Exploring the Quality and Application Potential of the Remaining Tea Stems after the Postharvest Tea Leaves: The Example of Lu’an Guapian Tea (Camellia sinensis L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Tea Leaf and Stem Quality Analysis
2.2.1. Color Differences between Tea Leaves and Stems and Their Infusions
2.2.2. Tea Taste and Quality
2.3. Detection of Major Chemical Compounds
2.3.1. Catechin, Caffeine, and Total Polyphenol Content
2.3.2. Amino Acid and Free Amino Acid Content
2.3.3. Monosaccharide, Disaccharide, and Total Soluble Sugar Content
2.3.4. Water-Soluble Substance, Crude Fiber, and Ash Content
2.4. Statistical Analysis
3. Results
3.1. Differences in Quality between Leaves and Stems
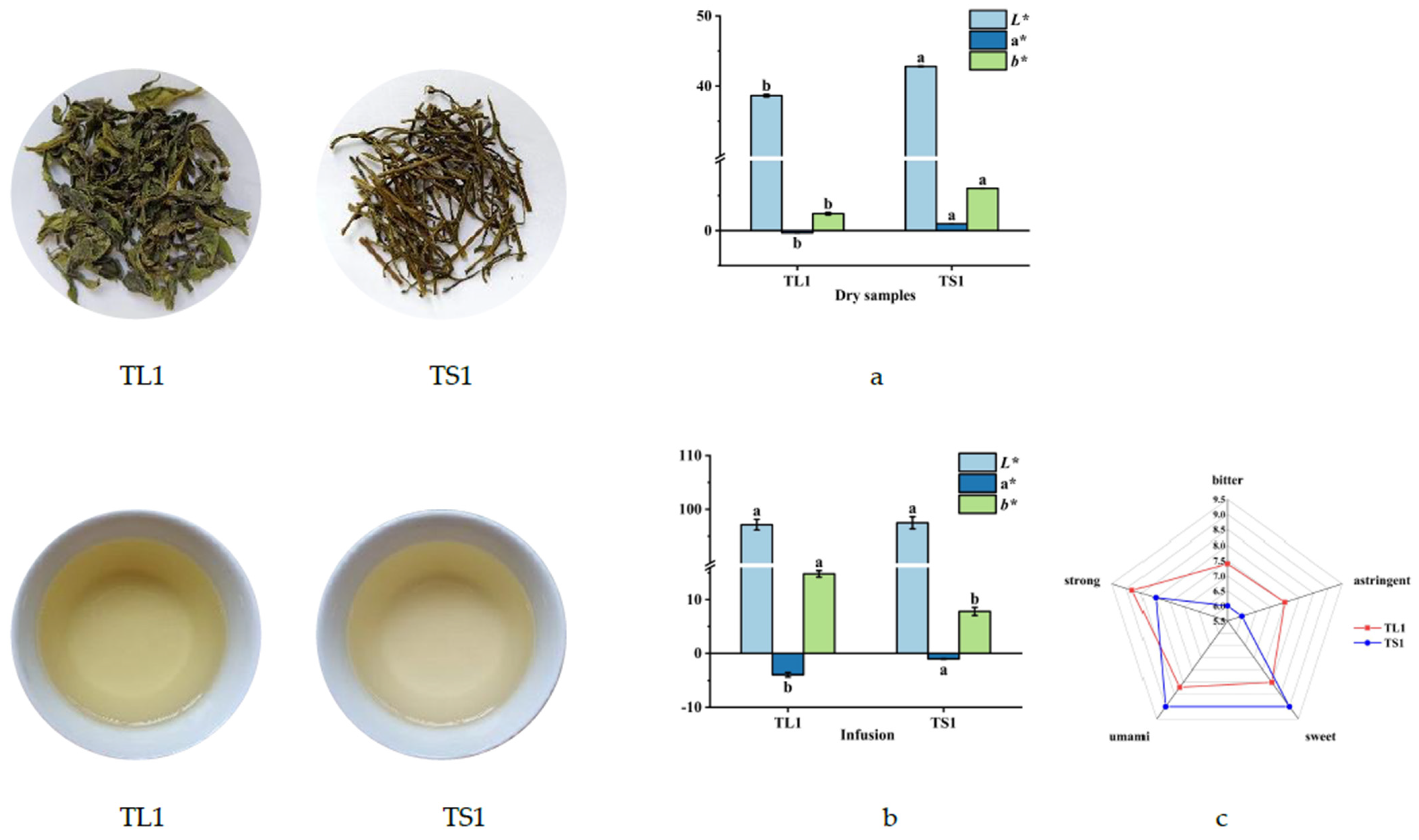
3.1.1. Color Differences between Tea Leaves and Stems and Their Infusions
3.1.2. Differences in Color and Taste among Tea Stems with Different Degrees of Tenderness
3.2. Differences in Primary Physicochemical Properties
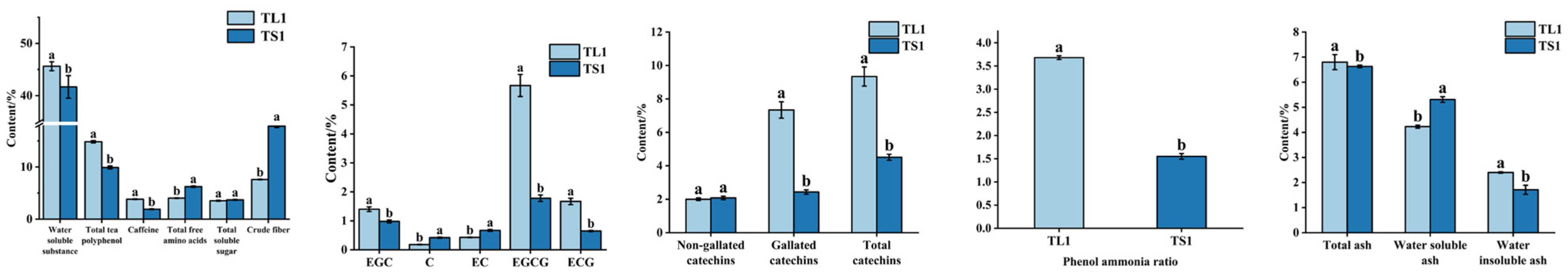
3.2.1. Differences in Primary Physicochemical Properties between Tea Leaves and Stems
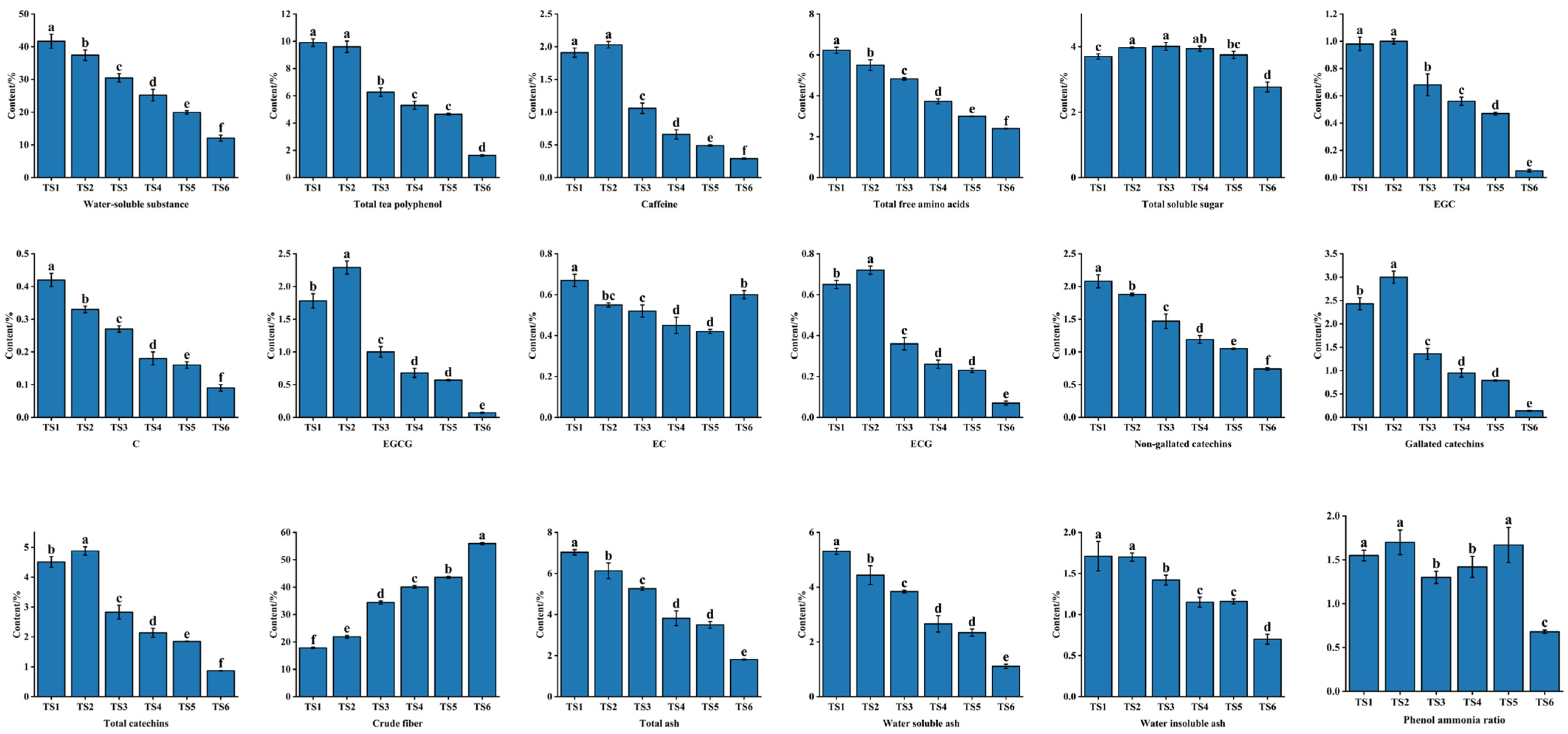
3.2.2. Differences in Primary Phytochemical Properties among Tea Stems with Different Degrees of Tenderness
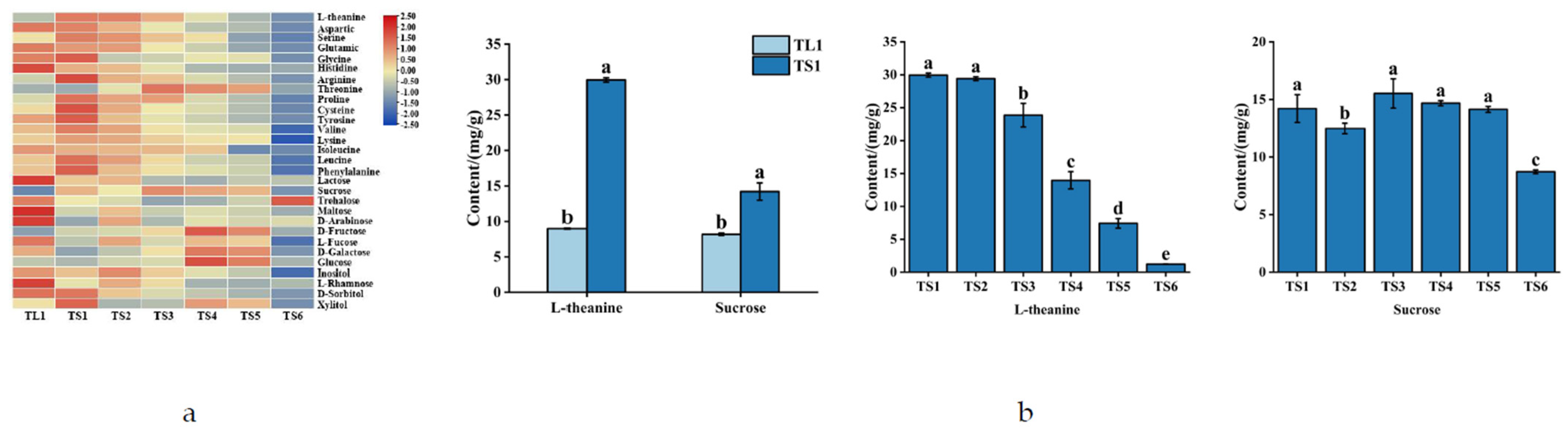
3.2.3. Differences in the Various Amino Acid, Monosaccharide, and Disaccharide Content of Tea Leaves and Stems
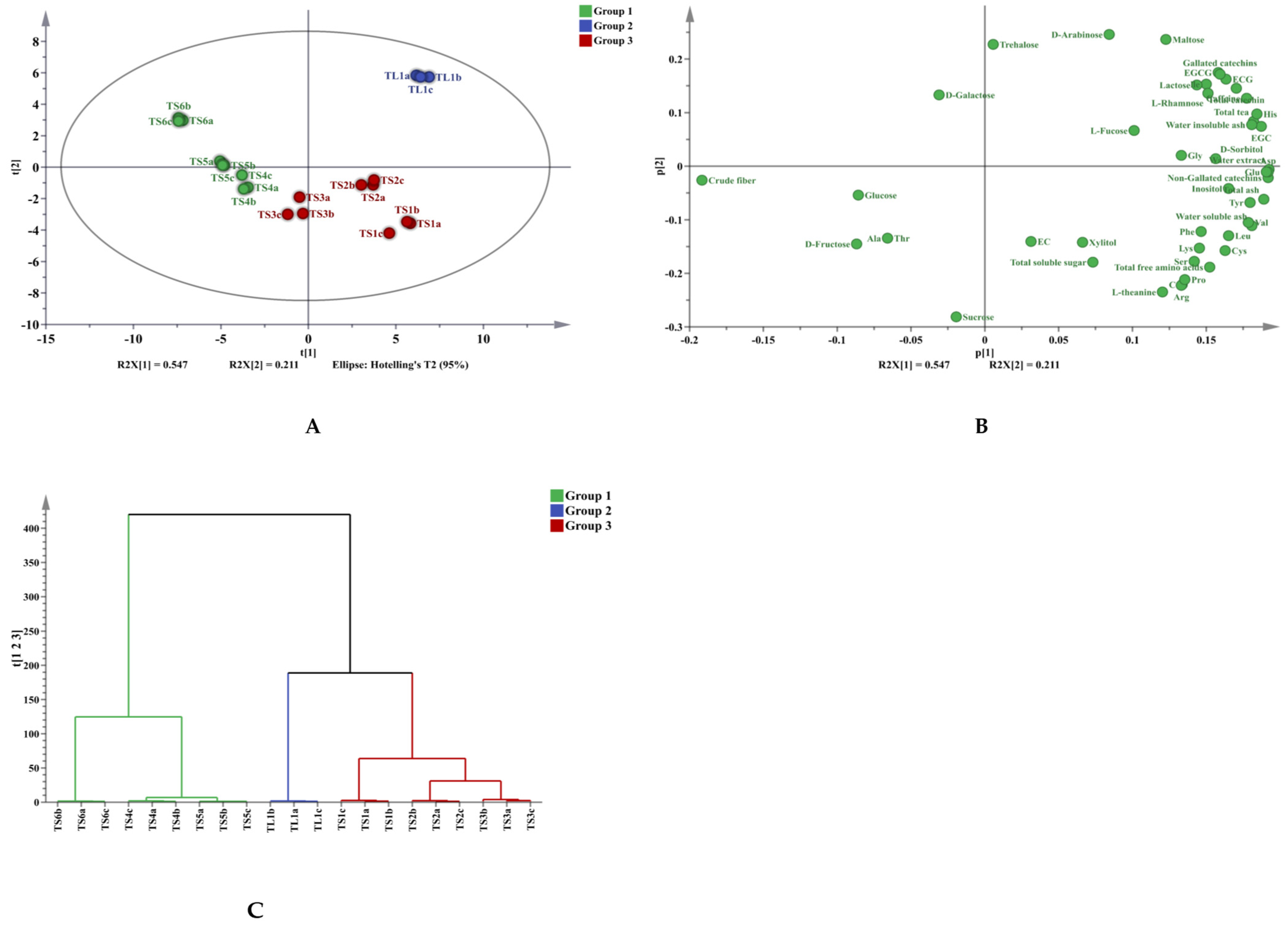
3.3. Multivariate Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, W.-X.; Wang, C.; Wang, Y.; Zheng, W.-J.; Wang, W.; Wan, X.-C.; Bao, G.-H. Novel Acylated Flavonol Tetraglycoside with Inhibitory Effect on Lipid Accumulation in 3T3-L1 Cells from Lu’an GuaPian Tea and Quantification of Flavonoid Glycosides in Six Major Processing Types of Tea. J. Agric. Food Chem. 2017, 65, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wu, W.; Wan, X.; Ning, J. Discriminating geographical origins of green tea based on amino acid, polyphenol, and caffeine content through high-performance liquid chromatography: Taking Lu’an guapian tea as an example. Food Sci. Nutr. 2019, 7, 2167–2175. [Google Scholar] [CrossRef]
- Sinija, V.R.; Mishra, H.N. Green tea: Health benefits. J. Nutr. Environ. Med. 2008, 17, 232–242. [Google Scholar] [CrossRef]
- Cooper, R. Green tea and theanine: Health benefits. Int. J. Food Sci. Nutr. 2011, 63, 90–97. [Google Scholar] [CrossRef]
- Dulloo, A.; Seydoux, J.; Girardier, L.; Chantre, P.; Vandermander, J. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Int. J. Obes. 2000, 24, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, M.L. Chinese Spring Green Tea. Gastronomica 2001, 1, 80–83. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, C.; Lu, M.; Gan, N.; Chen, Z.; Deng, W.-W.; Zhang, Z.-Z. Metabolic Flux Enhancement and Transcriptomic Analysis Displayed the Changes of Catechins Following Long-Term Pruning in Tea Trees (Camellia sinensis). J. Agric. Food Chem. 2018, 66, 8566–8573. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liang, L.; Li, Y.; Yang, T.; Fan, Y.; Mao, X.; Wang, Y. Studies of quality development and major chemical composition of green tea processed from tea with different shoot maturity. LWT 2021, 142, 111055. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, B.-J.; Hwang, J.-A.; Ko, K.-S.; Chung, J.-O.; Kim, E.-H.; Lee, S.-J.; Hong, Y.-S. Metabolic Dependence of Green Tea on Plucking Positions Revisited: A Metabolomic Study. J. Agric. Food Chem. 2011, 59, 10579–10585. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Katai, H.; Ohnishi, T.; Morita, A.; Panda, S.K.; Ikka, T. Tissue-Dependent Variation Profiles of Tea Quality-Related Metabolites in New Shoots of Tea Accessions. Front. Nutr. 2021, 8, 659807. [Google Scholar] [CrossRef]
- Huang, D.; Qiu, Q.; Wang, Y.; Wang, Y.; Lu, Y.; Fan, D.; Wang, X. Rapid Identification of Different Grades of Huangshan Maofeng Tea Using Ultraviolet Spectrum and Color Difference. Molecules 2020, 25, 4665. [Google Scholar] [CrossRef] [PubMed]
- GB/T,2018. GB/T 23776-2018. Methodology for Sensory Evaluation of Tea. Available online: http://down.foodmate.net/standard/sort/3/52614.html (accessed on 15 July 2022).
- Ning, J.; Cao, Q.; Su, H.; Zhu, X.; Wang, K.; Wan, X.; Zhang, Z. Discrimination of six tea categories coming from different origins depending on polyphenols, caffeine, and theanine combined with different discriminant analysis. Int. J. Food Prop. 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Wang, L.-Y.; Zhou, J.; He, W.; Zeng, J.-M.; Jiang, Y.-W.; Cheng, H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012, 130, 720–724. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.-M.; Ding, D.; Song, Y.-S.; Zhang, Z.-Z.; Luo, X.; Wan, X.-C. Chemical constituents analysis of white tea of different qualities and different storage times. Eur. Food Res. Technol. 2016, 242, 2093–2104. [Google Scholar] [CrossRef]
- Guo, X.; Long, P.; Meng, Q.; Ho, C.-T.; Zhang, L. An emerging strategy for evaluating the grades of Keemun black tea by combinatory liquid chromatography-Orbitrap mass spectrometry-based untargeted metabolomics and inhibition effects on α-glucosidase and α-amylase. Food Chem. 2018, 246, 74–81. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Duan, J.; Shi, J.; Xue, S.; Kakuda, Y. Variation in catechin contents in relation to quality of ‘Huang Zhi Xiang’ Oolong tea (Camellia sinensis) at various growing altitudes and seasons. Food Chem. 2010, 119, 648–652. [Google Scholar] [CrossRef]
- GB/T,2018. GB/T 8313-2018: Determination of Total Polyphenols and Catechins Content in Tea. Available online: http://down.foodmate.net/standard/sort/3/53218.html (accessed on 15 July 2022).
- Boogers, I.; Plugge, W.; Stokkermans, Y.Q.; Duchateau, A.L. Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J. Chromatogr. A 2008, 1189, 406–409. [Google Scholar] [CrossRef]
- GB/T,2017. GB/T 23193-2017. Determination of Theanine in Tea—Using High Performance Liquid Chromatography. Available online: http://down.foodmate.net/standard/sort/3/51404.html (accessed on 15 July 2022).
- GB/T,2013. GB/T 8314-2013. Tea—Determination of Free Amino Acid Content. Available online: http://down.foodmate.net/standard/sort/3/39356.html (accessed on 15 July 2022).
- Wei, Y.; Fang, S.; Jin, G.; Ni, T.; Hou, Z.; Li, T.; Deng, W.; Ning, J. Effects of two yellowing process on colour, taste and nonvolatile compounds of bud yellow tea. Int. J. Food Sci. Technol. 2020, 55, 2931–2941. [Google Scholar] [CrossRef]
- Gómez-González, S.; Ruiz-Jiménez, J.; Priego-Capote, F.; de Castro, M.D.L. Qualitative and Quantitative Sugar Profiling in Olive Fruits, Leaves, and Stems by Gas Chromatography−Tandem Mass Spectrometry (GC-MS/MS) after Ultrasound-Assisted Leaching. J. Agric. Food Chem. 2010, 58, 12292–12299. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Simoneit, B.R. Analysis of sugars in environmental samples by gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1141, 271–278. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Quan, J.; Zheng, Q.; Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Xie, J.; Su, Y. Simultaneous determination of rhamnose, xylitol, arabitol, fructose, glucose, inositol, sucrose, maltose in jujube (Zizyphus jujube Mill.) extract: Comparison of HPLC–ELSD, LC–ESI–MS/MS and GC–MS. Chem. Central J. 2016, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.; Su, H.; Fang, S.; Chen, X.; Ning, J.; Ho, C.; Wan, X. Effects of roasting treatment on non-volatile compounds and taste of green tea. Int. J. Food Sci. Technol. 2018, 53, 2586–2594. [Google Scholar] [CrossRef]
- GB/T,2013. GB/T 8305-2013. Tea—Determination of Water-Soluble Substance Content. Available online: http://down.foodmate.net/standard/sort/3/39348.html (accessed on 15 July 2022).
- GB/T,2013. GB/T 8310-2013. Tea—Determination of Crude Fiber Content. Available online: http://down.foodmate.net/standard/sort/3/39353.html (accessed on 15 July 2022).
- GB,2016. GB 5009.4-2016: Determination of Ash Content in Food. Available online: http://down.foodmate.net/standard/sort/3/49326.html (accessed on 15 July 2022).
- Xiao, Z.; Tao, M.; Liu, Z. Effects of stem removal on physicochemical properties and sensory quality of tencha beverages (Camellia sinensis; Chuanxiaoye). J. Food Sci. 2021, 86, 327–333. [Google Scholar] [CrossRef]
- Wang, L.-F.; Park, S.-C.; Chung, J.-O.; Baik, J.-H. The Compounds Contributing to the Greenness of Green Tea. J. Food Sci. 2004, 69, S301–S305. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and ecophysiology of tree stems: Corticular and wood photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-D.; Yang, J.-H.; Hsieh, Y.-J.; Liu, E.-H.; Mau, J.-L. Effect of Different Brewing Methods on Quality of Green Tea. J. Food Processing Preserv. 2014, 38, 1234–1243. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Fan, C.; Han, L. A method for producing superfine black tea powder with enhanced infusion and dispersion property. Food Chem. 2017, 214, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Ding, Z.; Ding, S.; Wang, H.; Bi, C.; Wang, L. Metabolic analyses reveal growth characteristics of young tea shoots in spring. Sci. Hortic. 2019, 246, 478–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Zhang, Z.; Huang, X.; Zhang, L.; Sun, P. Cellulose accumulation in the secondary cell walls is negatively correlated with the tenderness of new shoots in Camellia sinensis. Physiol Plant 2021, 172, 1700–1710. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Teng, R.-M.; Wang, W.-L.; Wang, Y.; Shen, W.; Zhuang, J. Identification of genes revealed differential expression profiles and lignin accumulation during leaf and stem development in tea plant (Camellia sinensis (L.) O. Kuntze). Protoplasma 2019, 256, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Janet, T.C.; John, W.K.; Thomas, K.; Kelvin, M.O.; Francis, W.N. Effect of Seasons on Theanine Levels in Different Kenyan Commercially Released Tea Cultivars and Its Variation in Different Parts of the Tea Shoot. Food Nutr. Sci. 2015, 06, 1450–1459. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Yuikawa, N.; Tanihiro, N.; Michihata, T.; Enomoto, T. The Effects of Roasting Conditions on the Physical Appearance Traits and Aroma and Taste Components of Roasted Stem Tea. Food Sci. Technol. Res. 2020, 26, 643–654. [Google Scholar] [CrossRef]
- Sasaki, T.; Koshi, E.; Take, H.; Michihata, T.; Maruya, M.; Enomoto, T. Characterisation of odorants in roasted stem tea using gas chromatography-mass spectrometry and gas chromatography-olfactometry analysis. Food Chem. 2017, 220, 177–183. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Guo, W.; He, C.; Tao, M.; Liu, Z. Exploring the Quality and Application Potential of the Remaining Tea Stems after the Postharvest Tea Leaves: The Example of Lu’an Guapian Tea (Camellia sinensis L.). Foods 2022, 11, 2357. https://doi.org/10.3390/foods11152357
Zhang W, Guo W, He C, Tao M, Liu Z. Exploring the Quality and Application Potential of the Remaining Tea Stems after the Postharvest Tea Leaves: The Example of Lu’an Guapian Tea (Camellia sinensis L.). Foods. 2022; 11(15):2357. https://doi.org/10.3390/foods11152357
Chicago/Turabian StyleZhang, Wenjun, Wenli Guo, Changxu He, Meng Tao, and Zhengquan Liu. 2022. "Exploring the Quality and Application Potential of the Remaining Tea Stems after the Postharvest Tea Leaves: The Example of Lu’an Guapian Tea (Camellia sinensis L.)" Foods 11, no. 15: 2357. https://doi.org/10.3390/foods11152357
APA StyleZhang, W., Guo, W., He, C., Tao, M., & Liu, Z. (2022). Exploring the Quality and Application Potential of the Remaining Tea Stems after the Postharvest Tea Leaves: The Example of Lu’an Guapian Tea (Camellia sinensis L.). Foods, 11(15), 2357. https://doi.org/10.3390/foods11152357





