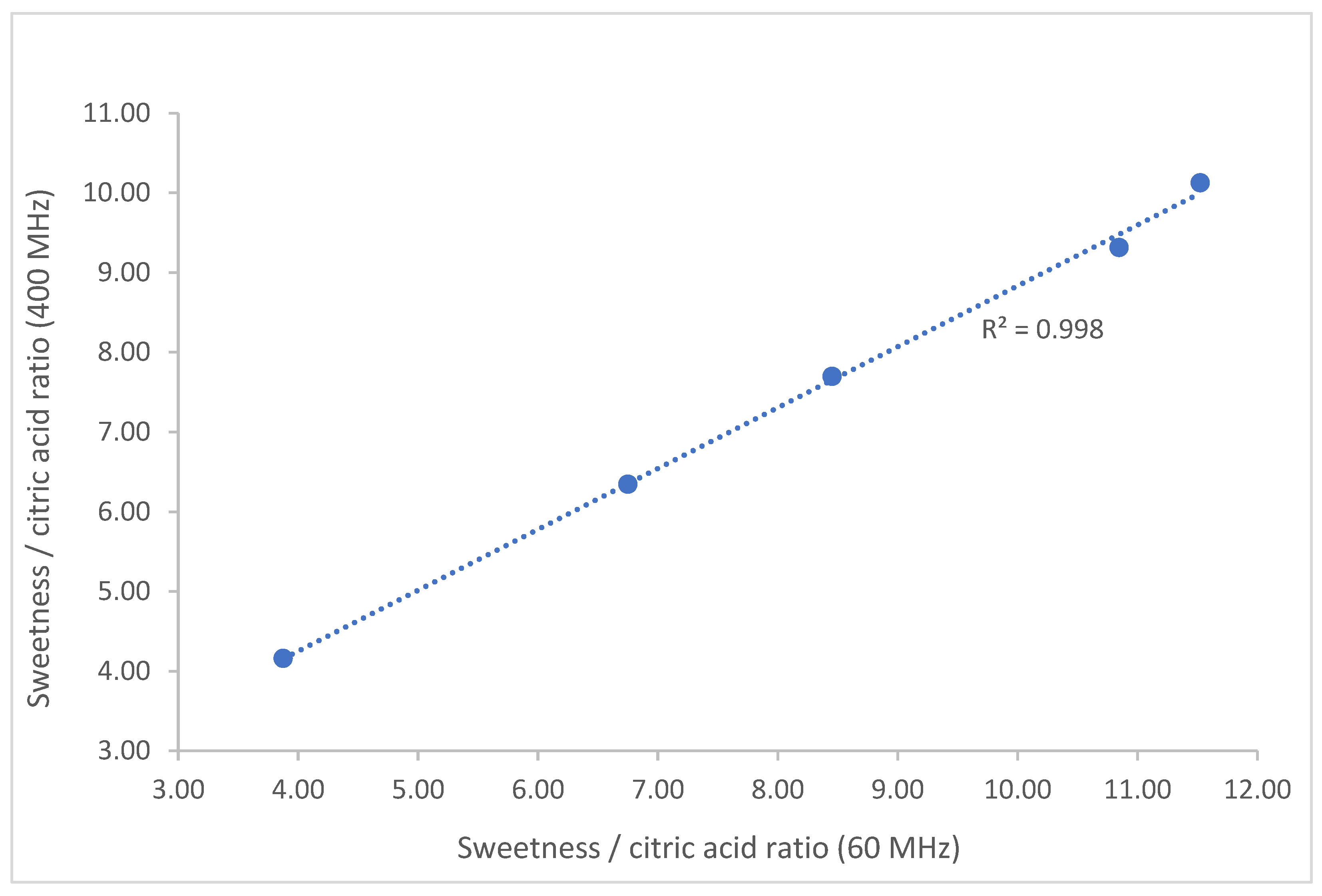
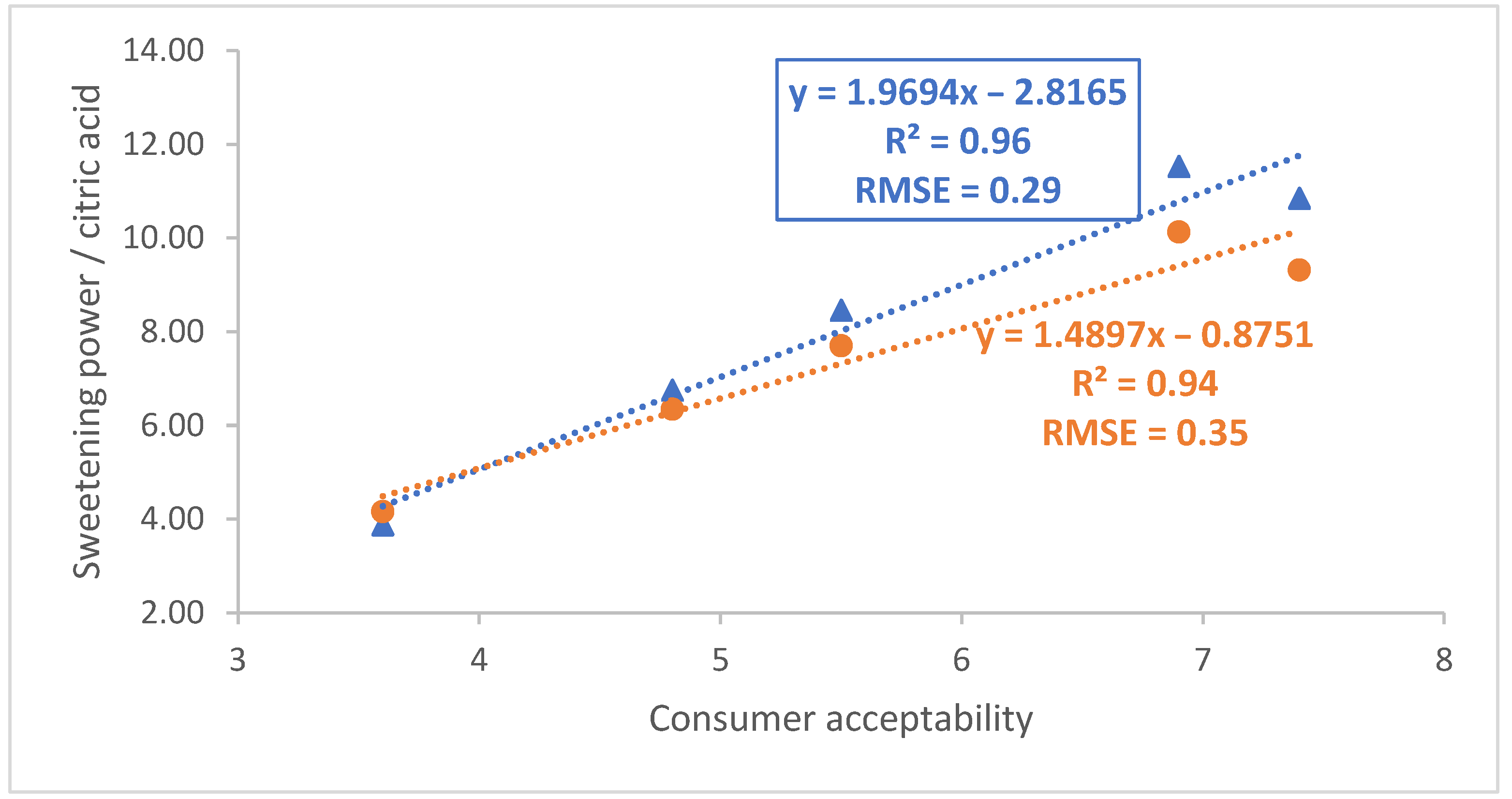
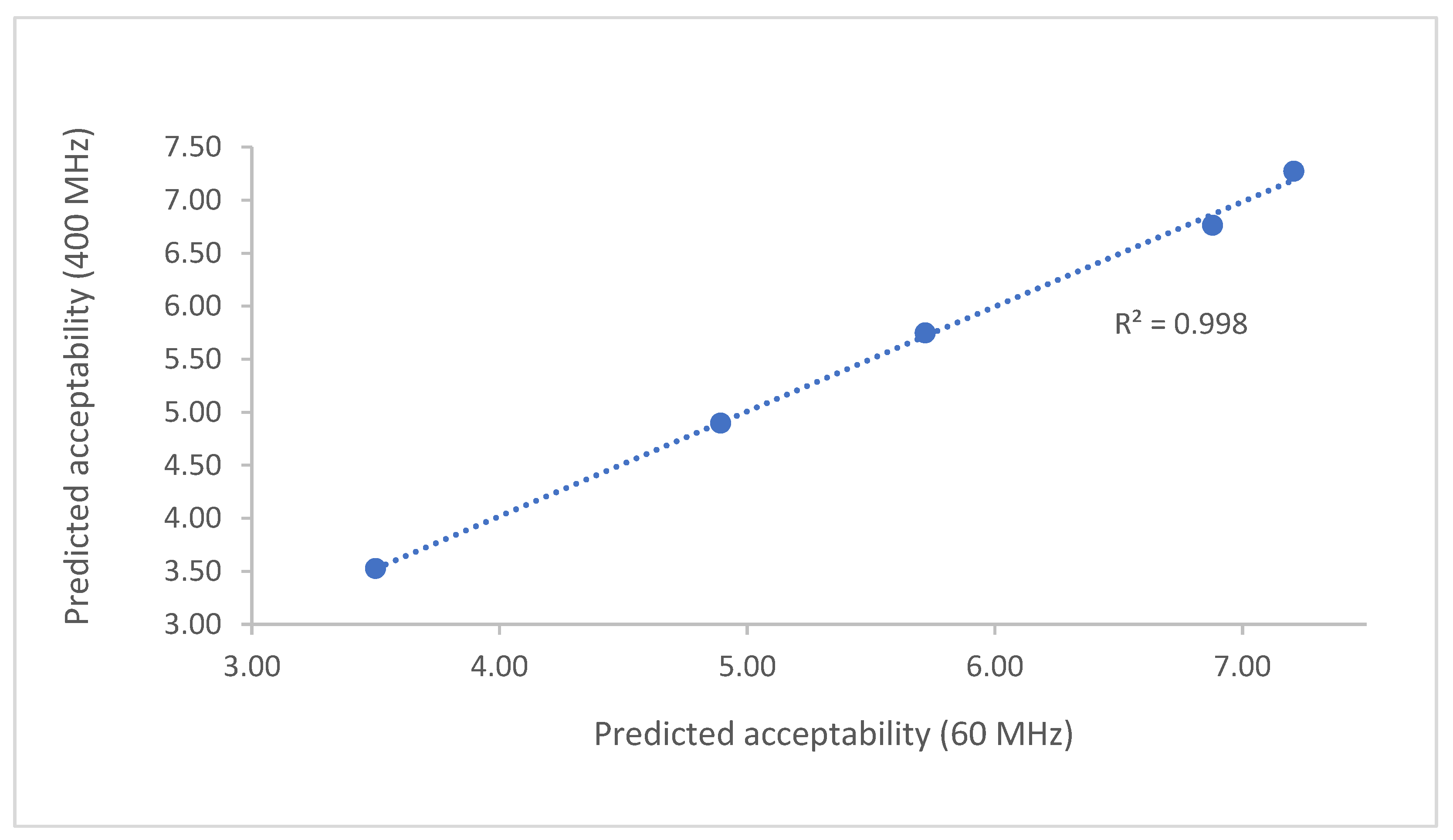
| Variety | Acceptability | 60 MHz | 400 MHz | ||||
|---|---|---|---|---|---|---|---|
| Sweetening Power/Citric Acid * | Predicted Acceptability | RMSE | Sweetening Power/Citric Acid * | Predicted Acceptability | RMSE | ||
| B475B | 7.4 | 10.85 | 6.88 | 0.29 | 9.31 | 6.76 | 0.35 |
| F7P3 | 6.9 | 11.53 | 7.21 | 10.12 | 7.27 | ||
| B475A | 5.5 | 8.45 | 5.72 | 7.70 | 5.75 | ||
| B79 | 4.8 | 6.75 | 4.89 | 6.34 | 4.90 | ||
| M16 | 3.6 | 3.88 | 3.50 | 4.16 | 3.53 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migues, I.; Rivas, F.; Moyna, G.; Kelly, S.D.; Heinzen, H. Predicting Mandarin Fruit Acceptability: From High-Field to Benchtop NMR Spectroscopy. Foods 2022, 11, 2384. https://doi.org/10.3390/foods11162384
Migues I, Rivas F, Moyna G, Kelly SD, Heinzen H. Predicting Mandarin Fruit Acceptability: From High-Field to Benchtop NMR Spectroscopy. Foods. 2022; 11(16):2384. https://doi.org/10.3390/foods11162384
Chicago/Turabian StyleMigues, Ignacio, Fernando Rivas, Guillermo Moyna, Simon D. Kelly, and Horacio Heinzen. 2022. "Predicting Mandarin Fruit Acceptability: From High-Field to Benchtop NMR Spectroscopy" Foods 11, no. 16: 2384. https://doi.org/10.3390/foods11162384
APA StyleMigues, I., Rivas, F., Moyna, G., Kelly, S. D., & Heinzen, H. (2022). Predicting Mandarin Fruit Acceptability: From High-Field to Benchtop NMR Spectroscopy. Foods, 11(16), 2384. https://doi.org/10.3390/foods11162384








