Structural Characterization, Rheological Properties and Protection of Oxidative Damage of an Exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Chemicals
2.2. Determination of Growth Curve
2.3. EPS Characterization
2.3.1. Production of EPS
2.3.2. Chemical Composition Analysis
2.3.3. Monosaccharide Composition Analysis and Molecular Weight (Mw) Determination
2.3.4. Spectra Analysis
2.3.5. Congo Red Test
2.3.6. 1D- and 2D-NMR Spectra Analysis
2.3.7. Scanning Electron Microscopy (SEM) Analysis
2.4. Rheological Properties of EPS-1
2.5. Antioxidant Properties of EPS-1
2.5.1. ABTS Free Radical Scavenging Activity
2.5.2. Ferric Ion Reducing Activity
2.5.3. Metal Ion Chelating Activity
2.6. Effect of EPS-1 on Yeast Resistance to Oxidative Stress
2.7. Statistical Analysis
3. Results and Discussion
3.1. Time Course of Bacterial Growth and EPS Production by Lc. citreum 1.2461
3.2. EPS-1 Characterization
3.2.1. Isolation and Purification of EPS-1
3.2.2. Monosaccharide Composition and Mw of the EPS-1
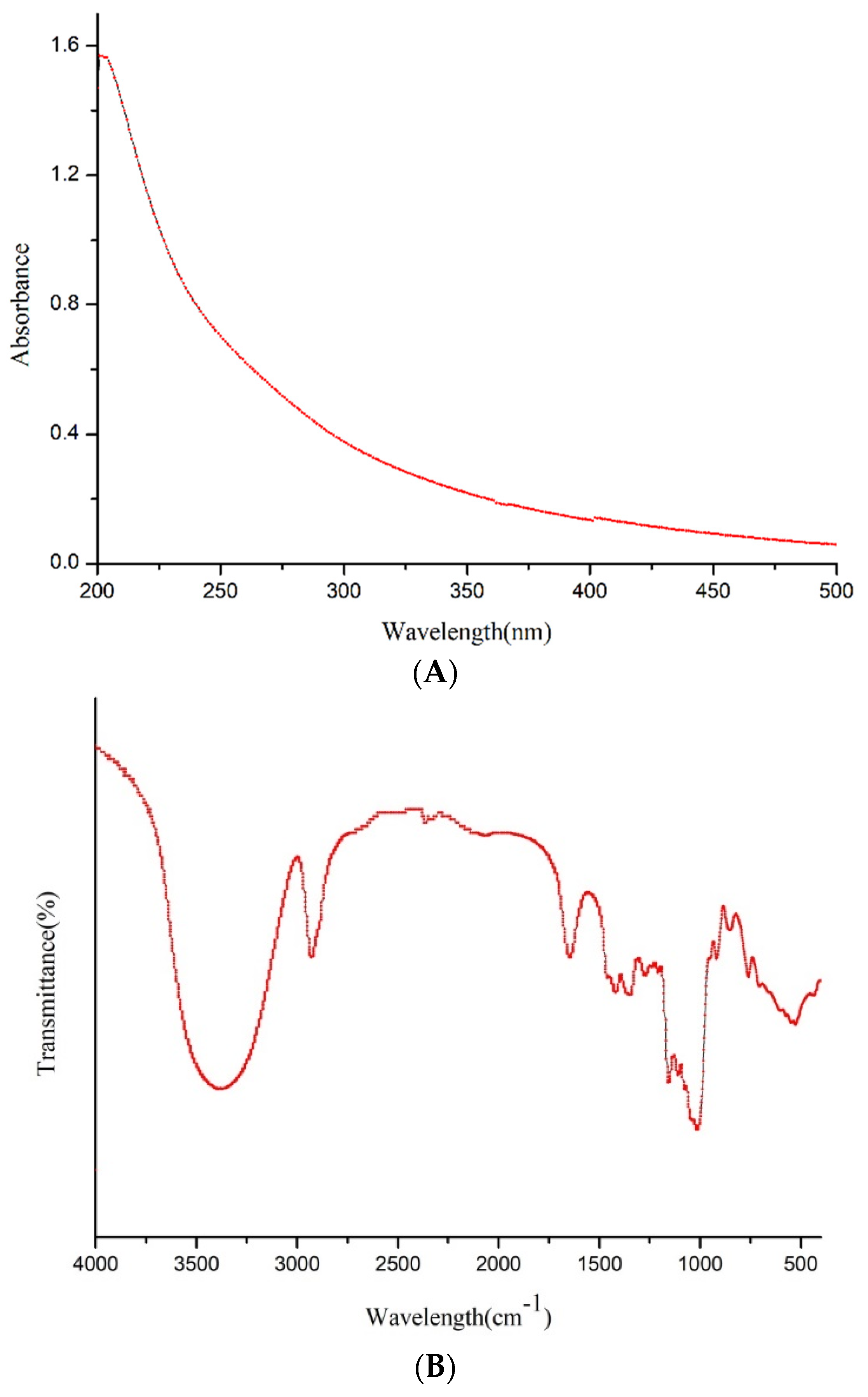
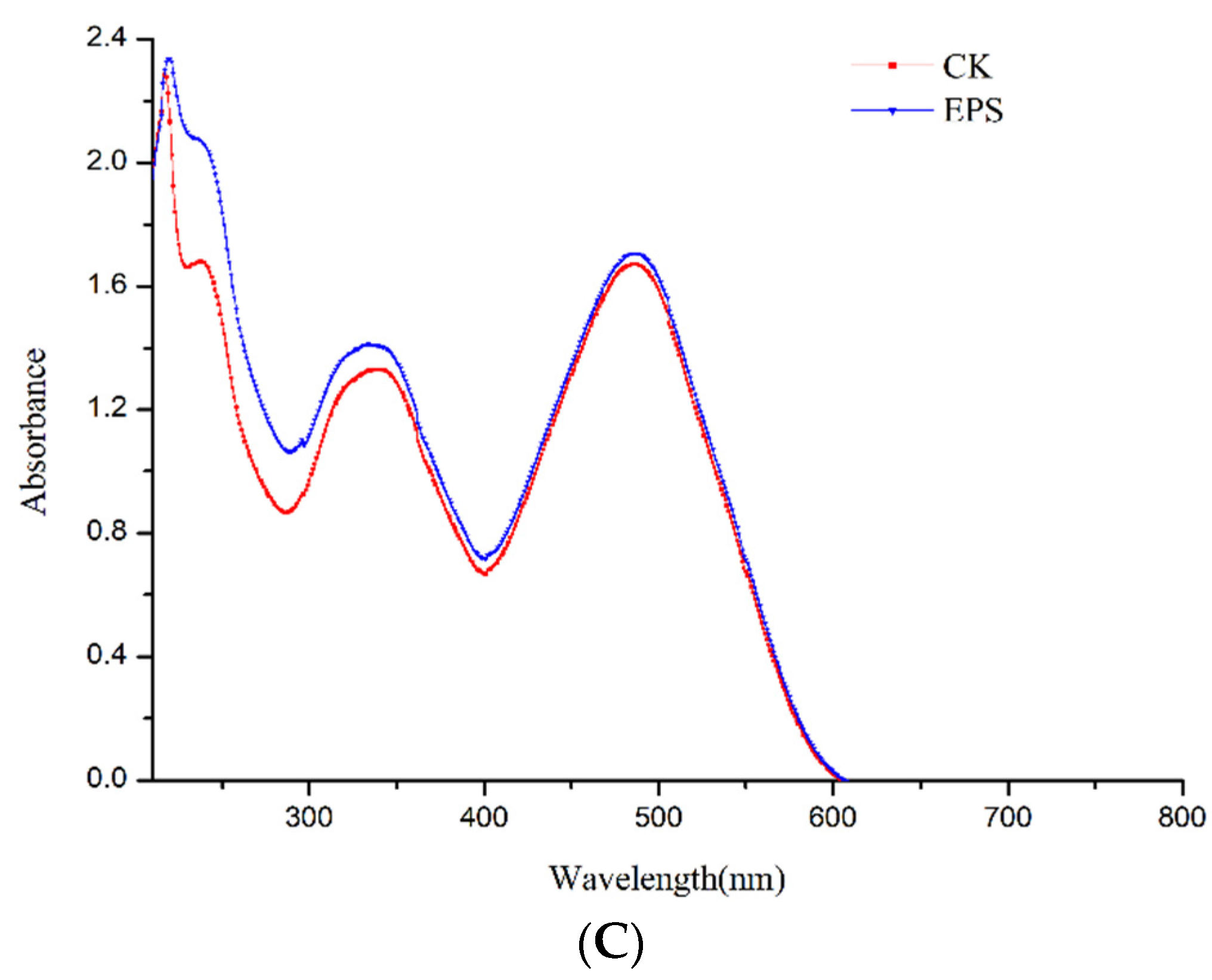
3.2.3. UV–Vis, FT-IR Analysis and Congo Red Test
3.2.4. NMR Spectroscopy Analysis
3.2.5. SEM Analysis
3.3. Rheological Measurement
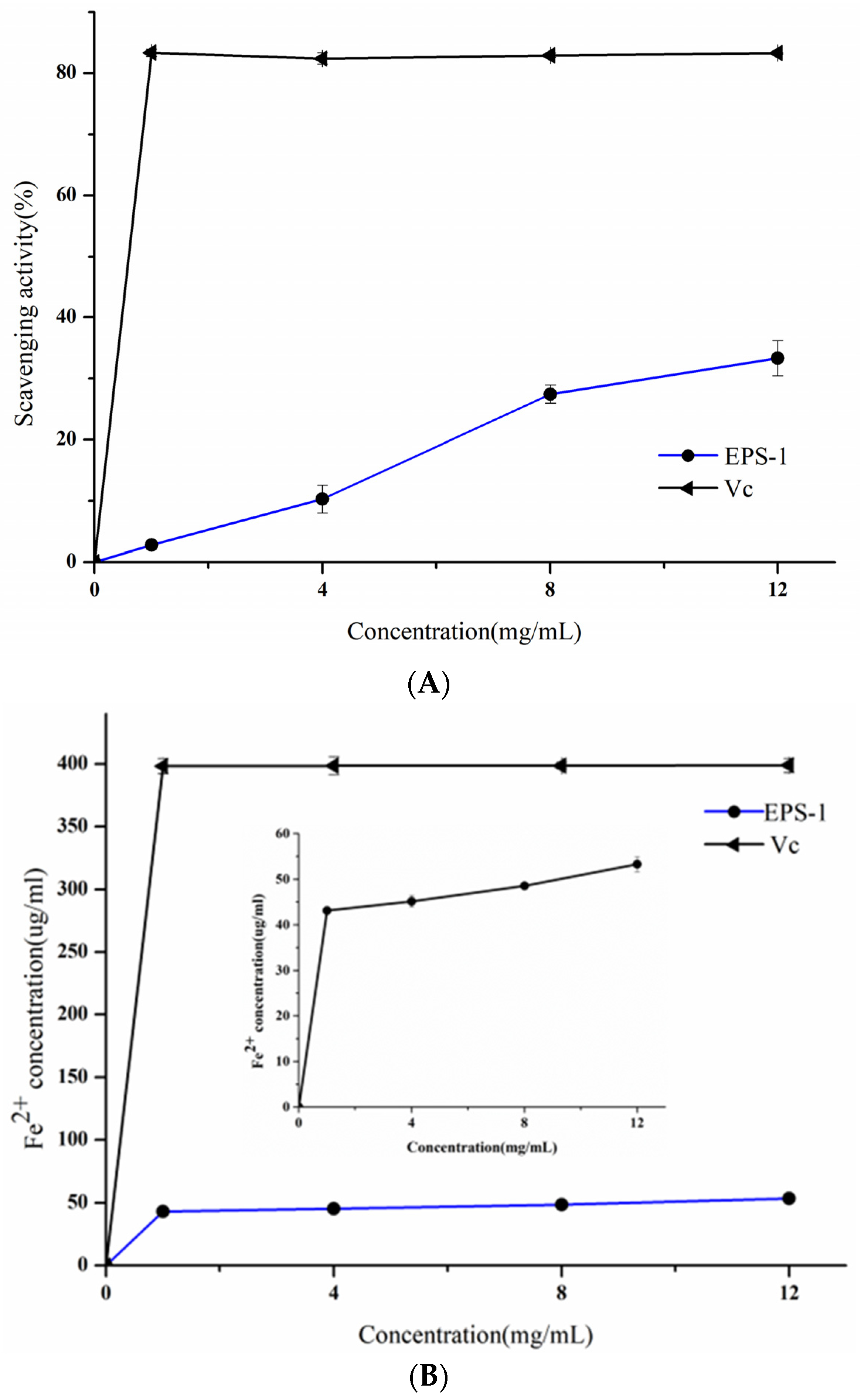
3.4. Antioxidant Activities of EPS-1
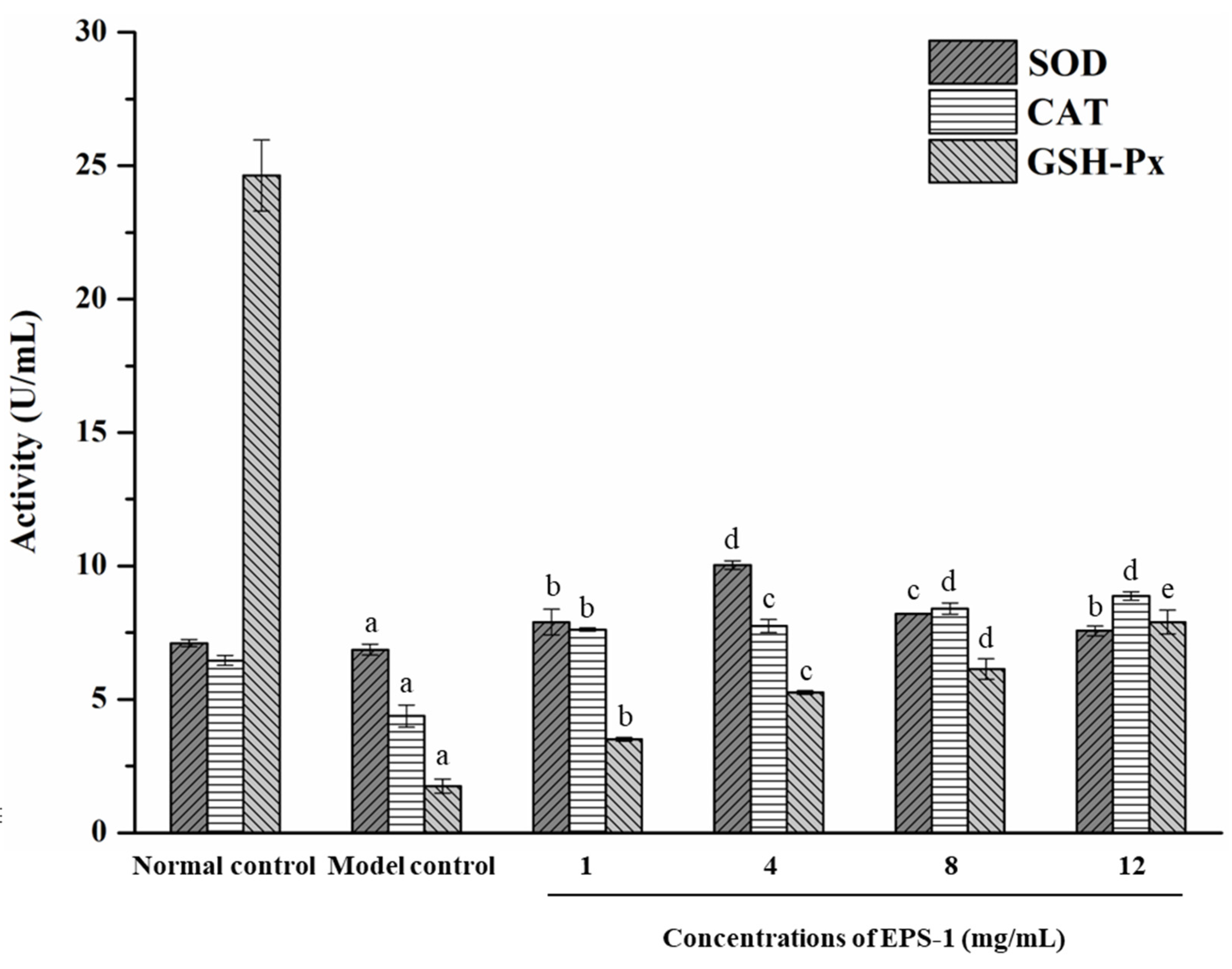
3.5. Effect of EPS on Yeast Resistance to Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Shen, M.; Yu, Q.; Chen, Y.; Huang, L.; Xie, J. The water-soluble non-starch polysaccharides from natural resources against excessive oxidative stress: A potential health-promoting effect and its mechanisms. Int. J. Biol. Macromol. 2021, 171, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Fukuda, K.; Shi, T.; Nagami, K.; Leo, F.; Nakamura, T.; Yasuda, K.; Senda, A.; Motoshima, H.; Urashima, T. Effects of carbohydrate source on physicochemical properties of the exopolysaccharide produced by Lactobacillus fermentum TDS030603 in a chemically defined medium. Carbohydr. Polym. 2010, 79, 1040–1045. [Google Scholar] [CrossRef]
- Li, R.; Xintong, J.; Youshuang, Z.; Yuran, Z.; Jing, G. Precipitation of proteins from soybean whey wastewater by successive foaming and defoaming. Chem. Eng. Process. 2018, 128, 124–131. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Chua, J.Y.; Liu, S.Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Joyce, O.T.; Chakraborty, S.K.; Tripathi, M.K.; Kotwaliwale, N.; Chandra, P. Quality characteristics of sauerkraut fermented by using a Lactobacillus paracasei starter culture grown in tofu whey. Food Sci. Technol. Int. 2018, 24, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ounis, W.B.; Champagne, C.P.; Makhlouf, J.; Bazinet, L. Utilization of tofu whey pre-treated by electromembrane process as a growth medium for Lactobacillus plantarum LB17. Desalination 2008, 229, 192–203. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Li, Q.; Chen, H.; Zhang, W.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2-induced injury RAW264. 7 cells. Int. J. Biol. Macromol. 2018, 118, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT-Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, Z.; Zhang, Z.; Shi, X. Characterization on structure and bioactivities of an exopolysaccharide from Lactobacillus curvatus SJTUF 62116. Int. J. Biol. Macromol. 2022, 210, 504–517. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Lobo, R.E.; Gómez, M.I.; de Valdez, G.F.; Torino, M.I. Physicochemical and antioxidant properties of a gastroprotective exopolysaccharide produced by Streptococcus thermophilus CRL1190. Food Hydrocoll. 2019, 96, 625–633. [Google Scholar] [CrossRef]
- Si, J.; Meng, G.; Wu, Y.; Ma, H.F.; Cui, B.K.; Dai, Y.C. Medium composition optimization, structural characterization, and antioxidant activity of exopolysaccharides from the medicinal mushroom Ganoderma lingzhi. Int. J. Biol. Macromol. 2019, 124, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Wang, X.; Miao, J.; Tian, Y.T. Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Q.; Wang, W.; Li, X.; Zhang, J. A polysaccharide isolated from Cynomorium songaricum Rupr. protects PC12 cells against H2O2-induced injury. Int. J. Biol. Macromol. 2016, 87, 222–228. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Pichyangkura, R.; Nepogodiev, S.A.; Wonganan, P.; Mahalapbutr, P.; Field, R.A. Characterization of a nanoparticulate exopolysaccharide from Leuconostoc holzapfelii KM01 and its potential application in drug encapsulation. Int. J. Biol. Macromol. 2021, 187, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Characterization and biocompatibility of glucan: A safe food additive from probiotic Lactobacillus plantarum DM5. J. Sci. Food Agric. 2014, 94, 683–690. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterization of a novel water-soluble polysaccharide from Leuconostoc citreum SK24. 002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Purama, R.K.; Goswami, P.; Khan, A.T.; Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr. Polym. 2009, 76, 30–35. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Taşdemir, V.; Sagdic, O.; Dertli, E. Characterisation and functional roles of a highly branched dextran produced by a bee pollen isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45, 101330. [Google Scholar] [CrossRef]
- Amari, M.; Arango, L.F.G.; Gabriel, V.; Robert, H.; Morel, S.; Moulis, C.; Gabriel, B.; Remaud-Siméon, M.; Fontagné-Faucher, C. Characterization of a novel dextransucrase from Weissella confusa isolated from sourdough. Appl. Microbiol. Biotechnol. 2013, 97, 5413–5422. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Ningsanond, S.; Cui, S.W.; Goff, H.D. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll. 2005, 19, 793–801. [Google Scholar] [CrossRef]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Xiao, L.; Han, S.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Chen, X.; Zhang, Q.; Li, W. Preparation, characterization and antioxidant activities of derivatives of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 145, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Li, T.; Hua, Q.; Li, N.; Cui, Y.; Zhao, M. Protective effect of a polysaccharide from Dipsacus asper Wall on streptozotocin (STZ)-induced diabetic nephropathy in rat. Int. J. Biol. Macromol. 2019, 133, 1194–1200. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]


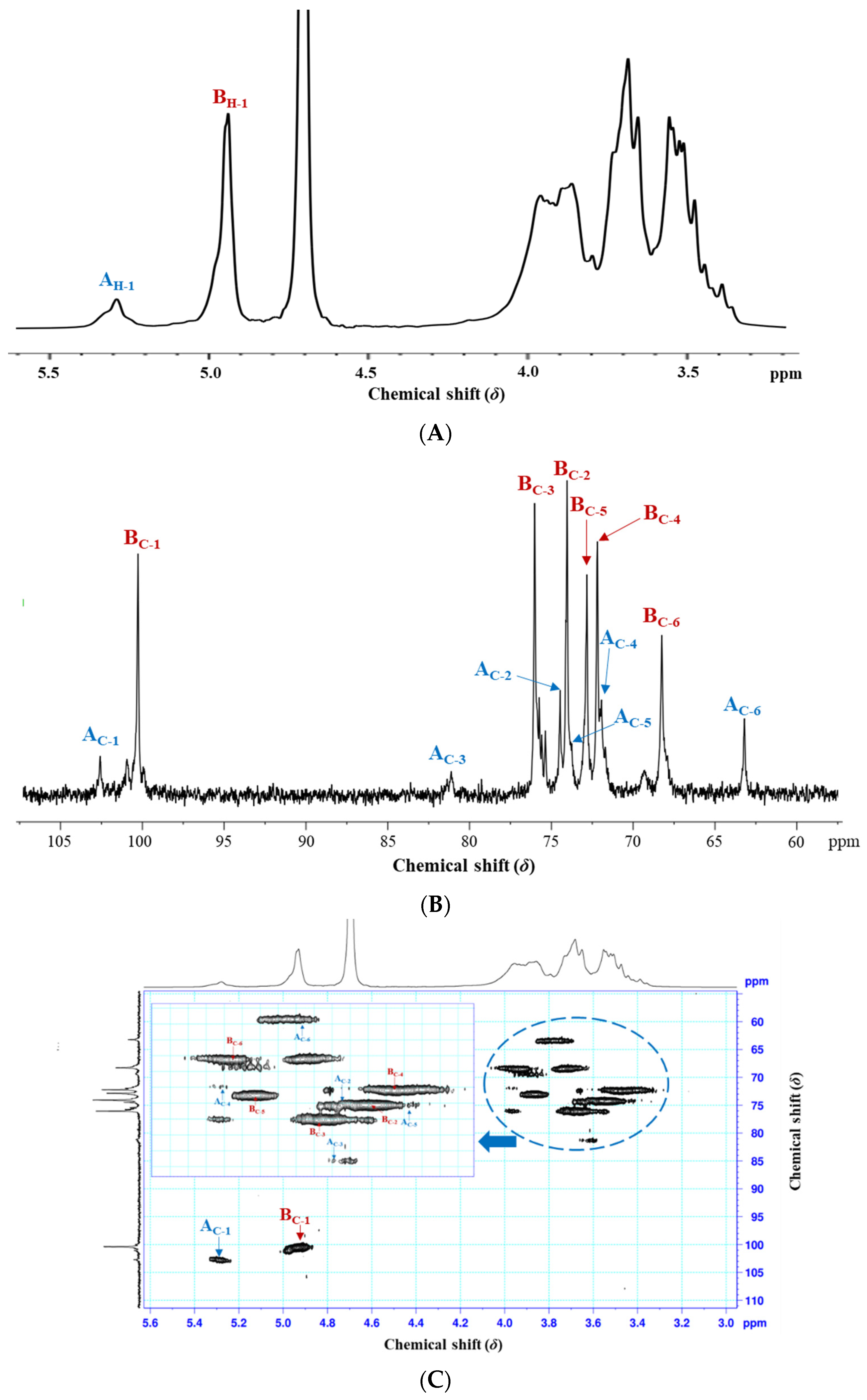





| Glycosyl Residues | H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 |
|---|---|---|---|---|---|---|
| A | 5.28 | 3.62 | 3.65 | 3.95 | 3.44 | 3.73 |
| →3)-α-D-Glcp (1→ | 102.69 | 74.49 | 81.16 | 71.96 | 73.79 | 63.19 |
| B | 4.93 | 3.54 | 3.68 | 3.47 | 3.86 | 3.92 |
| →6)-α-D-Glcp (1→ | 100.36 | 74.07 | 76.05 | 72.20 | 72.85 | 68.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, L.; Tian, J.; Wang, X.; Zhang, X.; Fang, Y.; Li, W. Structural Characterization, Rheological Properties and Protection of Oxidative Damage of an Exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey. Foods 2022, 11, 2283. https://doi.org/10.3390/foods11152283
Li Y, Xiao L, Tian J, Wang X, Zhang X, Fang Y, Li W. Structural Characterization, Rheological Properties and Protection of Oxidative Damage of an Exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey. Foods. 2022; 11(15):2283. https://doi.org/10.3390/foods11152283
Chicago/Turabian StyleLi, Yingying, Luyao Xiao, Juanjuan Tian, Xiaomeng Wang, Xueliang Zhang, Yong Fang, and Wei Li. 2022. "Structural Characterization, Rheological Properties and Protection of Oxidative Damage of an Exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey" Foods 11, no. 15: 2283. https://doi.org/10.3390/foods11152283
APA StyleLi, Y., Xiao, L., Tian, J., Wang, X., Zhang, X., Fang, Y., & Li, W. (2022). Structural Characterization, Rheological Properties and Protection of Oxidative Damage of an Exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey. Foods, 11(15), 2283. https://doi.org/10.3390/foods11152283







