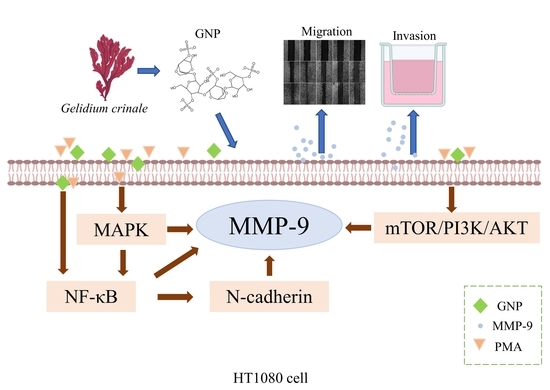
A Sulfated Polysaccharide from Red Algae (Gelidium crinale) to Suppress Cells Metastasis and MMP-9 Expression of HT1080 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cells Activity Assay (CCK-8)
2.3. Cell Wound Healing Assay
2.4. Trans Well Matrigel Invasion Assay
2.5. Gelatin Zymography
2.6. Quantitative Real Time PCR (qPCR)
2.7. Immunocytochemistry
2.8. Western Blot
2.9. Statistical Analysis
3. Results
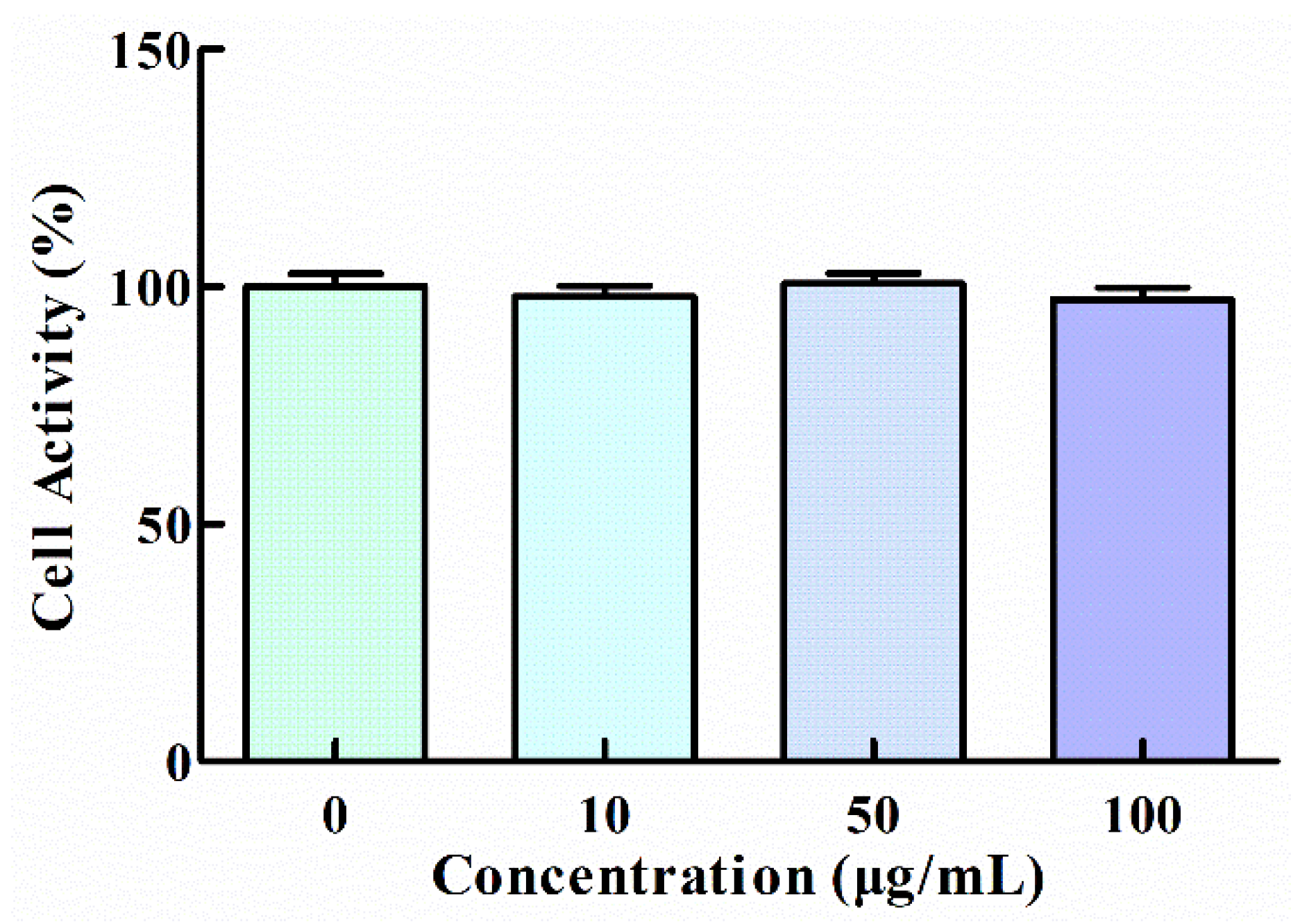
3.1. Effect of GNP on the Viability of HT1080 Cells
3.2. Effect of GNP on the Migration and Invasion Ability of HT1080 Cells
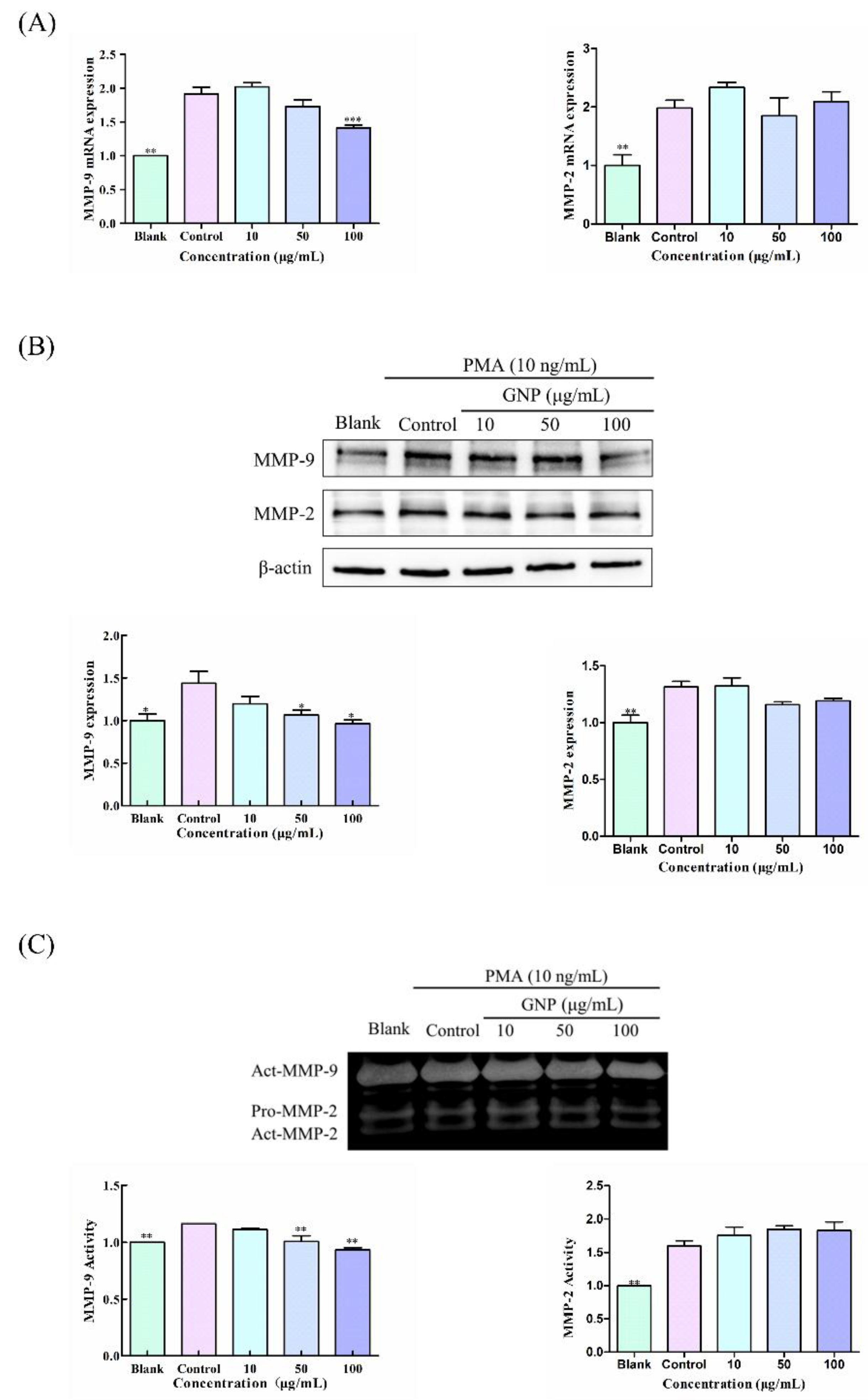
3.3. Effect of GNP on the Expression and Activity of MMP-9 and MMP-2 in HT1080 Cells Induced by PMA
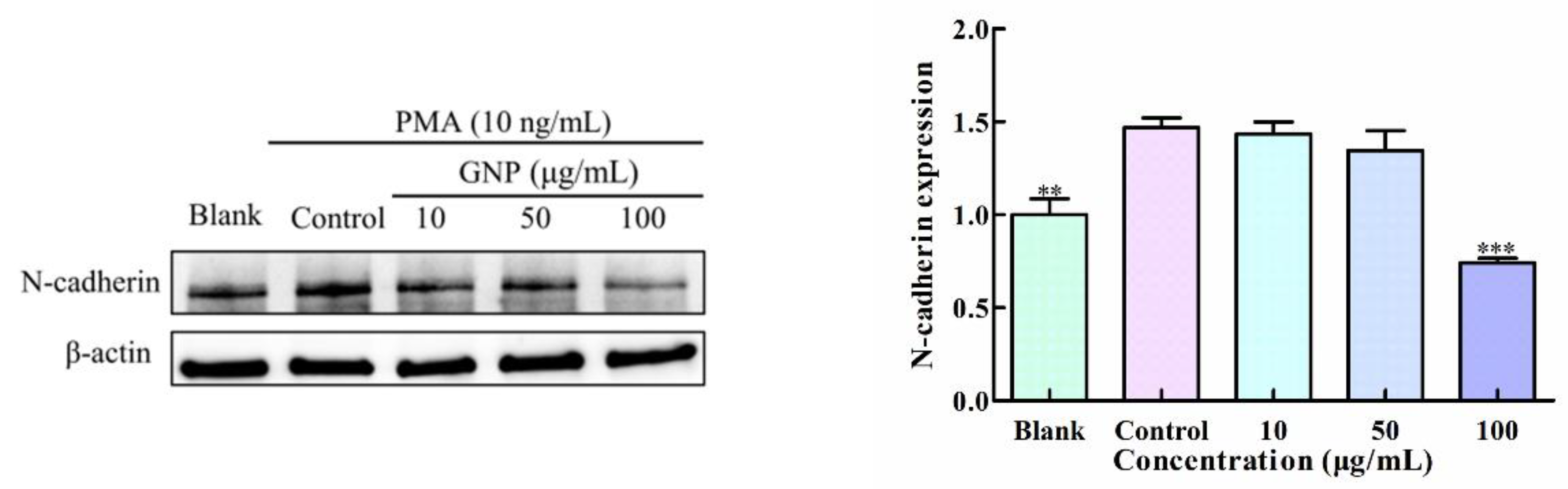
3.4. Effect of GNP on the Expression of N-Cadherin in HT1080 Cells by PMA
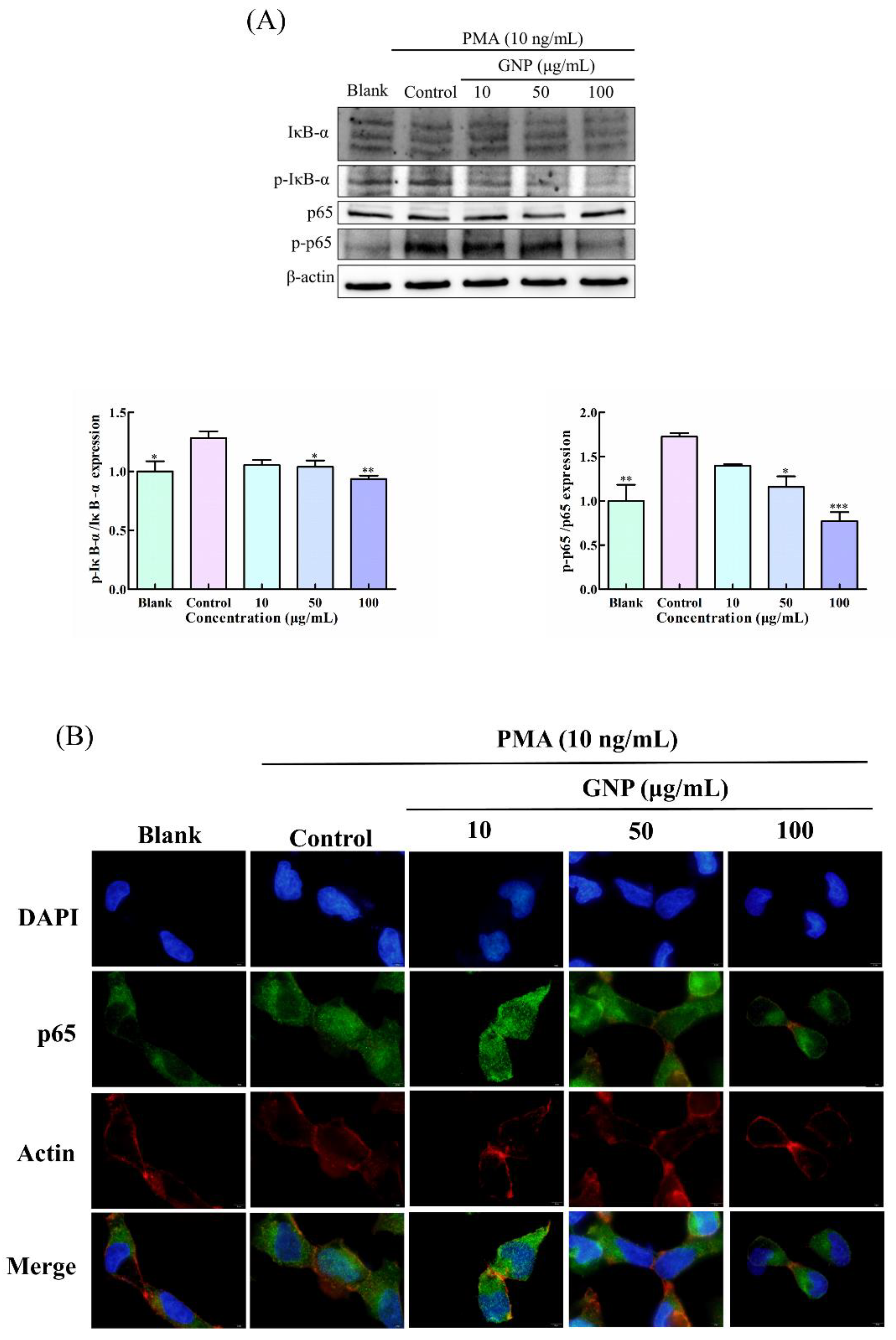
3.5. Effect of GNP on NF-κB Pathway in PMA-Induced HT1080 Cells
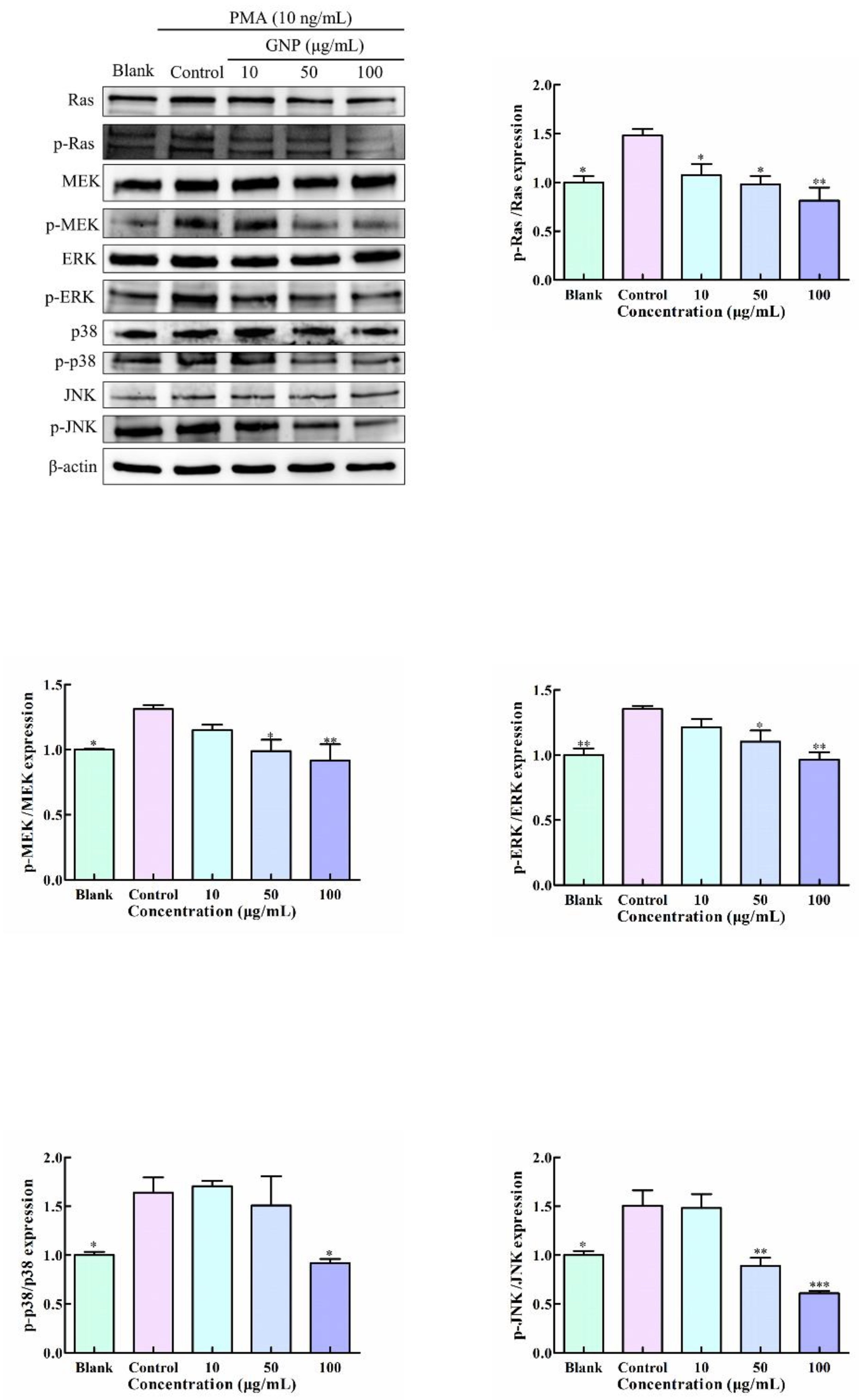
3.6. Effect of GNP on MAPK Pathway in PMA-Induced HT1080 Cells
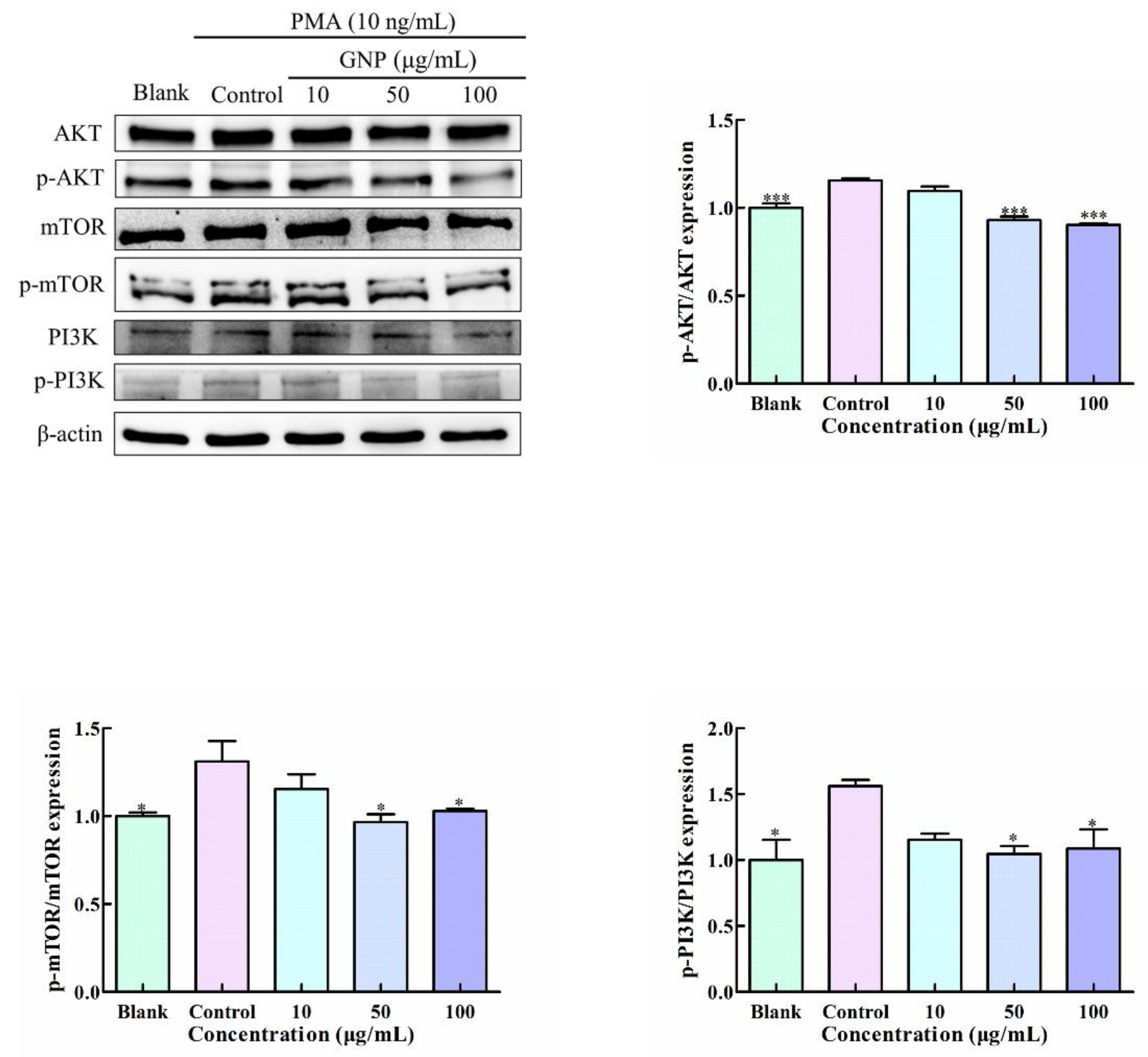
3.7. Effect of GNP on PI3K/AKT/mTOR Pathway in PMA-Induced HT1080 Cells
4. Discussion
| Source | Cell | Effective Concentration of Migration or Invasion | Mechanism | Reference |
|---|---|---|---|---|
| Antrodia cinnamomea | Human lung cancer A549 cells | Migration: 200 μg/mL | Promoting degradation of TGFRs and inhibiting Smad and non-Smad signaling pathways. | [37] |
| Gracilaria fisheri | CCA cells (HuCCA-1 and RMCCA-1) established from CCA tissue fragments of Thai patients | Migration: 10 μg/mL | Mediated by inhibition of MAPK/ERK signal transduction pathway | [38] |
| Codium isthmocladum | B16-F10 murine melanoma cell line | Invasion: 100 μg/mL | - | [33] |
| Undaria pinnatifida | OC cell lines (SKOV3, A2780) | Migration and Invasion: 100 μg/mL | Inhibits Hh pathway conduction in OC cells | [39] |
| Undaria pinnatifida sporophylls | Mouse Hca-F hepatocarcinoma cell line | Invasion: 500 μg/mL | Mediated through the mechanism involving inactivation of the NF-κB pathway | [40] |
| Stichopus variegatus | Human breast cancer cells MDA-MB-231 | Migration: 100 ug/mL | - | [41] |
| Ascophyllum nodosum | Murine B16 melanoma | Migration: 10 ug/mL Invasion: 5 ug/mL | Inhibition of EMT, reduced the expression of MMP-9 | [42] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, F.; Chen, M.F.; Zhang, Y.Y.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Sun, S.L.; Qian, Z.J. A Novel Peptide from Abalone (Haliotis discus hannai) to Suppress Metastasis and Vasculogenic Mimicry of Tumor Cells and Enhance Anti-Tumor Effect In Vitro. Mar. Drugs 2019, 17, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutry, J.; Tissot, S.; Ujvari, B.; Capp, J.P.; Giraudeau, M.; Nedelcu, A.M.; Thomas, F. The evolution and ecology of benign tumors. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188643. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wei, Y.; Liu, R.; Xu, R.; Xiang, L.; Du, J. Role of tumour-derived exosomes in metastasis. Biomed. Pharmacother. 2022, 147, 112657. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschlager, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, X.; Zhan, R.; Zhao, Z.; Xu, K.; Tang, B. Tricolor imaging of MMPs to investigate the promoting roles of inflammation on invasion and migration of tumor cells. Talanta 2021, 222, 121525. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, E.H.; Hwang, Y.P.; Choi, J.M.; Choi, C.Y.; Chung, Y.C.; Seo, J.K.; Jeong, H.G. Suppression of PMA-induced tumor cell invasion and metastasis by aqueous extract isolated from Prunella vulgaris via the inhibition of NF-kappaB-dependent MMP-9 expression. Food Chem. Toxicol. 2010, 48, 564–571. [Google Scholar] [CrossRef]
- Mook, O.R.; Frederiks, W.M.; van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar] [CrossRef]
- Umezawa, K.; Lin, Y. Inhibition of matrix metalloproteinase expression and cellular invasion by NF-kappaB inhibitors of microbial origin. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140412. [Google Scholar] [CrossRef]
- Hua, H.; Zhu, Y.; Song, Y.H. Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2018, 101, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Chen, M.F.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.J. Boiled Abalone Byproduct Peptide Exhibits Anti-Tumor Activity in HT1080 Cells and HUVECs by Suppressing the Metastasis and Angiogenesis in Vitro. J. Agric. Food Chem. 2019, 67, 8855–8867. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Xiao, Z.; Tang, Y.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.-J. Inhibition effects of 7-phloro-eckol from Ecklonia cava on metastasis and angiogenesis induced by hypoxia through regulation of AKT/mTOR and ERK signaling pathways. Arab. J. Chem. 2021, 14, 103187. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Ma, Y.; Huan, L.; Wang, Y.; Xia, B.; Wang, G. Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Res. 2020, 46, 101817. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.J. Structural Characterization of Sulfated Polysaccharide Isolated from Red Algae (Gelidium crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9, 794818. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Liu, S.; Pan, C.; Chen, S.; Li, L.; Qi, B.; Yang, X. Insights on preparation, structure and activities of Gracilaria lemaneiformis polysaccharide. Food Chem. X 2021, 12, 100153. [Google Scholar] [CrossRef] [PubMed]
- Batirel, S.; Yilmaz, A.M.; Sahin, A.; Perakakis, N.; Kartal Ozer, N.; Mantzoros, C.S. Antitumor and antimetastatic effects of walnut oil in esophageal adenocarcinoma cells. Clin. Nutr. 2018, 37, 2166–2171. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, M.; Dorfman, R.G.; Pan, Y.; Tang, D.; Xu, L.; Zhao, Z.; Zhou, Q.; Zhou, L.; Wang, Y.; et al. SIRT2 Promotes the Migration and Invasion of Gastric Cancer through RAS/ERK/JNK/MMP-9 Pathway by Increasing PEPCK1-Related Metabolism. Neoplasia 2018, 20, 745–756. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Momtaz, S.; Haratipour, P.; El-Senduny, F.F.; Panah, A.I.; Navabi, J.; Soheilikhah, Z.; Farzaei, M.H.; Rahimi, R. Molecular Mechanisms Underlying Cancer Preventive and Therapeutic Potential of Algal Polysaccharides. Curr. Pharm. Des. 2019, 25, 1210–1235. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Li, S.; Xin, Q.; Yuan, M.; Li, H.; Song, X.; Gao, H.; Pervaiz, N.; Sun, X.; et al. Quercetin Inhibits the Migration and Invasion of HCCLM3 Cells by Suppressing the Expression of p-Akt1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. 2018, 24, 2583–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.R.; Noh, E.M.; Oh, H.J.; Hur, H.; Kim, J.M.; Han, J.H.; Hwang, J.K.; Park, B.H.; Park, J.W.; Youn, H.J.; et al. Dihydroavenanthramide D inhibits human breast cancer cell invasion through suppression of MMP-9 expression. Biochem. Biophys. Res. Commun. 2011, 405, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delma, C.R.; Thirugnanasambandan, S.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Natarajan, M.; Aravindan, N. Fucoidan from marine brown algae attenuates pancreatic cancer progression by regulating p53—NFkappaB crosstalk. Phytochemistry 2019, 167, 112078. [Google Scholar] [CrossRef] [PubMed]
- Rajasinghe, L.D.; Pindiprolu, R.H.; Gupta, S.V. Delta-tocotrienol inhibits non-small-cell lung cancer cell invasion via the inhibition of NF-kappaB, uPA activator, and MMP-9. Onco Targets Ther. 2018, 11, 4301–4314. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Huang, S.F.; Wang, J.S.; Chu, W.K.; Nien, J.E.; Chen, W.S.; Chow, S.E. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer 2016, 16, 800. [Google Scholar] [CrossRef] [Green Version]
- Da, C.; Wu, K.; Yue, C.; Bai, P.; Wang, R.; Wang, G.; Zhao, M.; Lv, Y.; Hou, P. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget 2017, 8, 8131–8142. [Google Scholar] [CrossRef] [Green Version]
- Iroegbu, J.D.; Ijomone, O.K.; Femi-Akinlosotu, O.M.; Ijomone, O.M. ERK/MAPK signalling in the developing brain: Perturbations and consequences. Neurosci. Biobehav. Rev. 2021, 131, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Aroui, S.; Aouey, B.; Chtourou, Y.; Meunier, A.C.; Fetoui, H.; Kenani, A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem. Biol. Interact. 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Jung, J.S.; Jung, K.; Kim, D.H.; Kim, H.S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef]
- Dobbin, Z.C.; Landen, C.N. The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int. J. Mol. Sci. 2013, 14, 8213–8227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; He, D.; Zhang, C.; Bai, Y.; Zhang, C. The regulate function of polysaccharides and oligosaccharides that with sulfate group on immune-related disease. J. Funct. Foods 2022, 88, 104870. [Google Scholar] [CrossRef]
- Bellan, D.L.; Biscaia, S.M.P.; Rossi, G.R.; Cristal, A.M.; Goncalves, J.P.; Oliveira, C.C.; Simas, F.F.; Sabry, D.A.; Rocha, H.A.O.; Franco, C.R.C.; et al. Green does not always mean go: A sulfated galactan from Codium isthmocladum green seaweed reduces melanoma metastasis through direct regulation of malignancy features. Carbohydr. Polym. 2020, 250, 116869. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the structure-bioactivity relationships of marine sulfated polysaccharides: A review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Lu, M.K.; Lin, T.Y.; Chao, C.H.; Hu, C.H.; Hsu, H.Y. Molecular mechanism of Antrodia cinnamomea sulfated polysaccharide on the suppression of lung cancer cell growth and migration via induction of transforming growth factor beta receptor degradation. Int. J. Biol. Macromol. 2017, 95, 1144–1152. [Google Scholar] [CrossRef]
- Sae-Lao, T.; Luplertlop, N.; Janvilisri, T.; Tohtong, R.; Bates, D.O.; Wongprasert, K. Sulfated galactans from the red seaweed Gracilaria fisheri exerts anti-migration effect on cholangiocarcinoma cells. Phytomedicine 2017, 36, 59–67. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Xu, Y.; Chen, G.; Qiu, Y. Sulfated Polysaccharide from Undaria Pinnatifida Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Ovarian Cancer via Suppressing the Hedgehog Signaling Pathway. Front. Mater. 2021, 8, 795061. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Liu, X.; Teng, H.; Zhang, C.; Hou, L.; Zou, X. Anti-metastasis effect of fucoidan from Undaria pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS ONE 2014, 9, e106071. [Google Scholar] [CrossRef] [Green Version]
- Thinh, P.D.; Ly, B.M.; Usoltseva, R.V.; Shevchenko, N.M.; Rasin, A.B.; Anastyuk, S.D.; Malyarenko, O.S.; Zvyagintseva, T.N.; San, P.T.; Ermakova, S.P. A novel sulfated fucan from Vietnamese sea cucumber Stichopus variegatus: Isolation, structure and anticancer activity in vitro. Int. J. Biol. Macromol. 2018, 117, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732. [Google Scholar] [CrossRef] [PubMed]

| Primer | Primer Sequence (5′-3′) |
|---|---|
| MMP-9 | F: 5′-TCCTGGTGCTCCTGGTGCTG-3′ |
| R: 5′-CTGCCTGTCGGTGAGATTGGTTC-3′ | |
| MMP-2 | F: 5′-AGCCAAGCGGTCTAAGTCCAGAG-3′ |
| R: 5′-GGAATGAAGCACAGCAGGTCTCAG-3′ | |
| β-actin | F: 5′-CCTGGCACCCAGCACAAT-3′ |
| R: 5′-GGGCCGGACTCGTCATAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Pei, Y.; He, Y.-L.; Liu, Y.; Chen, M.; Hong, P.; Zhou, C.; Qian, Z.-J. A Sulfated Polysaccharide from Red Algae (Gelidium crinale) to Suppress Cells Metastasis and MMP-9 Expression of HT1080 Cells. Foods 2022, 11, 2360. https://doi.org/10.3390/foods11152360
Zheng H, Pei Y, He Y-L, Liu Y, Chen M, Hong P, Zhou C, Qian Z-J. A Sulfated Polysaccharide from Red Algae (Gelidium crinale) to Suppress Cells Metastasis and MMP-9 Expression of HT1080 Cells. Foods. 2022; 11(15):2360. https://doi.org/10.3390/foods11152360
Chicago/Turabian StyleZheng, Haiyan, Yu Pei, Yuan-Lin He, Yi Liu, Minqi Chen, Pengzhi Hong, Chunxia Zhou, and Zhong-Ji Qian. 2022. "A Sulfated Polysaccharide from Red Algae (Gelidium crinale) to Suppress Cells Metastasis and MMP-9 Expression of HT1080 Cells" Foods 11, no. 15: 2360. https://doi.org/10.3390/foods11152360
APA StyleZheng, H., Pei, Y., He, Y.-L., Liu, Y., Chen, M., Hong, P., Zhou, C., & Qian, Z.-J. (2022). A Sulfated Polysaccharide from Red Algae (Gelidium crinale) to Suppress Cells Metastasis and MMP-9 Expression of HT1080 Cells. Foods, 11(15), 2360. https://doi.org/10.3390/foods11152360