Intelligent Evaluation of Stone Cell Content of Korla Fragrant Pears by Vis/NIR Reflection Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Korla Fragrant Pears and Pretreatment
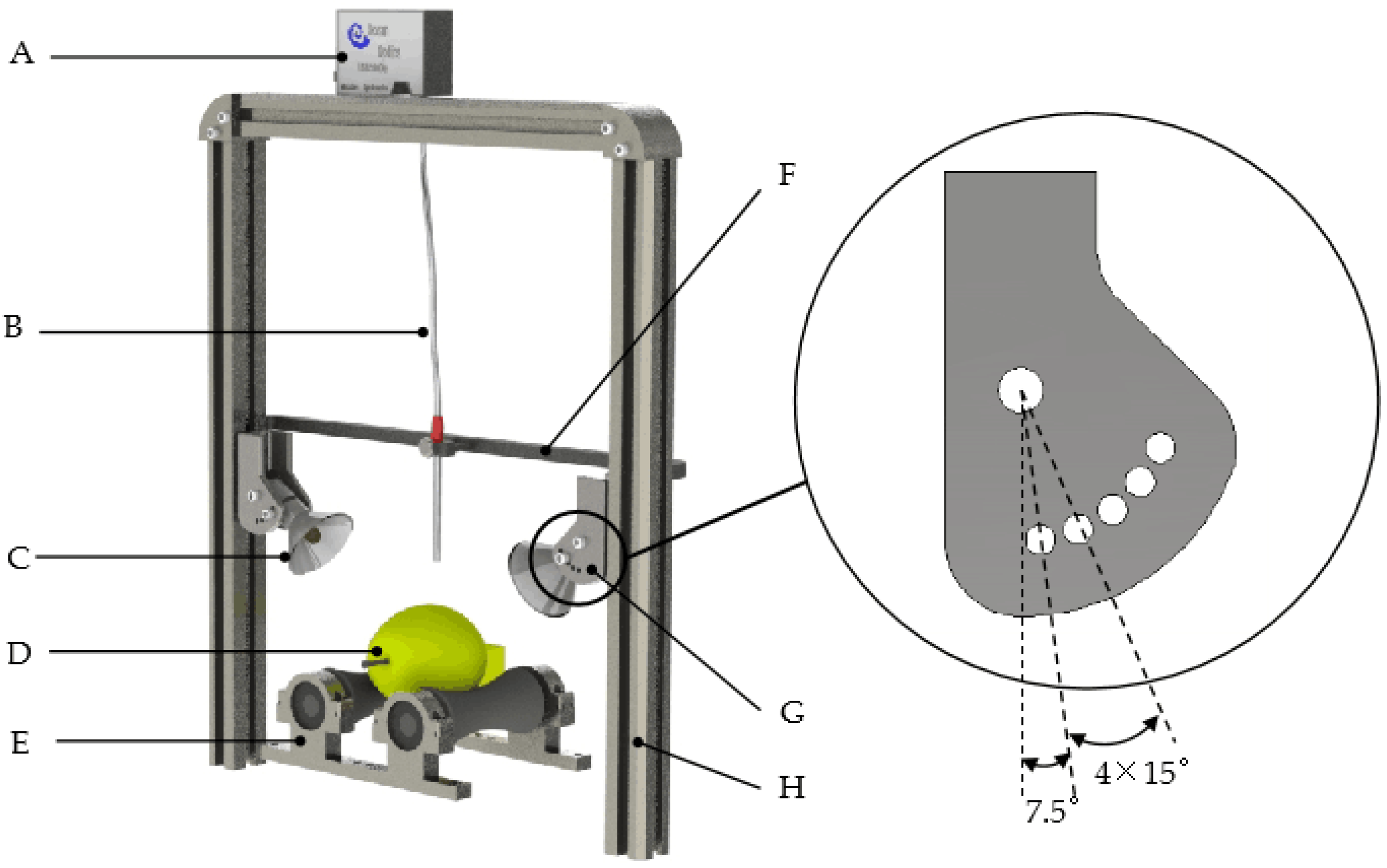
2.2. Vis/NIR Spectroscopy System and Diffuse Reflectance Spectra Acquisition
2.3. Measurement of SCC
2.4. Spectral Preprocessing and Sample Set Division
2.5. Algorithms of Selecting Characteristic Wavelengths
2.5.1. SPA
2.5.2. UVE Combined with Monte Carlo Sampling (MCUVE) and PLSR
2.6. Modeling Algorithm
3. Results
3.1. Statistics of SCC Measured Values
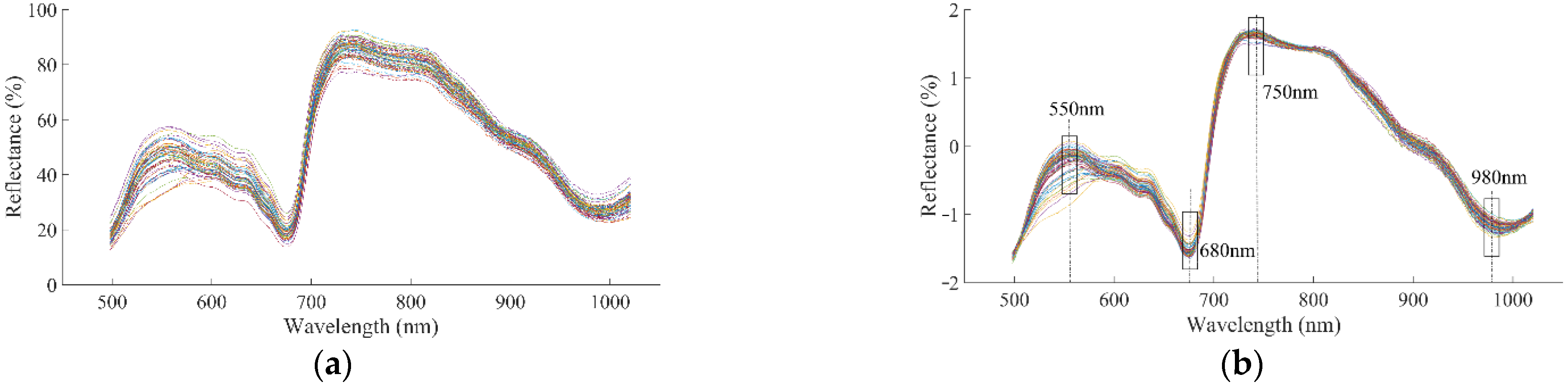
3.2. Spectral Characteristics and Different Preprocessing Methods
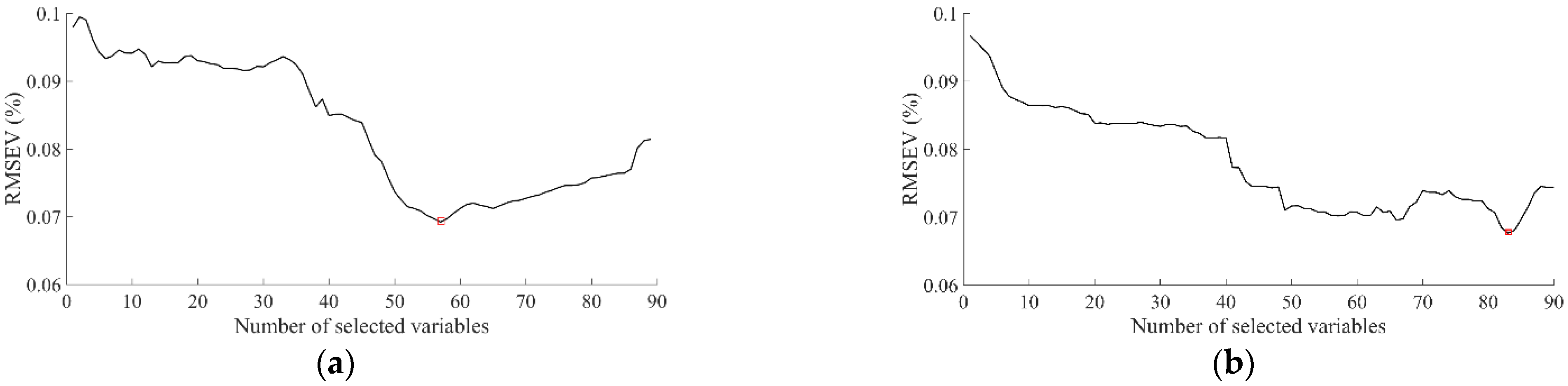
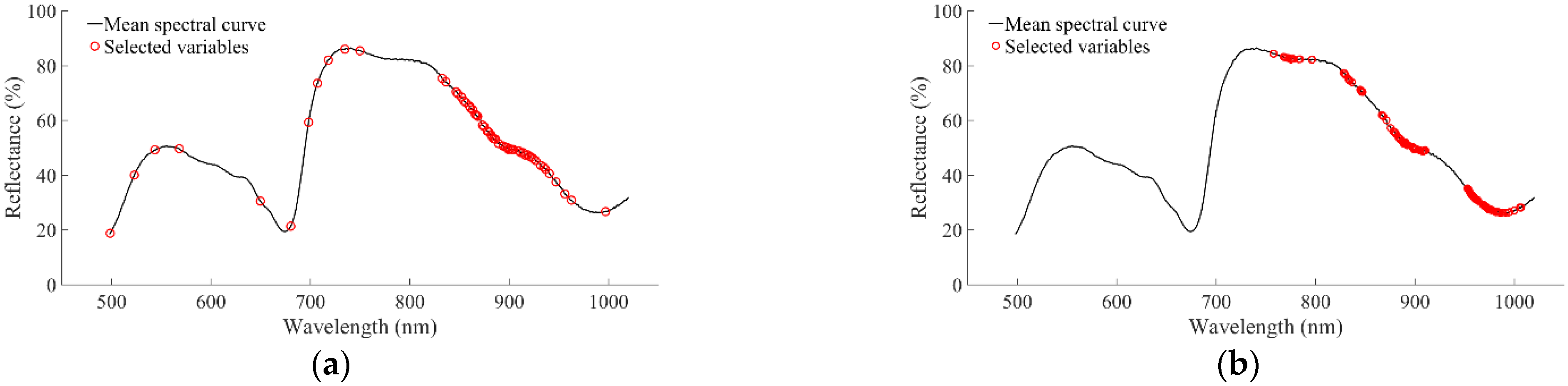
3.3. Characteristic Wavelengths
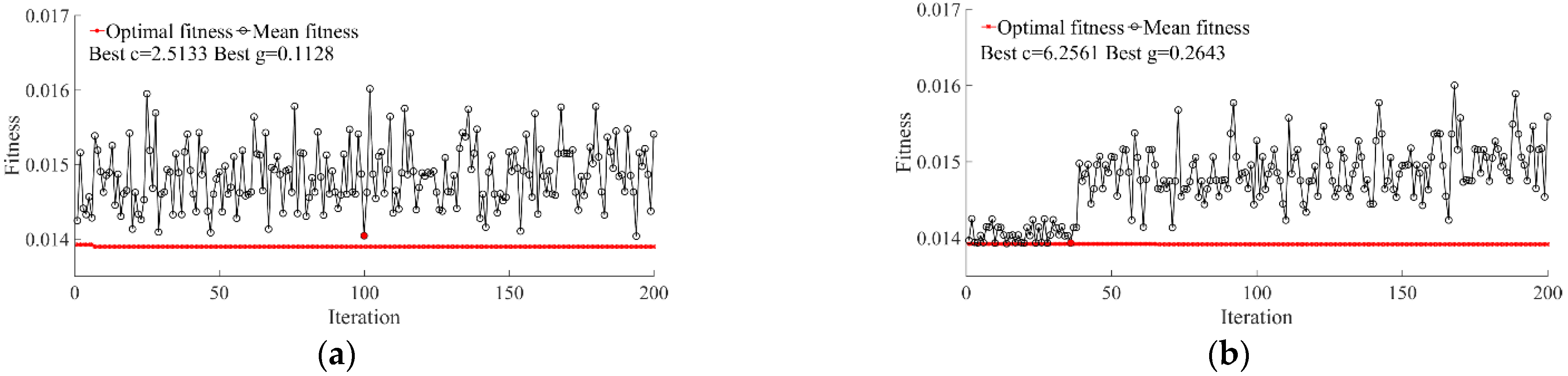
3.4. SCC Evaluation Based on PSO-SVR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.S.; Xu, S.Z.; Xue, C.; Wang, R.Z.; Zhang, M.Y.; Li, J.M.; Zhang, S.L.; Wu, J. Pear-process: A new phenotypic tool for stone cell trait evaluation in pear fruit. J. Integr. Agric. 2020, 19, 1625–1634. [Google Scholar] [CrossRef]
- Li, D.H.; Meng, X.T.; Xu, M.Q.; Che, F.B.; Pan, Y.; Zhao, D.Y. Research on identification features of quality classification of Korla fragrantpear (Pyrus sinkiangensis Yu). Storage Process 2018, 18, 5–15. [Google Scholar]
- Xu, H.B.; Wu, J.; Wang, Z.P.; Gao, Y.M.; Wang, Z.P.; Zhao, Z.Q. Characteristics of the vibration spectral response of rough peel pear among Korla pear. Sci. Technol. Food Ind. 2015, 36, 57–66. [Google Scholar]
- Zhang, J.Y.; Li, J.M.; Xue, C.; Wang, R.Z.; Zhang, M.Y.; Qi, K.J.; Fan, J.; Hu, H.J.; Zhang, S.L.; Wu, j. The variation of stone cell content in 236 germplasms of sand pear (Pyrus pyrifolia) and identification of related candidate genes. Hortic. Plant J. 2021, 7, 108–116. [Google Scholar] [CrossRef]
- Tao, S.T.; Khanizadeh, S.; Zhang, H.; Zhang, S.L. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci. 2009, 176, 413–419. [Google Scholar] [CrossRef]
- Jin, Q.; Yan, C.C.; Qiu, J.X.; Zhang, N.; Lin, Y.; Cai, Y.P. Structural characterization and deposition of stone cell lignin in Dangshan Su pear. Sci. Hortic. 2013, 155, 123–130. [Google Scholar] [CrossRef]
- Yan, C.C.; Yin, M.; Zhang, N.; Jin, Q.; Fang, Z.; Lin, Y.; Cai, Y.P. Stone cell distribution and lignin structure in various pear varieties. Sci. Hortic. 2014, 174, 142–150. [Google Scholar] [CrossRef]
- Fan, S.X.; Wang, Q.Y.; Tian, X.; Yang, G.Y.; Xia, Y.; Li, J.B.; Huang, W.Q. Non-destructive evaluation of soluble solids content of apples using a developed portable Vis/NIR device. Biosyst. Eng. 2020, 193, 138–148. [Google Scholar] [CrossRef]
- Yuan, L.M.; Mao, F.; Chen, X.J.; Li, L.M.; Huang, G.Z. Non-invasive measurements of ‘Yunhe’ pears by vis/NIRS technology coupled with deviation fusion modeling approach. Postharvest Biol. Technol. 2020, 160, 111067. [Google Scholar] [CrossRef]
- João, P.S.N.; Mateua, W.D.A.; Izabella, P.C.; Luis, C.C.J.; Gustavo, H.A.T. Determination of ‘Palmer’ mango maturity indices using portable near infrared (VIS/NIR) spectrometer. Postharvest Biol. Technol. 2017, 130, 75–80. [Google Scholar]
- Wang, J.Y.; Guo, Z.M.; Zou, C.X.; Jiang, S.Q.; EI-Seedi, H.R.; Zou, X.B. General Model of Multi-Quality Detection for Apple from Different Origins by Vis/NIR Transmittance Spectroscopy. J. Food Meas. Charact. 2022; accepted. [Google Scholar] [CrossRef]
- Guo, Z.M.; Wang, M.M.; Agyekum, A.A.; Wu, J.Z.; Chen, X.B.; Zou, M.; EI-Seedi, H.R.; Tao, F.F.; Qin, O.Y.; Zou, X.B. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Li, J.B.; Zhang, H.L.; Zhan, B.S.; Zhang, Y.F.; Li, R.L.; Li, J.B. Nondestructive firmness measurement of the multiple cultivars of pears by Vis/NIR spectroscopy coupled with multivariate calibration analysis and MC-UVE-SPA method. Infrared Phys. Technol. 2020, 104, 103154. [Google Scholar] [CrossRef]
- Nur, F.M.K.; Puneet, M.; Rob, E.S.; Ernst, J.W.; Martin, P.B. Assessing firmness in mango comparing broadband and miniature spectrophotometers. Infrared Phys. Technol. 2021, 115, 103733. [Google Scholar]
- René, M.; Carolina, C.; Magnólia, L.S.N.; Emanuel, J.N.M.; Juan, P.Z.; Sergio, T.F. Non-destructive evaluation and detection of internal physiological disorders in ‘Keitt’ mango using a hand-held Vis/NIR spectrometer. Postharvest Biol. Technol. 2020, 167, 111251. [Google Scholar]
- Bizzani, M.; Flores, D.W.M.; Colnago, L.A.; Ferreira, M.D. Non-invasive spectroscopic methods to estimate orange firmness, peel thickness, and total pectin content. Microchem. J. 2017, 133, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Annelisa, A.B.; Fernanda, C.; Abadia, R.N.; Clarissa, D.; Flávio, A.S.; Gustavo, H.A.T.; Luis, C.C.J. Non-destructive determination of color, titratable acidity, and dry matter in intact tomatoes using a portable Vis/NIR spectrometer. J. Food Compos. Anal. 2022, 107, 104288. [Google Scholar]
- Sylvia, T.; Marianne, G.B.; Karen, K.P.; Sergey, V.K. Evaluating pear (cv. Clara Frijs) dry matter and soluble solids content with near infrared spectroscopy. LWT-Food Sci. Technol. 2014, 59, 1107–1113. [Google Scholar]
- Guo, Z.M.; Barimah, A.O.; Yin, L.M.; Chen, Q.S.; Shi, J.Y.; EI-Seedi, H.R.; Zou, X.B. Intelligent evaluation of taste constituents and polyphenols-to-amino acids ratio in matcha tea powder using near infrared spectroscopy. Food Chem. 2021, 353, 129372. [Google Scholar] [CrossRef]
- Valber, E.A.; Adriano, A.G.; David, D.S.F.; Héctor, C.G.; Roberto, K.H.G.; Mario, C.U.A. Vis/NIR spectrometric determination of Brix and sucrose in sugar production samples using kernel partial least squares with interval selection based on the successive projections algorithm. Talanta 2018, 181, 38–43. [Google Scholar]
- Sun, Y.; Gu, X.Z.; Sun, K.; Hu, H.J.; Xu, M.; Wang, Z.J.; Tu, K.; Pan, L.Q. Hyperspectral reflectance imaging combined with chemometrics and successive projections algorithm for chilling injury classification in peaches. LWT-Food Sci. Technol. 2017, 75, 557–564. [Google Scholar] [CrossRef]
- Jan, P.M.A.; Yvan, V.H.; Lutgarde, M.C.B. Improved variable reduction in partial least squares modelling by Global-Minimum Error Uninformative-Variable Elimination. Anal. Chim. Acta 2017, 982, 37–47. [Google Scholar]
- Li, C.; Zhao, T.L.; Li, C.; Mei, L.; Yu, E.; Dong, Y.T.; Chen, J.H.; Zhu, S.J. Determination of gossypol content in cottonseeds by near infrared spectroscopy based on Monte Carlo uninformative variable elimination and nonlinear calibration methods. Food Chem. 2017, 221, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Huang, W.Q.; Wang, Z.L.; Liu, S.Q.; He, X.; Fan, S.X. Calibration transfer between developed portable Vis/NIR devices for detection of soluble solids contents in apple. Postharvest Biol. Technol. 2022, 183, 111720. [Google Scholar] [CrossRef]
- Pedro, S.S.; Ana, S.A.; Carla, M.B. Use of artificial neural network model for rice quality evaluation based on grain physical parameters. Foods 2021, 10, 3016. [Google Scholar]
- Wang, H.W.; Shangguan, L.Y.; Wu, J.J.; Guan, R. Multiple linear regression modeling for compositional data. Neurocomputing 2013, 122, 490–500. [Google Scholar] [CrossRef]
- Nadya, V.; Claudia, M.; Jimy, O.; Tony, C.; Himer, A.; Wilson, C. Comparison between artificial neural network and partial least squares regression models for hardness modeling during the ripening process of Swiss-type cheese using spectral profiles. J. Food Eng. 2018, 219, 8–15. [Google Scholar]
- Sahar, R.; Hossein, M.; Bahareh, J.; Aslan, A.; Mohammad, S. Achieving a robust Vis/NIR model for microbial contamination detection of Persian leek by spectral analysis based on genetic, iPLS algorithms and VIP scores. Postharvest Biol. Technol. 2021, 175, 111413. [Google Scholar]
- Jair, C.; Farid, G.L.; Lisbeth, R.M.; Asdrubal, L. A comprehensive survey on support vector machine classification:Applications, challenges and trends. Neurocomputing 2020, 408, 189–215. [Google Scholar]
- Onuwa, O.; Christopher, E.N. Deep support vector machine for hyperspectral image classification. Pattern Recognit. 2020, 103, 107298. [Google Scholar]
- Ji, Y.M.; Sun, L.J.; Li, Y.S.; Li, J.; Liu, S.C.; Xie, X.; Xu, Y.T. Non-destructive classification of defective potatoes based on hyperspectral imaging and support vector machine. Infrared Phys. Technol. 2019, 99, 71–79. [Google Scholar] [CrossRef]
- Camilo, L.M.M.; Kássio, M.G.L.; Francis, L.M. Uncertainty estimation and misclassification probability for classification models based on discriminant analysis and support vector machines. Anal. Chim. Acta 2019, 1063, 40–46. [Google Scholar]
- Xu, S.X.; Zhao, Y.C.; Wang, M.Y.; Shi, X.Z. Determination of rice root density from Vis–NIR spectroscopy by support vector machine regression and spectral variable selection techniques. Catena 2017, 157, 12–23. [Google Scholar] [CrossRef]
- Alireza, S.; Adel, B.; Miguel, G. Evaluation of banana quality indices from color features using support vector regression. Talanta 2016, 148, 54–61. [Google Scholar]
- Li, P.; Ma, J.C.; Zhong, N. Raman spectroscopy combined with support vector regression and variable selection method for accurately evaluating salmon fillets storage time. Optik 2021, 247, 167879. [Google Scholar] [CrossRef]
- Nie, J.Y.; Li, J.; Yang, Z.F.; Zhang, H.J.; Li, M.Q. Study on the conditions for measuring stone cell content in pear flesh by freezing method. J. Fruit Sci. 2006, 01, 133–135. [Google Scholar]
- Liu, Q.; Chen, S.X.; Zhou, D.D.; Chao, D.; Wang, J.H.; Zhou, H.S.; Tu, K.; Pan, L.Q.; Li, P.X. Nondestructive detection of weight loss rate, surface color, vitamin C content, and firmness in mini-Chinese cabbage with nanopackaging by fourier transform-near infrared spectroscopy. Foods 2021, 10, 2309. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.J.; Yang, G.J. Development of a non-destructive method for detection of the juiciness of pear via VIS/NIR spectroscopy combined with chemometric methods. Foods 2020, 9, 1778. [Google Scholar] [CrossRef]
- Fu, X.S.; Hong, X.Z.; Liao, J.Y.; Ji, Q.G.; Li, C.F.; Zhang, M.Z.; Ye, Z.H.; Yu, X.P. Fingerprint approaches coupled with chemometrics to discriminate geographic origin of imported salmon in China’s consumer market. Foods 2021, 10, 2986. [Google Scholar] [CrossRef]
- Mario, C.U.A.; Teresa, C.B.S.; Roberto, K.H.G.; Takashi, Y.; Henrique, C.C.; Valeria, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar]
- Esfandiarpour-Boroujeni, I.; Karimi, E.; Shirani, H.; Esmaeilizadeh, M.; Mosleh, Z. Yield evaluation of apricot using a hybrid particle swarm optimizationimperialist competitive algorithm- support vector regression (PSO-ICA-SVR) method. Sci. Hortic. 2019, 257, 108756. [Google Scholar] [CrossRef]
- Cen, H.; He, Y.; Huang, M. Combination and comparison of multivariate analysis for the identification of orange varieties using visible and near infrared reflectance spectroscopy. Eur. Food Res. Technol. 2007, 225, 699–705. [Google Scholar] [CrossRef]
- Jamshidi, M.; Hamdami, N.; Dohkani, S.; Keramat, J. Single-and multi-objective optimization of low fat ice-cream formulation, based on genetic algorithms. J. Agric. Sci. Technol. 2012, 14, 1285–1296. [Google Scholar]
- Tian, X.; Wang, Q.Y.; Li, J.B.; Peng, F.; Huang, W.Q. Non-destructive evaluation of soluble solids content of pear based on fruit surface feature classification and multivariate regression analysis. Infrared Phys. Technol. 2018, 92, 336–344. [Google Scholar] [CrossRef]

| Sample Set | Numbers | Min (%) | Max (%) | Mean (%) | SD (%) | p |
|---|---|---|---|---|---|---|
| Cs | 90 | 0.240 | 0.657 | 0.486 | 0.100 | 0.008 |
| Vs | 30 | 0.315 | 0.652 | 0.481 | 0.083 |
| Frame Size | None | 3 | 5 | 7 | 9 | |
|---|---|---|---|---|---|---|
| Fitting Order | ||||||
| none | 0.8613 | |||||
| 0.8214 | ||||||
| 1 | 0.8276 | 0.7867 | 0.7403 | 0.7012 | ||
| 0.8007 | 0.7616 | 0.7150 | 0.6710 | |||
| 2 | 0.8306 | 0.7928 | 0.7789 | |||
| 0.8035 | 0.7710 | 0.7458 | ||||
| 3 | 0.8414 | 0.8227 | 0.8023 | |||
| 0.8137 | 0.8006 | 0.7853 | ||||
| 4 | 0.8527 | 0.8419 | ||||
| 0.8195 | 0.8059 | |||||
| 5 | 0.8926 | 0.8647 | ||||
| 0.8210 | 0.8100 | |||||
| 6 | 0.8589 | |||||
| 0.8128 | ||||||
| 7 | 0.8527 | |||||
| 0.8026 | ||||||
| Parameter | Preprocessing Algorithm | Factor Number | RC | RMSEC (%) | RV | RMSEV (%) |
|---|---|---|---|---|---|---|
| Stone cell content (%) | None | 9 | 0.8613 | 0.0360 | 0.8214 | 0.0412 |
| MSC | 10 | 0.9191 | 0.0277 | 0.8879 | 0.0325 | |
| SNV | 10 | 0.9189 | 0.0277 | 0.8935 | 0.0315 | |
| S-G(7, 5) | 10 | 0.8926 | 0.0319 | 0.8210 | 0.0409 | |
| S-G(7, 5)& MSC | 10 | 0.9001 | 0.0308 | 0.8614 | 0.0361 | |
| S-G(7, 5)& SNV | 10 | 0.8999 | 0.0308 | 0.8641 | 0.0356 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhang, Y.; Liu, Y.; Zhang, Z.; Yan, T. Intelligent Evaluation of Stone Cell Content of Korla Fragrant Pears by Vis/NIR Reflection Spectroscopy. Foods 2022, 11, 2391. https://doi.org/10.3390/foods11162391
Wang T, Zhang Y, Liu Y, Zhang Z, Yan T. Intelligent Evaluation of Stone Cell Content of Korla Fragrant Pears by Vis/NIR Reflection Spectroscopy. Foods. 2022; 11(16):2391. https://doi.org/10.3390/foods11162391
Chicago/Turabian StyleWang, Tongzhao, Yixiao Zhang, Yuanyuan Liu, Zhijuan Zhang, and Tongbin Yan. 2022. "Intelligent Evaluation of Stone Cell Content of Korla Fragrant Pears by Vis/NIR Reflection Spectroscopy" Foods 11, no. 16: 2391. https://doi.org/10.3390/foods11162391
APA StyleWang, T., Zhang, Y., Liu, Y., Zhang, Z., & Yan, T. (2022). Intelligent Evaluation of Stone Cell Content of Korla Fragrant Pears by Vis/NIR Reflection Spectroscopy. Foods, 11(16), 2391. https://doi.org/10.3390/foods11162391





