Effect of Refining Degree on the Quality Changes and Lipid Oxidation of Camellia (Camellia oleifera) Oil during Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Basic Physical and Chemical Indicators
2.4. Lipid Extraction
2.5. LC–ESI–MS/MS Analysis
2.6. Data Processing and Lipid Identification
2.7. Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical Parameters and Fatty Acid Composition
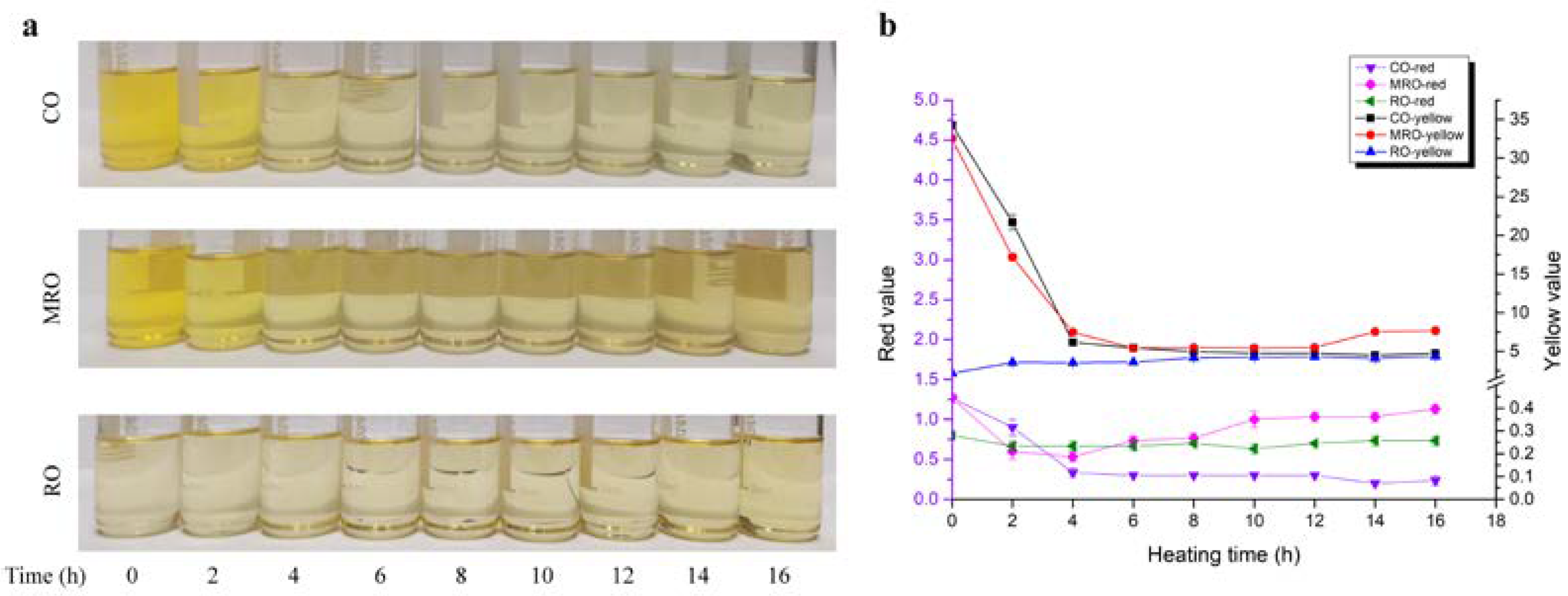
3.1.1. Color Variation
3.1.2. Iodine Value
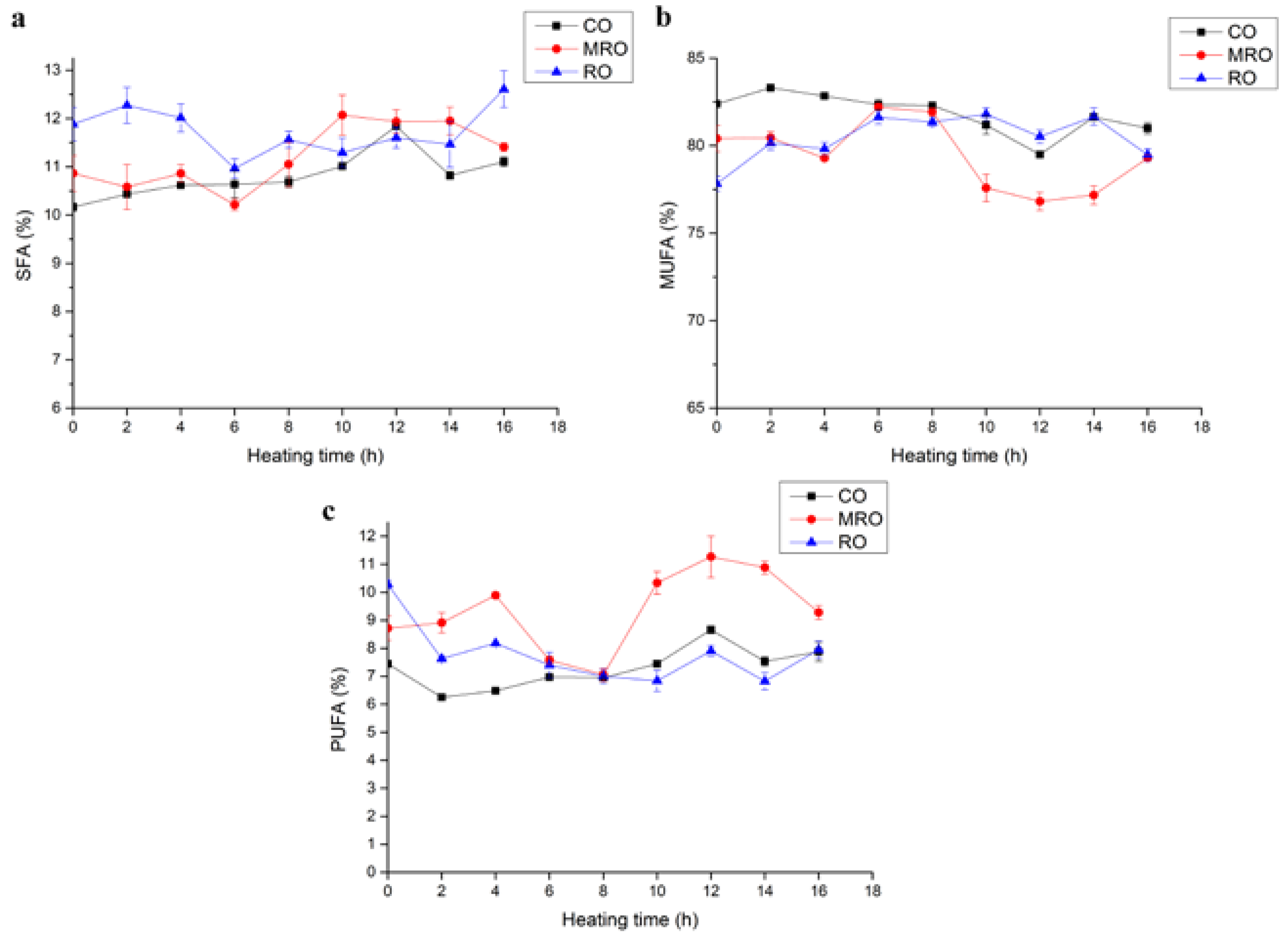
3.1.3. Fatty Acid Composition
3.2. Degree of Oxidation
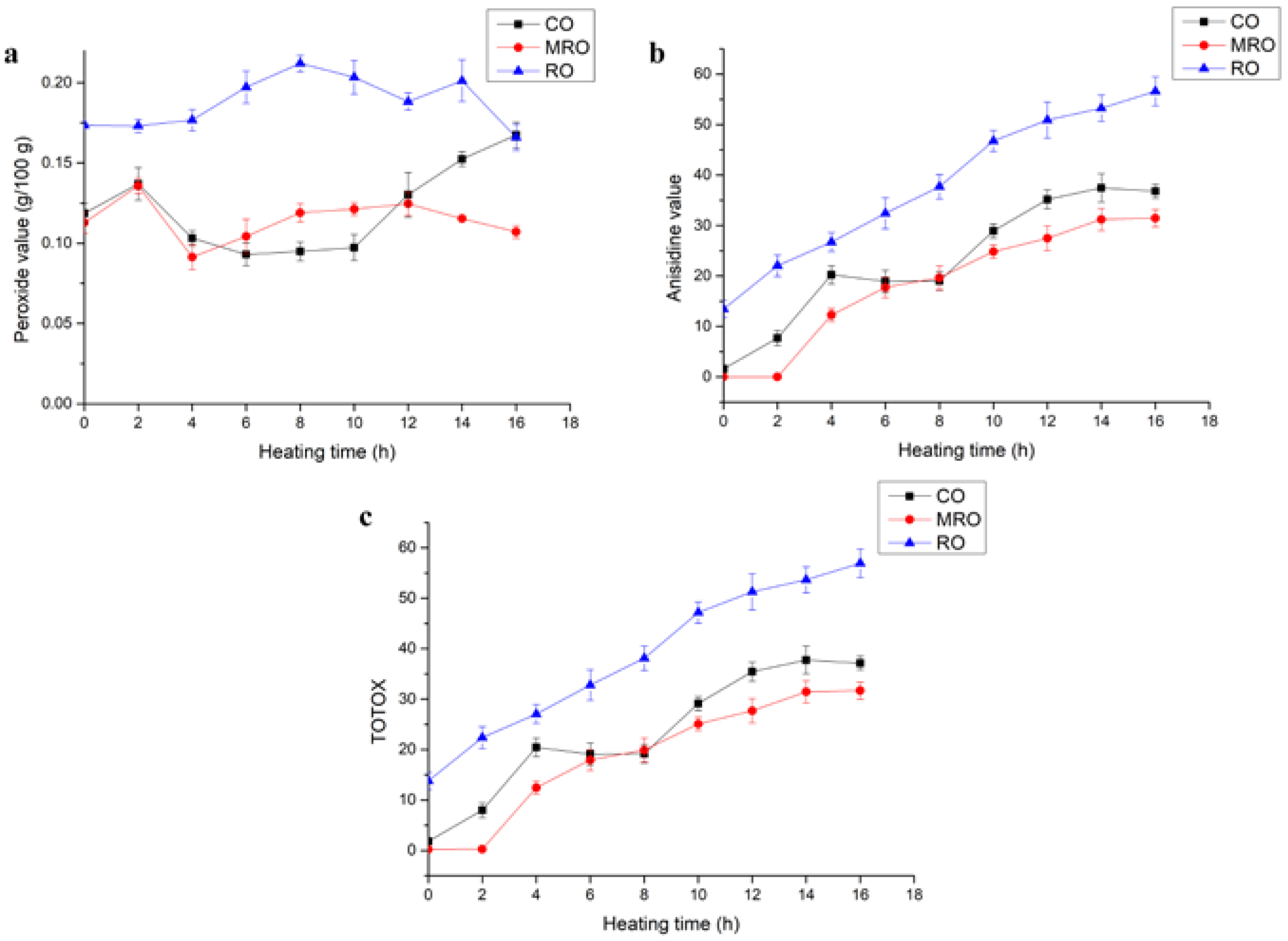
3.2.1. PV, p-AV, and Total Oxidation (TOTOX) Value
3.2.2. K232 and K268
3.2.3. Polar Components
3.3. Natural Antioxidant Substances
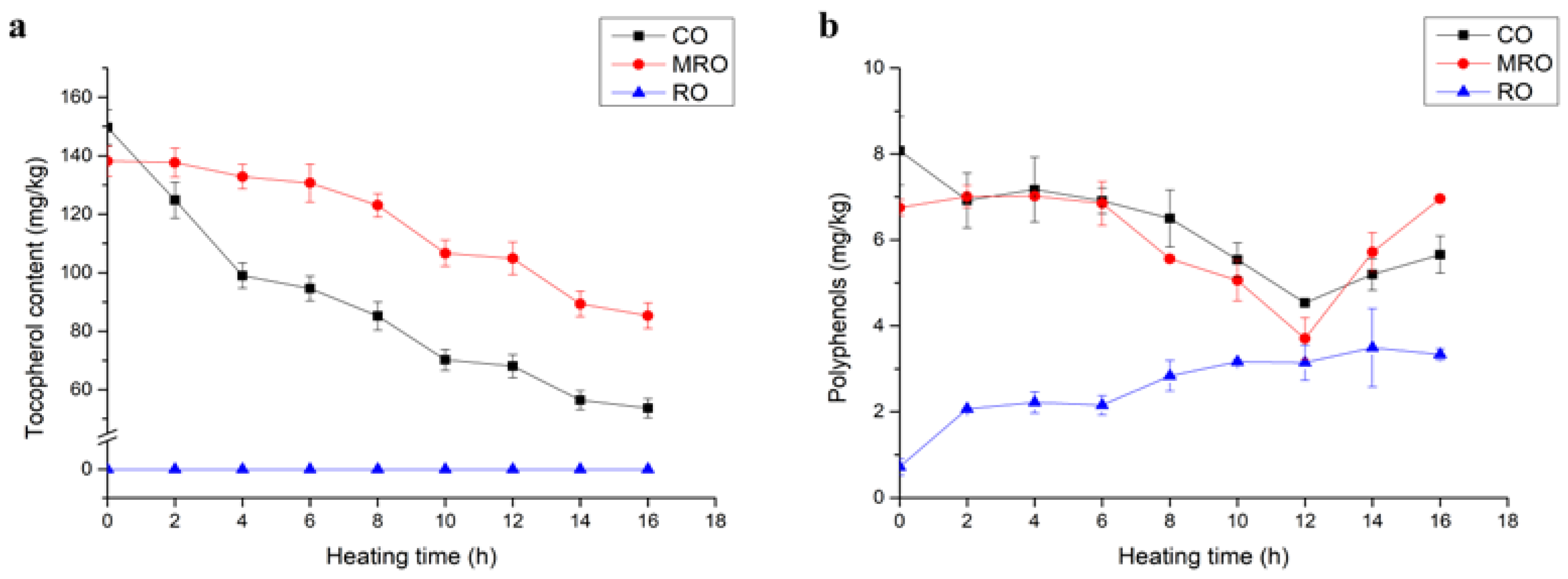
3.3.1. Tocopherol
3.3.2. Polyphenols
3.4. Lipidomic Analysis of Lipid Changes in Camellia Oil after Heating
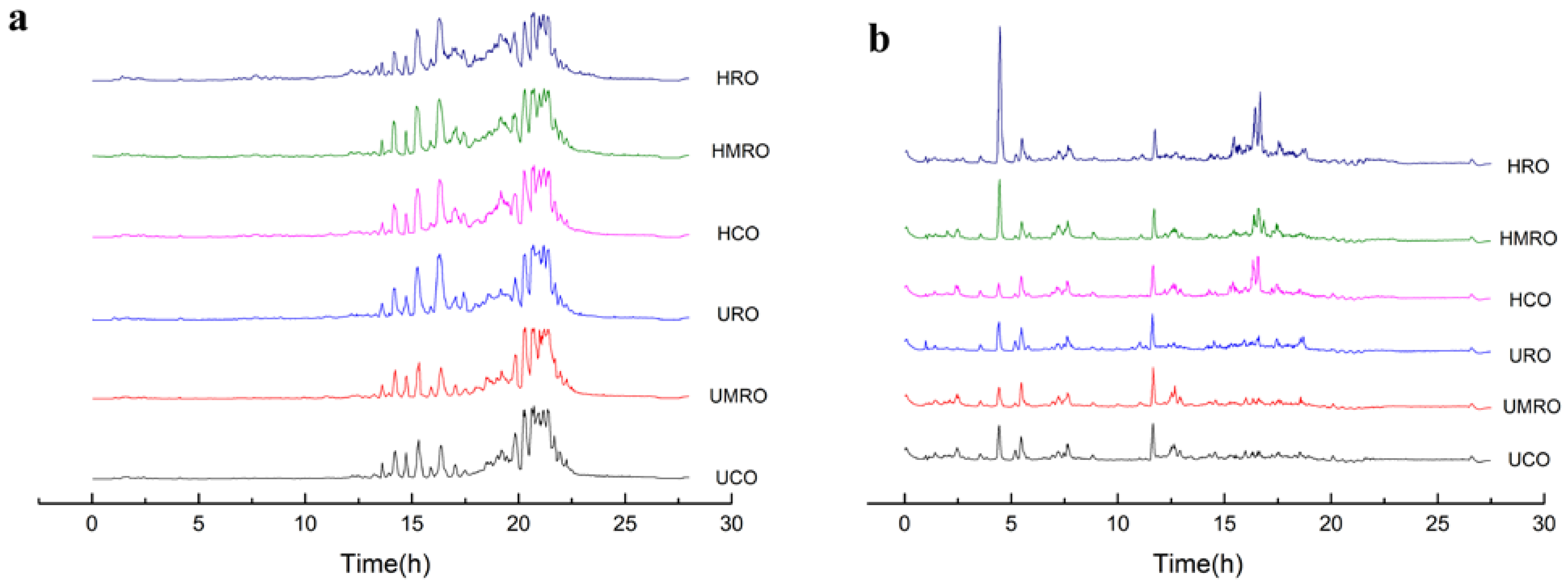
3.4.1. Identified Lipids Based on LC–ESI–MS/MS
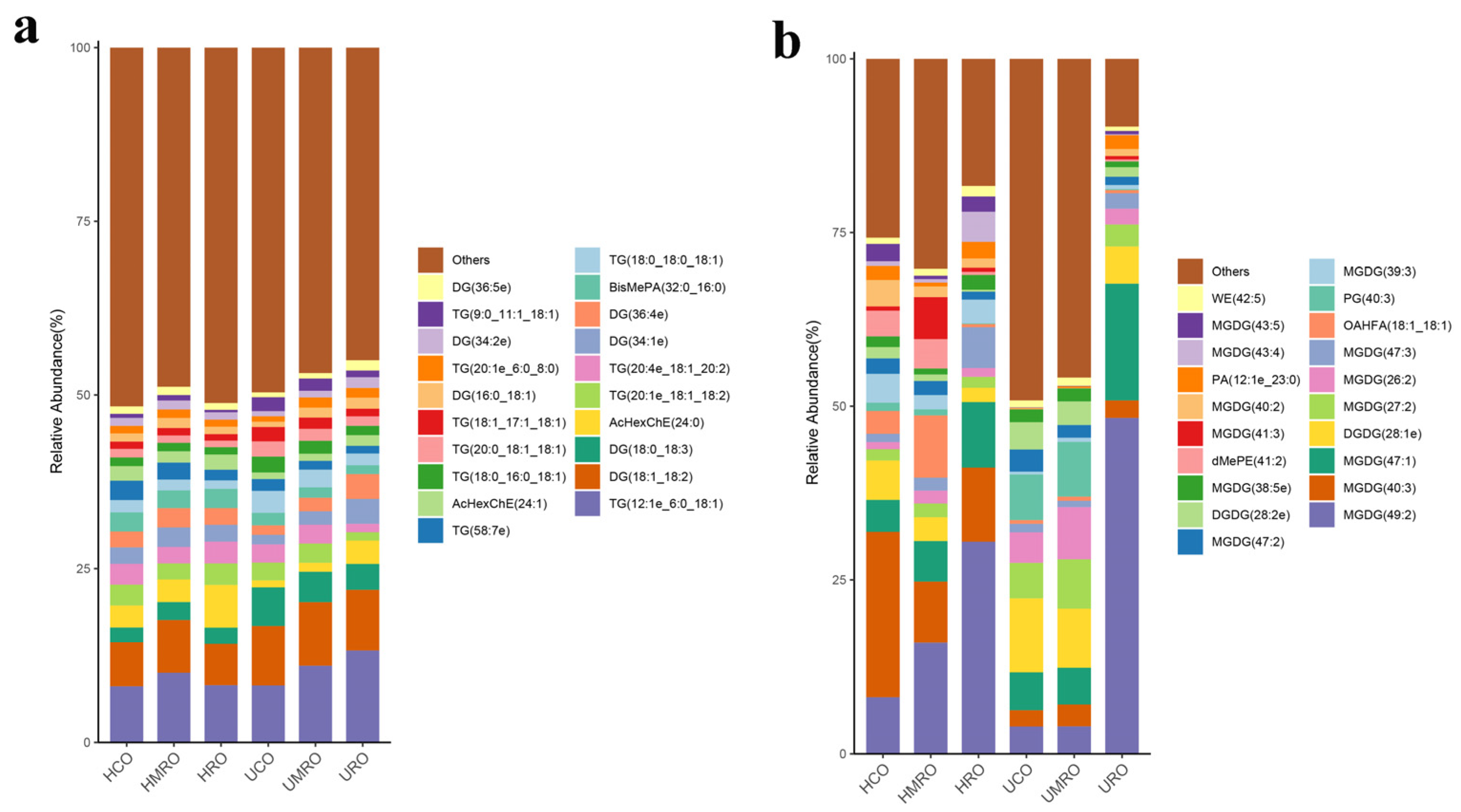
3.4.2. Lipids Changes in Camellia Oils after Heating
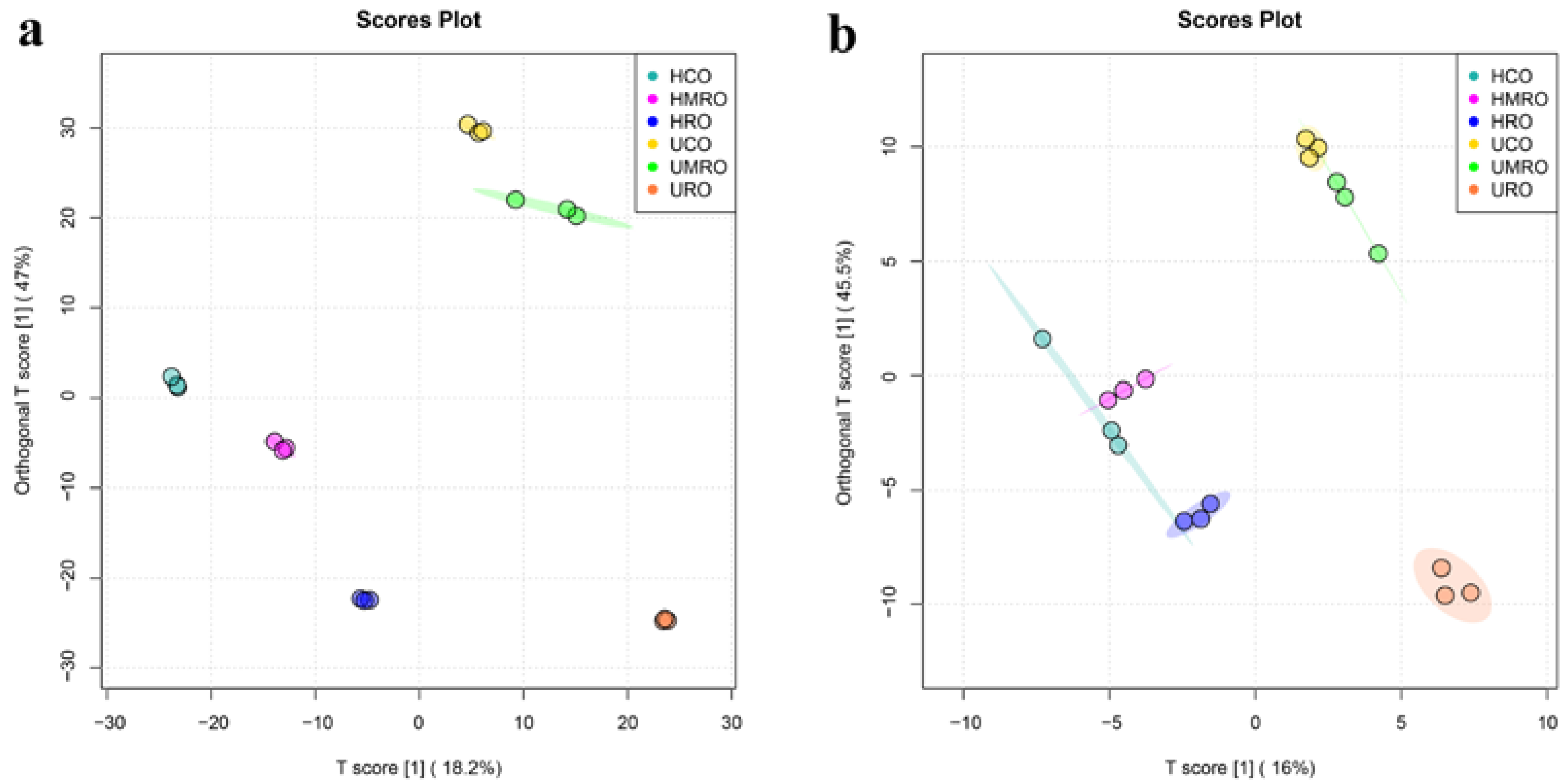
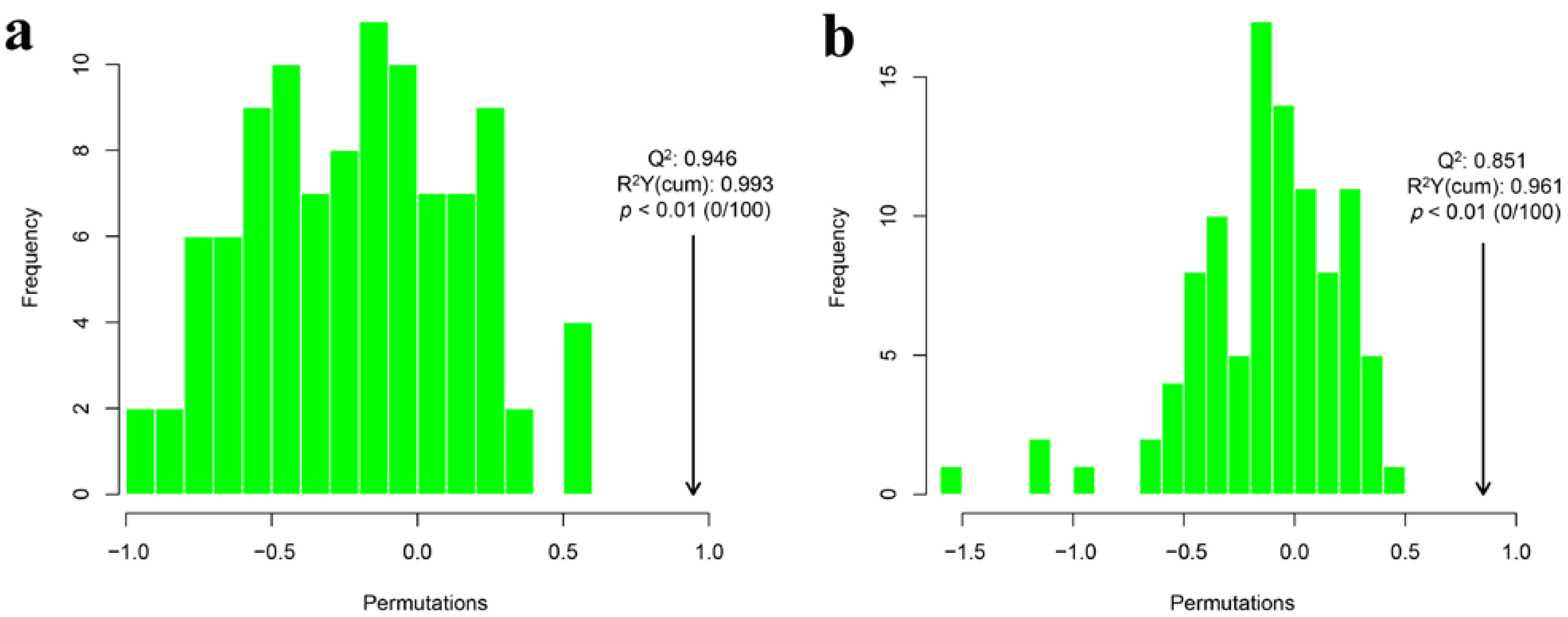
3.4.3. Clustering Analysis
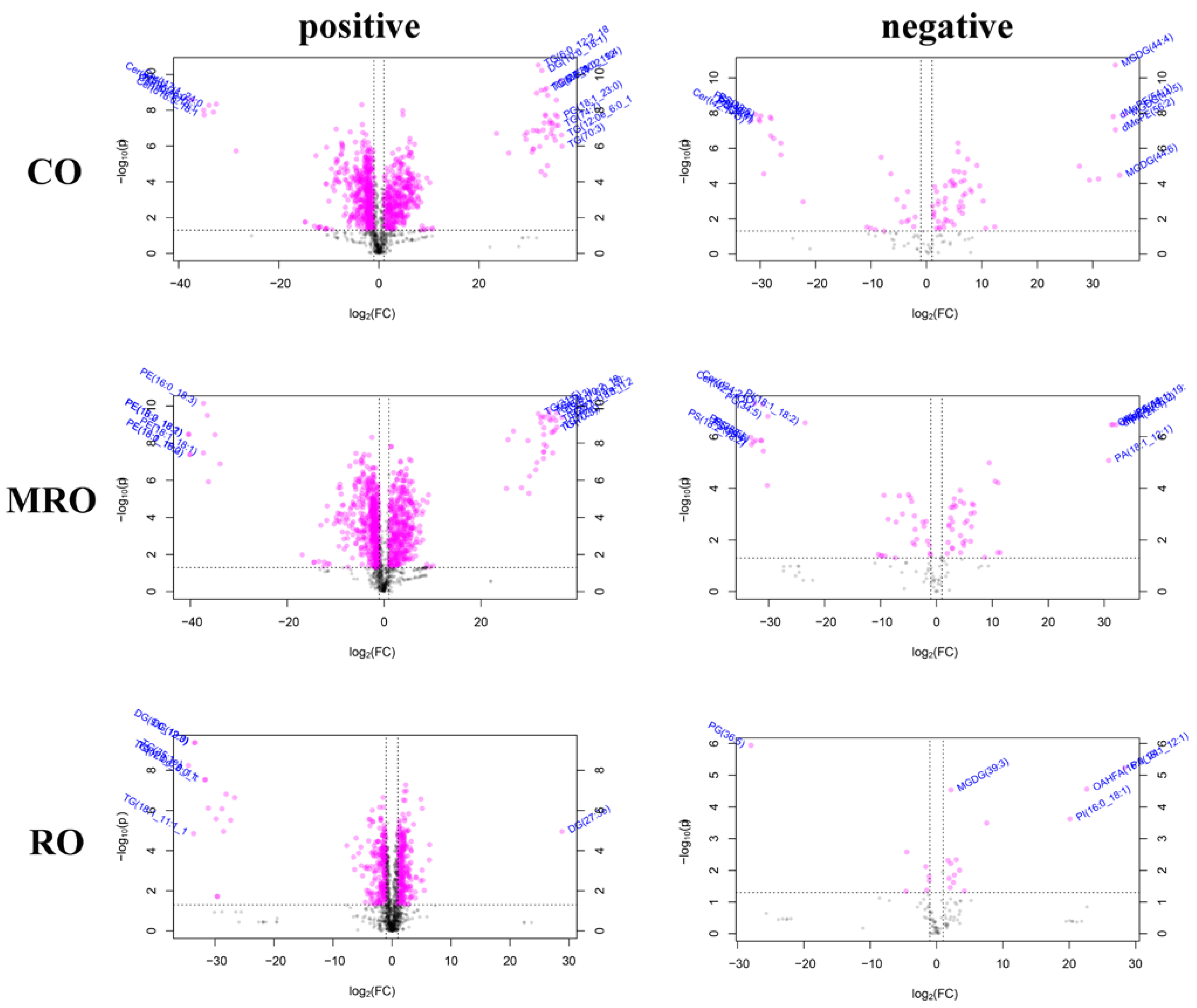
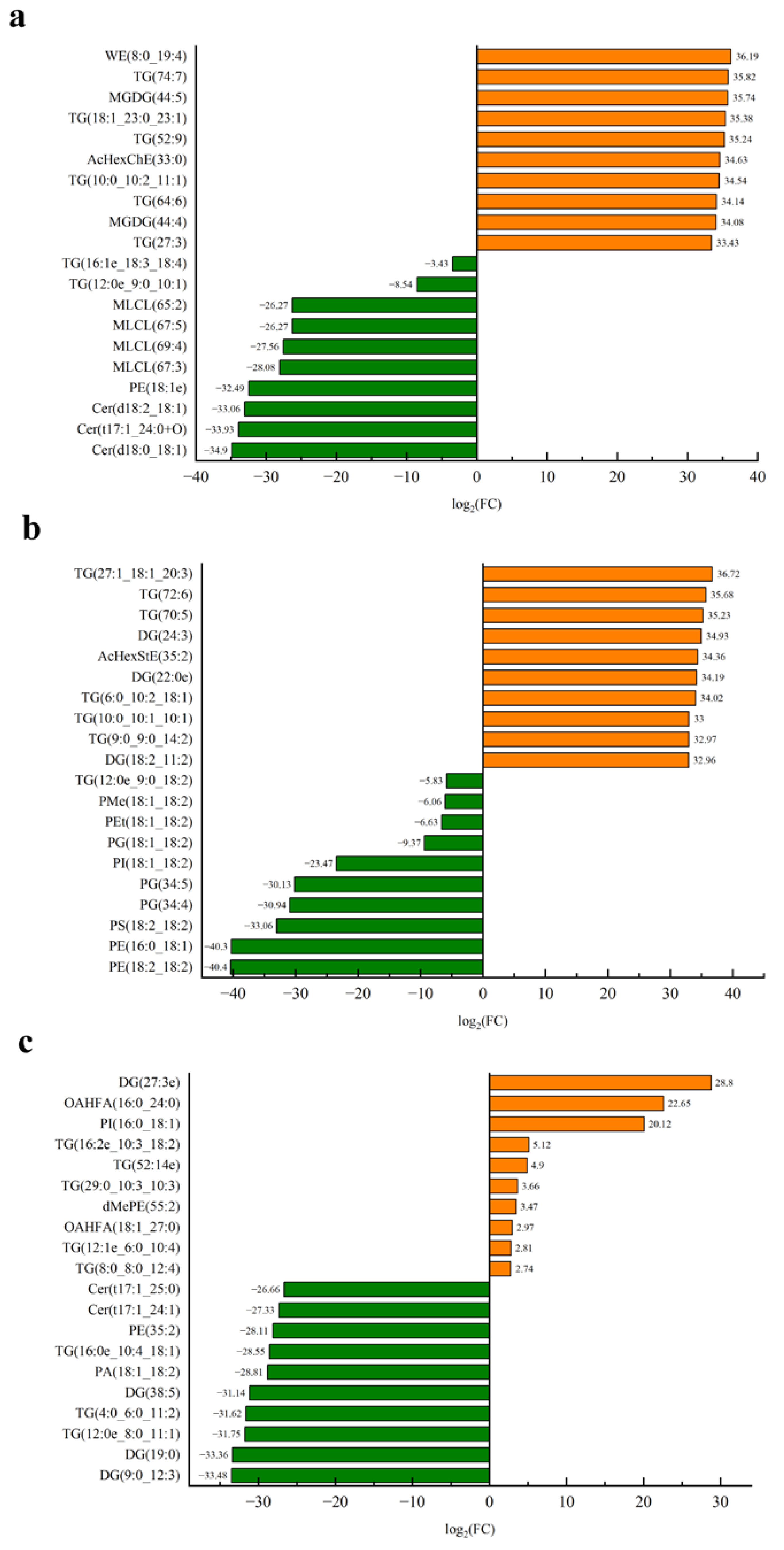
3.4.4. Screening of Differential Lipids
Common Differential Lipids
Unique Differential Lipids
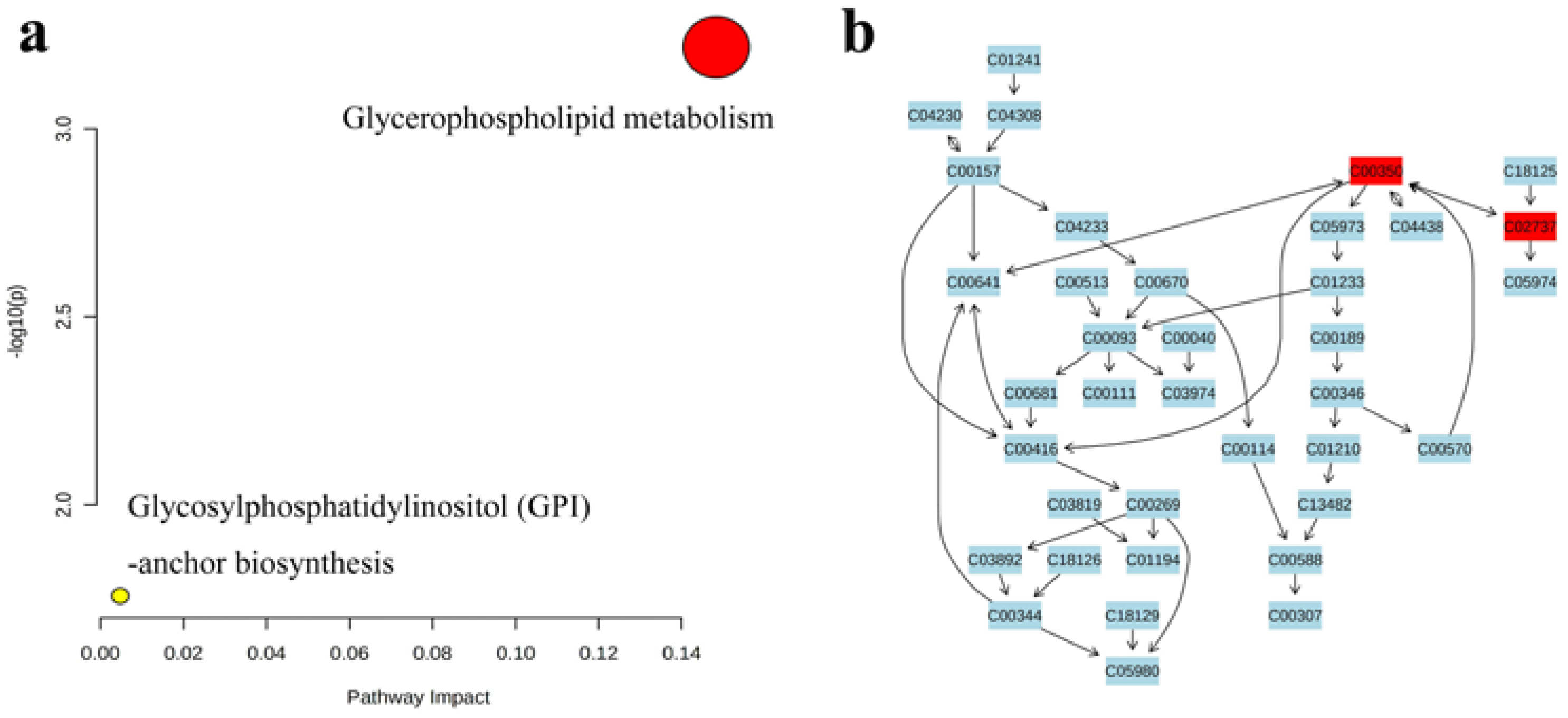
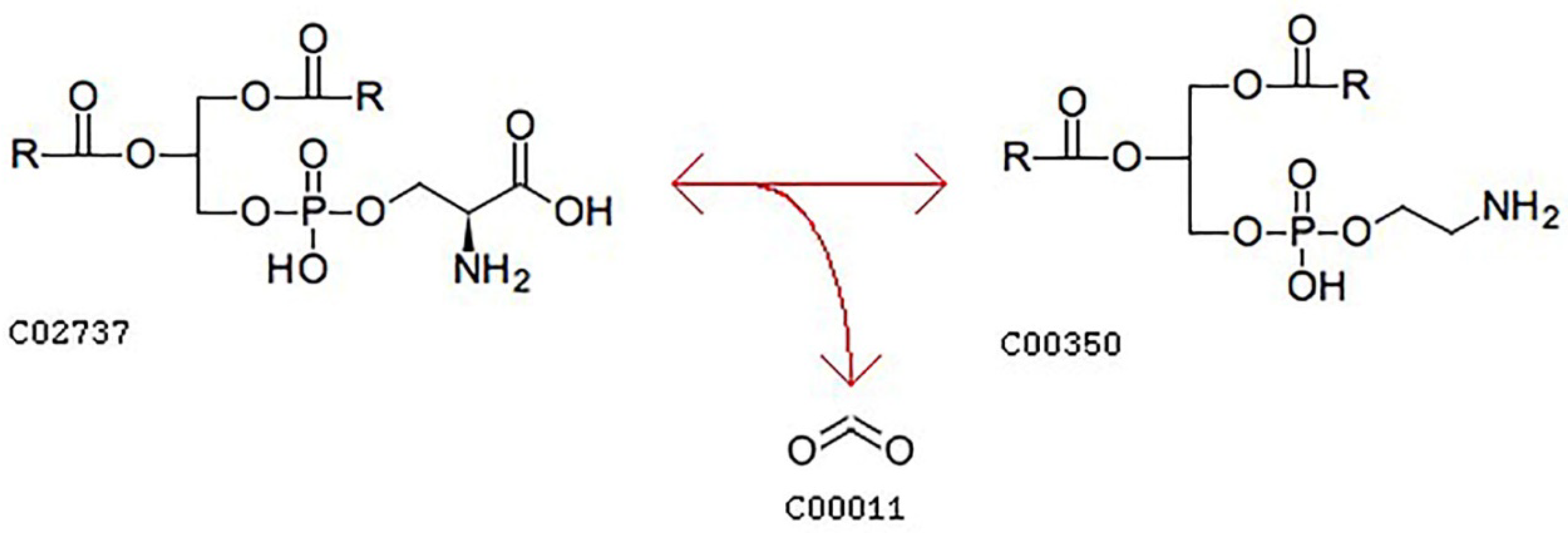
3.4.5. Pathways Analyses of Lipids in Camellia Oil
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO | crude oil |
| MRO | moderately refined oil |
| RO | refined oil |
| HCO | heated crude oil |
| HMRO | heated moderately refined oil |
| HRO | heated refined oil |
| UCO | unheated crude oil |
| UMRO | unheated moderately refined oil |
| URO | unheated refined oil |
| PV | peroxide Value |
| p-AV | p-anisidine value |
| PCA | principal components analysis |
| PLS-DA | partial least squares discriminant analysis |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| RF | random forest |
| SVM | support vector machine |
| VIP | value importance in projection |
| FC | fold change |
| ORA | over representation analysis |
| SFA | saturated fatty acids |
| MUFA | monounsaturated fatty acids |
| PUFA | polyunsaturated fatty acids |
| UFA | unsaturated fatty acids |
| TOTOX | total oxidation values |
| TIC | total Ion Chromatography |
| TG | triacylglycerol |
| DG | diacylglycerols |
| MGDG | monogalactosyl diacylglycerols |
| PA | phosphatidic acids |
| Cer | ceramides |
| MLCL | monolysocardiolipin |
| PS | phosphatidylserines |
| LPE | lyso-phosphatidylethanolamines |
| PE | phosphatidylethanolamines |
| PG | phosphatidylglycerols |
| dMePE | dimethylphosphatidylethanolamine |
| WE | wax esters |
| SQMG | sulphoquinovosyldiacylglycerols |
| CmE | campesterol ester |
| PI | phosphatidylinositols |
References
- Ye, M.; Zhou, H.; Hao, J.; Chen, T.; Liu, X. Microwave pretreatment on microstructure, characteristic compounds and oxidative stability of Camellia seeds. Ind. Crops Prod. 2021, 161, 113193. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Wei, W.; Huang, C.; Xi, C.; Xi, Z.; Feng, F. Triacylglycerols of camellia oil: Composition and positional distribution of fatty acids. Eur. J. Lipid Sci. Technol. 2016, 118, 1254–1255. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, M.Y.; Yoon, S.H. Optimization of the refining process of camellia seed oil for edible purposes. Food Sci. Biotechnol. 2014, 23, 65–73. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Qiu, X.; Hu, W.; Sun, H.; Chen, X.; Bai, Y.; Gu, X.; Wang, C.; Chen, H.; et al. Optimizing refining temperatures to reduce the loss of essential fatty acids and bioactive compounds in tea seed oil. Food and Bioproducts Processing. Trans. Inst. Chem. Eng. Part C 2015, 94, 136–146. [Google Scholar]
- Strieder, M.M.; Engelmann, J.I.; Pohndorf, R.S.; Rodrigues, P.A.; Juliano, R.S.; Dotto, G.L.; Pinto, L.A.A. The effect of temperature on rice oil bleaching to reduce oxidation and loss in bioactive compounds. Grasas Aceites Int. J. Fats Oils 2019, 70, e287. [Google Scholar] [CrossRef]
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Optimization of Degumming Parameters in Chemical Refining Process to Reduce Phosphorus Contents in Kenaf Seed Oil. Sep. Purif. Technol. 2017, 188, 379–385. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, Q.; Pan, J.; Liu, M.; Long, Y.; Ge, F. Effectively improve the quality of camellia oil by the combination of supercritical fluid extraction and molecular distillation (SFE-MD). Lebensm. Wiss. Technol. 2019, 110, 175–181. [Google Scholar] [CrossRef]
- Huang, D.; Men, K.; Tang, X.; Li, W.; Sherif, S.A. Microwave intermittent drying characteristics of camellia oleifera seeds. J. Food Process Eng. 2021, 44, e13608. [Google Scholar] [CrossRef]
- Aladedunye, A.F. Inhibiting thermo-oxidative degradation of oils during frying. In Doctor of Philosophy; University of Lethbridge: Lethbridge, AB, Canada, 2011. [Google Scholar]
- Leonardis, A.D.; Macciola, V. Heat-oxidation stability of palm oil blended with extra virgin olive oil. Food Chem. 2012, 135, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Keller, M.A.; Sailer, S.; Seppi, D.; Golderer, G.; Werner-Felmayer, G.; Zoeller, R.A.; Watschinger, K. A Novel Assay for the Introduction of the Vinyl Ether Double Bond into Plasmalogens Using Pyrene-Labelled Substrates. J. Lipid Res. 2018, 59, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalli, J.; Colas, R.A.; Walker, M.E.; Serhan, C.N. Lipid Mediator Metabolomics Via LC-MS/MS Profiling and Analysis. Clin. Metab. 2018, 1730, 59–72. [Google Scholar]
- Dasilva, G.; Muoz, S.; Lois, S.; Medina, I. Non-Targeted LC-MS/MS Assay for Screening Over 100 Lipid Mediators from ARA, EPA, and DHA in Biological Samples Based on Mass Spectral Fragmentations. Molecules 2019, 24, 2276. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effects of thermal processing on trans-cis-isomerization of b-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003, 83, 609–617. [Google Scholar] [CrossRef]
- Pumilia, G.; Cichon, M.J.; Cooperstone, J.L.; Giuffrida, D.; Dugo, G.; Schwartz, S.J. Changes in chlorophylls, chlorophyll degradation products and lutein in pistachio kernels (Pistacia vera L.) during roasting. Food Res. Int. 2014, 65, 193–198. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, Q.; Wu, S.; Wang, X. Contribution of phospholipids to the formation of fishy off-odor and oxidative stability of soybean oil. Eur. J. Lipid Sci. Technol. 2016, 118, 603–611. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, K.; Chen, X.; Yang, B.; Kan, J. Thermostability and kinetics analysis of oil color, carotenoids and capsai-cinoids in hotpot oil models (butter, rapeseed oil, and their blends). LWT-Food Sci. Technol. 2021, 152, 112216. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Yu, Y.; Zheng, X.; He, D. Prediction of Edible-oil Iodine Values Based on Raman and near Infrared Spectroscopy. In Proceedings of the 2017 2nd International Conference on Computational Modeling, Simulation and Applied Mathematics(CMSAM 2017), Beijing, China, 22 October 2017. [Google Scholar]
- Aniolowska, M.; Kita, A. The effect of frying on glycidyl esters content in palm oil. Food Chem. 2016, 203, 95–103. [Google Scholar] [CrossRef]
- Wan, T.W.; Saad, B.; Lim, B.P. Determination of TOTOX value in palm oleins using a FI-potentiometric analyzer. Food Chem. 2009, 113, 285–290. [Google Scholar]
- Shaker, M.A. Comparison between traditional deep-fat frying and air-frying for production of healthy fried potato strips. Int. Food Res. J. 2015, 22, 1557–1563. [Google Scholar]
- Guillén, M.a.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxi-dative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Labrinea, E.P.; Thomaidis, N.S.; Georgiou, C.A. Direct olive oil anisidine value determination by flow injection. Anal. Chim. Acta 2001, 448, 201–206. [Google Scholar] [CrossRef]
- Roman, O.; Heyd, B.; Broyart, B.; Matienzo, R.C.; Maillard, M.N. Oxydative reactivity of unsaturated fatty acids from sunflower, high oleic sunflower and rapeseed oils subjected to heat treatments, under controlled conditions. LWT-Food Sci. Technol. 2013, 52, 49–59. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F.; Jablonski, C.R. Comparison of standard and NMR methodologies for assessment of oxi-dative stability of canola and soybean oils. Food Chem. 1995, 52, 249–253. [Google Scholar] [CrossRef]
- Guillen, M.D.; Uriarte, P.S. Relationships between the evolution of the percentage in weight of polar compounds and that of the molar percentage of acyl groups of edible oils submitted to frying temperature. Food Chem. 2013, 138, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Giarnetti, M.; Summo, C.; Paradiso, V.M.; Cosmai, L.; Gomes, T. A comparative study on oxidative and hy-drolytic stability of monovarietal extra virgin olive oil in bakery products. Food Res. Int. 2013, 54, 1995–2000. [Google Scholar] [CrossRef]
- Houhoula, D.P.; Oreopoulou, V.; Tzia, C. A kinetic study of oil deterioration during frying and a comparison with heating. J. Am. Oil Chem. Soc. 2002, 79, 133–137. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, M.J.; Kim, Y.J.; Lee, J.H. Monitoring changes in acid value, total polar material, and antioxidant capacity of oils used for frying chicken. Food Chem. 2017, 220, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chang, C.; Hong, C.; Zhang, H.; Wang, X. Phenolic compounds as stabilizers of oils and antioxidative mechanisms under frying conditions: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 33–45. [Google Scholar] [CrossRef]
- Wu, G.; Han, S.; Zhang, Y.; Liu, T.; Karrar, E.; Jin, Q.; Zhang, H.; Wang, X. Effect of phenolic extracts from Camellia oleifera seed cake on the formation of polar compounds, core aldehydes, and monoepoxy oleic acids during deep-fat frying. Food Chem. 2022, 372, 131143. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Robledo, S.N.; Zachetti, V.; Zon, M.A.; Fernández, H. Quantitative determination of tocopherols in edible vegetable oils using electrochemical ultra-microsensors combined with chemometric tools. Talanta 2013, 116, 964–971. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, D.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Characterization of triacylglycerols in unfermented cocoa beans by HPLC-ESI mass spectrometry. Food Chem. 2018, 254, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Velzen, E.; Hoefsloot, H.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Szeto, S.; Reinke, S.N.; Sykes, B.D.; Lemire, B.D. Mutations in the Saccharomyces cerevisiae Succinate Dehydrogenase Result in Distinct Metabolic Phenotypes Revealed Through H-1 NMR-Based Metabolic Footprinting. J. Proteome Res. 2010, 9, 6729–6739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Z.; Zhang, H.; Wang, Y.; Liu, X.; Wang, Q.; Xue, J.; Zhao, Y.; Yang, S. Meat differentiation between pasture-fed and concentrate-fed sheep/goats by liquid chromatography quadrupole time-of-flight mass spectrometry combined with metabolomic and lipidomic profiling. Meat Sci. 2020, 173, 108374. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Liu, B.; Meng, X.; Li, K.; Guo, J.; Cai, Z. Visualization of lipids in cottonseeds by matrix-assisted laser desorption/ionization mass spectrometry imaging. Talanta 2021, 221, 121614. [Google Scholar] [CrossRef]
- Murano, Y.; Funabashi, T.; Takeuchi, H. Improvement in Storage Stability of Fish Fillets Using Dietary Soybean Phospha-tidylcholine and Phosphatidylethanolamine. Food Sci. Technol. Int. Tokyo 2009, 15, 163–170. [Google Scholar]
- Doert, M.; Krüger, S.; Morlock, G.E.; Kroh, L.W. Synergistic effect of lecithins for tocopherols: Formation and antioxidant effect of the phosphatidylethanolamine-L-ascorbic acid condensate. Z. Fur Lebensm. Unters. Und Forsch. A 2017, 243, 583–596. [Google Scholar] [CrossRef]
- Smeets, E.F.; Comfurius, P.; Bevers, E.M.; Zwaal, R. Contribution of different phospholipid classes to the prothrombin converting capacity of sonicated lipid vesicles. Thromb. Res. 1996, 81, 419–426. [Google Scholar] [CrossRef]
- Van, D.V.; Jelske, N.; Lingrell, S.; Mccloskey, N.; Leblond, N.D.; Galleguillos, D.; Zhao, Y.Y.; Curtis, J.M.; Sipione, S.; Fullerton, M.D.; et al. A role for phosphatidylcholine and phosphatidylethanolamine in hepatic insulin signaling. FASEB J. 2019, 33, 5045–5057. [Google Scholar]
- Joshi, A.S.; Thompson, M.N.; Fei, N.; Hüttemann, M.; Greenberg, M.L. Cardiolipin and Mitochondrial Phosphatidyleth-anolamine Have Overlapping Functions in Mitochondrial Fusion in Saccharomyces cerevisiae. J. Biol. Chem. 2012, 287, 17589–17597. [Google Scholar] [CrossRef] [Green Version]
- Folch, J. Brain cephalin, a mixture of phosphatides. Separation from it of phosphatidylserine, phosphatidylethanolamine, and a fraction containing an inositol phosphatide. J. Biol. Chem. 1942, 146, 35–44. [Google Scholar] [CrossRef]
- Delwaide, P.J.; Gyselynck-Mambourg, A.M.; Hurlet, A.; Ylieff, M. Double-blind randomized controlled study of phospha-tidylserine in senile demented patients. Acta Neurol. Scand. 1986, 73, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [Green Version]





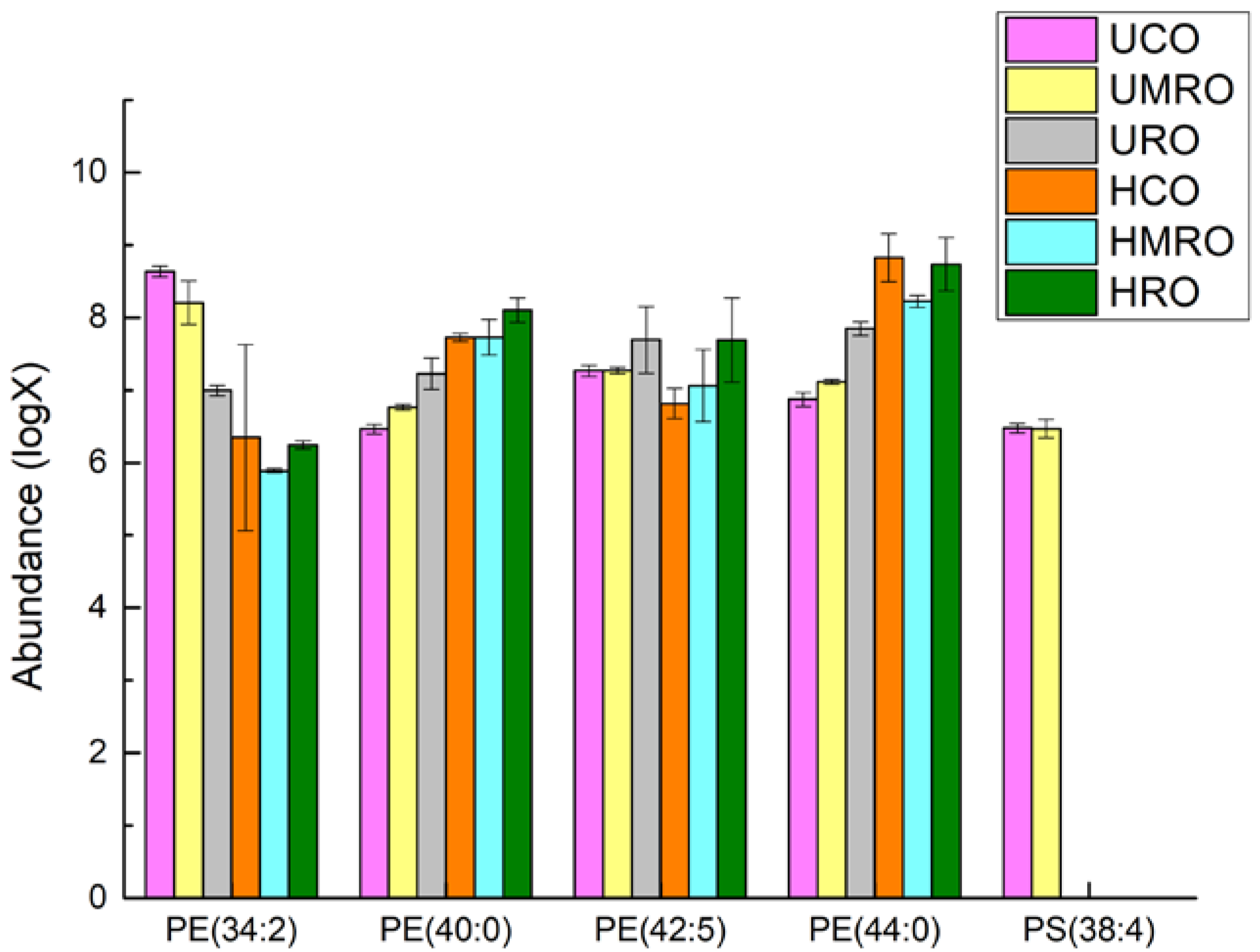
| Fatty Acid (%) | CO | MRO | RO |
|---|---|---|---|
| C16:0 | 7.84 ± 0.11 | 8.48 ± 0.35 | 9.41 ± 0.17 |
| C18:0 | 2.28 ± 0.07 | 2.38 ± 0.22 | 2.48 ± 0.18 |
| C18:1 | 81.79 ± 0.20 | 79.87 ± 0.75 | 77.83 ± 0.42 |
| C18:2 | 7.17 ± 0.05 | 8.26 ± 0.44 | 10.28 ± 0.15 |
| C18:3 | 0.27 ± 0.02 | 0.45 ± 0.02 | ND |
| C20:1 | 0.51 ± 0.03 | 0.53 ± 0.02 | ND |
| Correlation Coefficient | Tocopherols | Polyphenols | TOTOX | K232 | K268 | Polar Components |
|---|---|---|---|---|---|---|
| Tocopherols | 1 | |||||
| Polyphenols | 0.880 ** | 1 | ||||
| TOTOX | −0.737 ** | −0.533 ** | 1 | |||
| K232 | −0.919 ** | −0.727 ** | 0.877 ** | 1 | ||
| K268 | −0.900 ** | −0.737 ** | 0.780 ** | 0.928 ** | 1 | |
| Polar components | −0.826 ** | −0.652 ** | 0.838 ** | 0.955 ** | 0.813 ** | 1 |
| No. | HCO | HMRO | HRO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AcHexChE (33:0) | 21 | TG (9:0_9:0_14:2) | 41 | WE (3:0_20:2) | 1 | AcHexChE (33:0) | 21 | TG (6:0_12:2_18:3) | 1 | DG (27:3e) |
| 2 | AcHexCmE (8:0) | 22 | TG (6:0_10:2_18:1) | 42 | WE (8:0_19:4) | 2 | AcHexCmE (8:0) | 22 | TG (41:1) | 2 | OAHFA (16:0_24:0) |
| 3 | AcHexStE (35:2) | 23 | TG (14:1e_10:4_10:4) | 43 | MGDG (35:4) | 3 | AcHexStE (35:2) | 23 | TG (18:2e_8:0_18:1) | 3 | PA (18:1_12:1) |
| 4 | AcHexZyE (36:3) | 24 | TG (6:0_12:2_18:3) | 44 | MGDG (44:4) | 4 | AcHexZyE (36:3) | 24 | TG (19:1_6:0_20:2) | ||
| 5 | DG (18:2) | 25 | TG (18:0_9:0_16:0) | 45 | MGDG (44:5) | 5 | CmE (0:0) | 25 | TG (18:1_11:1_18:2) | ||
| 6 | DG (22:0e) | 26 | TG (18:2e_8:0_18:1) | 46 | MGDG (44:6) | 6 | DG (22:0e) | 26 | TG (18:1_23:0_23:1) | ||
| 7 | DG (27:3) | 27 | TG (52:9) | 47 | PA (27:1) | 7 | DG (24:3) | 27 | TG (28:1_18:1_18:2) | ||
| 8 | DG (10:0_18:1) | 28 | TG (57:8) | 48 | PA (18:1_12:1) | 8 | DG (18:2_11:2) | 28 | TG (27:1_18:1_20:3) | ||
| 9 | DG (18:1_11:2) | 29 | TG (18:1_23:0_23:1) | 49 | SQMG (20:1) | 9 | DG (18:3e_18:2) | 29 | TG (70:5) | ||
| 10 | PG (18:1_23:0) | 30 | TG (28:1_18:1_18:2) | 50 | dMePE (54:1) | 10 | PE (62:2) | 30 | TG (71:3) | ||
| 11 | TG (27:3) | 31 | TG (64:6) | 51 | dMePE (56:2) | 11 | PG (18:1_23:0) | 31 | TG (72:6) | ||
| 12 | TG (6:0_10:2_12:1) | 32 | TG (27:1_18:1_20:3) | 12 | TG (4:0_6:0_10:3) | 32 | TG (30:0_20:2_24:2) | ||||
| 13 | TG (12:0e_6:0_11:3) | 33 | TG (69:2) | 13 | TG (29:4) | 33 | WE (10:0_18:3) | ||||
| 14 | TG (10:0_10:1_10:1) | 34 | TG (70:2) | 14 | TG (10:0_10:1_10:1) | 34 | Cer (d32:4) | ||||
| 15 | TG (6:0_10:2_14:4) | 35 | TG (70:3) | 15 | TG (31:5) | 35 | OAHFA (18:1_19:1) | ||||
| 16 | TG (6:0_11:1_14:2) | 36 | TG (28:1_18:1_24:2) | 16 | TG (10:0_10:2_11:4) | 36 | PA (27:1) | ||||
| 17 | TG (10:0_10:2_11:1) | 37 | TG (71:3) | 17 | TG (6:0_6:0_20:2) | 37 | PA (18:1_12:1) | ||||
| 18 | TG (8:0_11:2_12:1) | 38 | TG (24:2_23:0_24:2) | 18 | TG (9:0_9:0_14:2) | 38 | dMePE (54:1) | ||||
| 19 | TG (10:0_10:2_11:4) | 39 | TG (72:6) | 19 | TG (6:0_10:2_18:1) | 39 | dMePE (56:2) | ||||
| 20 | TG (6:0_6:0_20:2) | 40 | TG (74:7) | 20 | TG (14:1e_10:4_10:4) | ||||||
| No. | HCO | HMRO | HRO |
|---|---|---|---|
| 1 | Cer (d18:0_18:1) | Cer (d18:2_25:0+O) | BisMePA (22:4_18:2) |
| 2 | Cer (d18:2_18:1) | PA (34:1) | DG (19:0) |
| 3 | Cer (d18:2_25:0+O) | PE (18:1e) | DG (9:0_12:3) |
| 4 | PE (18:1e) | PE (18:0_16:0) | DG (38:5) |
| 5 | TG (35:1e) | PE (16:0_18:1) | PA (18:1_18:2) |
| 6 | Cer (d34:2+O) | PE (16:0_18:3) | PE (16:0_18:3) |
| 7 | Cer (t18:1_18:1) | PE (18:1_18:1) | PE (35:2) |
| 8 | Cer (t17:1_24:0+O) | PE (18:1_18:2) | PE (40:5) |
| 9 | Cer (t42:1+O) | PE (18:2_18:2) | TG (4:0_6:0_11:2) |
| 10 | Cer (t42:2) | Cer (d34:2+O) | TG (12:0e_8:0_11:1) |
| 11 | LPE (18:1) | Cer (t18:1_18:1) | TG (35:1e) |
| 12 | LPE (18:2) | Cer (t42:1+O) | TG (16:0e_10:4_18:1) |
| 13 | MLCL (65:2) | LPE (18:1) | TG (18:1_11:1_18:2) |
| 14 | MLCL (67:3) | LPE (18:2) | Cer (t17:1_24:1) |
| 15 | MLCL (67:5) | PG (34:4) | Cer (t17:1_25:0) |
| 16 | MLCL (69:4) | PG (34:5) | PG (36:5) |
| 17 | PG (36:7) | PG (36:7) | |
| 18 | PI (18:1_18:2) | PI (18:1_18:2) | |
| 19 | PS (38:4) | PS (18:2_18:2) | |
| 20 | PS (38:5) | PS (38:4) | |
| 21 | PS (38:6) | PS (38:5) | |
| 22 | PS (38:6) |
| Types of Lipids | CO | MRO | RO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Quantity | Upward Adjustment | Downward Adjustment | Total Quantity | Upward Adjustment | Downward Adjustment | Total Quantity | Upward Adjustment | Downward Adjustment | |
| Phospholipids | 15 | 3 | 12 | 21 | 3 | 18 | 6 | 2 | 4 |
| Sphingolipid | 9 | 1 | 8 | 7 | 2 | 5 | 2 | 0 | 2 |
| Neutral lipids | 29 | 24 | 5 | 29 | 26 | 3 | 72 | 38 | 34 |
| Fatty acyl, other phospholipids | 11 | 11 | 0 | 16 | 13 | 3 | 3 | 2 | 1 |
| Glyceroglycolipid | 17 | 16 | 1 | 14 | 10 | 4 | 9 | 6 | 3 |
| Derivative lipids | 2 | 2 | 0 | 3 | 3 | 0 | 2 | 2 | 0 |
| Total | 83 | 57 | 26 | 90 | 57 | 33 | 94 | 50 | 44 |
| Types of Lipids | CO | MRO | RO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Quantity | Upward Adjustment | Downward Adjustment | Total Quantity | Upward Adjustment | Downward Adjustment | Total Quantity | Upward Adjustment | Downward Adjustment | |
| Phospholipids | 5 | 0 | 5 | 9 | 0 | 9 | 3 | 1 | 2 |
| Sphingolipid | 3 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 2 |
| Neutral lipids | 19 | 15 | 4 | 21 | 18 | 3 | 68 | 36 | 32 |
| Fatty acyl, other phospholipids | 4 | 4 | 0 | 9 | 6 | 3 | 3 | 2 | 1 |
| Glyceroglycolipid | 6 | 6 | 0 | 5 | 2 | 3 | 4 | 2 | 2 |
| Derivative lipids | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Total | 37 | 25 | 12 | 46 | 28 | 18 | 81 | 42 | 39 |
| No. | Compound | m/z | RT/min | Chemical Formula | VIP | log2(FC) |
|---|---|---|---|---|---|---|
| 1 | AcHexChE (33:0) | 1042.9372 | 20.91 | C66H124O7N1 | 1.1552 | 34.63 |
| 2 | AcHexSiE (18:2) | 856.7025 | 18.20 | C53H94O7N1 | 1.1552 | −3.32 |
| 3 | AcHexZyE (36:3) | 1076.9216 | 19.79 | C69H122O7N1 | 1.1550 | 31.51 |
| 4 | Cer (d18:0_18:1) | 566.5507 | 14.74 | C36H72O3N1 | 1.1553 | −34.90 |
| 5 | Cer (d18:2_18:1) | 562.5194 | 13.51 | C36H68O3N1 | 1.1553 | −33.06 |
| 6 | Cer (t17:1_24:0+O) | 712.6097 | 16.99 | C42H82O7N1 | 1.1553 | −33.93 |
| 7 | DG (10:0_18:1) | 528.4623 | 12.06 | C31H62O5N1 | 1.1554 | 32.52 |
| 8 | DG (18:1_11:2) | 538.4466 | 10.75 | C32H60O5N1 | 1.1553 | 31.47 |
| 9 | MGDG (35:4) | 763.5366 | 9.51 | C44H75O10 | 1.1761 | 27.61 |
| 10 | MGDG (40:6) | 829.5835 | 16.02 | C49H81O10 | 1.1722 | 6.86 |
| 11 | MGDG (42:6) | 857.6148 | 16.53 | C51H85O10 | 1.1692 | 4.71 |
| 12 | MGDG (44:4) | 889.6774 | 17.47 | C53H93O10 | 1.1794 | 34.08 |
| 13 | MGDG (44:5) | 887.6618 | 16.40 | C53H91O10 | 1.1793 | 35.74 |
| 14 | MGDG (47:2) | 935.7557 | 18.48 | C56H103O10 | 1.1635 | 1.83 |
| 15 | MLCL (65:2) | 1347.9700 | 13.65 | C74H141O16P2 | 1.1776 | −26.27 |
| 16 | MLCL (67:3) | 1373.9857 | 13.70 | C76H143O16P2 | 1.1788 | −28.08 |
| 17 | MLCL (67:5) | 1369.9544 | 12.57 | C76H139O16P2 | 1.1784 | −26.27 |
| 18 | MLCL (69:4) | 1400.0013 | 13.73 | C78H145O16P2 | 1.1787 | −27.56 |
| 19 | OAHFA (18:1_18:0) | 563.5045 | 17.52 | C36H67O4 | 1.1716 | 4.27 |
| 20 | OAHFA (18:1_18:1) | 561.4888 | 16.37 | C36H65O4 | 1.1757 | 4.97 |
| 21 | OAHFA (18:2_18:1) | 559.4732 | 15.34 | C36H63O4 | 1.1755 | 6.86 |
| 22 | PE (18:1e) | 480.3085 | 3.72 | C23H47O7N1P1 | 1.1553 | −32.49 |
| 23 | TG (10:0_10:2_11:1) | 580.4572 | 12.05 | C34H62O6N1 | 1.1551 | 34.54 |
| 24 | TG (12:0e_9:0_10:1) | 570.5092 | 12.29 | C34H68O5N1 | 1.1551 | −8.54 |
| 25 | TG (16:1e_18:3_18:4) | 855.6837 | 17.96 | C55H92O5Na1 | 1.1553 | −3.43 |
| 26 | TG (18:0_9:0_16:0) | 754.6919 | 20.04 | C46H92O6N1 | 1.1552 | 33.27 |
| 27 | TG (18:1_23:0_23:1) | 1049.9447 | 20.59 | C67H126O6Na1 | 1.1551 | 35.38 |
| 28 | TG (20:2e_14:2_18:3) | 857.6993 | 18.21 | C55H94O5Na1 | 1.1551 | −3.20 |
| 29 | TG (27:3) | 529.3500 | 5.75 | C30H50O6Na1 | 1.1554 | 33.43 |
| 30 | TG (28:1_18:1_24:2) | 1125.0519 | 22.77 | C73H138O6N1 | 1.1553 | 33.22 |
| 31 | TG (52:9) | 845.6654 | 14.16 | C55H89O6 | 1.1552 | 35.24 |
| 32 | TG (6:0_10:2_12:1) | 543.3656 | 7.25 | C31H52O6Na1 | 1.1553 | 33.14 |
| 33 | TG (6:0_10:2_14:4) | 543.3680 | 7.25 | C33H51O6 | 1.1554 | 32.40 |
| 34 | TG (64:6) | 1019.9001 | 20.93 | C67H119O6 | 1.1552 | 34.14 |
| 35 | TG (69:2) | 1115.0675 | 23.21 | C72H140O6N1 | 1.1551 | 30.80 |
| 36 | TG (74:7) | 1180.0229 | 21.62 | C77H136O6Na1 | 1.1551 | 35.82 |
| 37 | WE (8:0_19:4) | 420.3836 | 9.15 | H50C27O2N1 | 1.1554 | 36.19 |
| No. | Compound | m/z | RT/Min | Chemical Formula | VIP | log2(FC) |
|---|---|---|---|---|---|---|
| 1 | AcHexStE (35:2) | 1075.9263 | 20.15 | C70H123O7 | 1.1320 | 34.36 |
| 2 | Cer (d32:4) | 548.4320 | 7.62 | C33H58O5N1 | 1.1319 | 25.58 |
| 3 | CmE (0:0) | 401.3778 | 12.59 | C28H49O1 | 1.1320 | 32.71 |
| 4 | DG (18:2_11:2) | 536.4310 | 10.07 | C32H58O5N1 | 1.1319 | 32.96 |
| 5 | DG (22:0e) | 437.3601 | 18.55 | C25H50O4Na1 | 1.1319 | 34.19 |
| 6 | DG (24:3) | 451.3418 | 16.43 | C27H47O5 | 1.1319 | 34.93 |
| 7 | dMePE (41:2) | 840.6488 | 15.41 | C48H91O8N1P1 | 1.2068 | 9.45 |
| 8 | MGDG (29:5) | 677.4270 | 7.63 | C38H61O10 | 1.1718 | −1.84 |
| 9 | MGDG (34:2) | 753.5522 | 15.29 | C43H77O10 | 1.1571 | −2.21 |
| 10 | MGDG (41:3) | 849.6461 | 16.84 | C50H89O10 | 1.1988 | 11.02 |
| 11 | MGDG (43:6) | 871.6305 | 16.86 | C52H87O10 | 1.1995 | 10.52 |
| 12 | OAHFA (18:1_19:1) | 575.5045 | 15.22 | C37H67O4 | 1.2073 | 31.30 |
| 13 | OAHFA (18:1_23:0) | 633.5827 | 19.45 | C41H77O4 | 1.1765 | 6.73 |
| 14 | OAHFA (18:1_24:0) | 647.5984 | 19.73 | C42H79O4 | 1.1889 | 4.28 |
| 15 | OAHFA (18:1_26:1) | 673.6140 | 19.65 | C44H81O4 | 1.1747 | 6.16 |
| 16 | OAHFA (18:2_24:0) | 645.5827 | 19.22 | C42H77O4 | 1.1906 | 6.40 |
| 17 | OAHFA (38:2) | 589.5201 | 17.19 | C38H69O4 | 1.1838 | 2.98 |
| 18 | PA (18:0_18:1) | 701.5127 | 15.04 | C39H74O8N0P1 | 1.1856 | −4.44 |
| 19 | PE (16:0_18:1) | 740.5201 | 12.25 | C39H76O8N1P1Na1 | 1.1319 | −40.30 |
| 20 | PE (18:2_18:2) | 740.5225 | 12.25 | C41H75O8N1P1 | 1.1319 | −40.40 |
| 21 | PEt (18:1_18:2) | 725.5127 | 12.99 | C41H74O8N0P1 | 1.1957 | −6.63 |
| 22 | PG (18:1_18:2) | 771.5182 | 12.13 | C42H76O10N0P1 | 1.1992 | −9.37 |
| 23 | PG (34:4) | 741.4712 | 13.68 | C40H70O10N0P1 | 1.2059 | −30.94 |
| 24 | PG (34:5) | 739.4556 | 12.50 | C40H68O10N0P1 | 1.2078 | −30.13 |
| 25 | PG (40:3) | 827.5808 | 15.02 | C46H84O10N0P1 | 1.1682 | −2.41 |
| 26 | PI (18:1_18:2) | 859.5342 | 11.77 | C45H80O13N0P1 | 1.2086 | −23.47 |
| 27 | PMe (18:1_18:1) | 713.5127 | 13.81 | C40H74O8N0P1 | 1.1958 | −4.66 |
| 28 | PMe (18:1_18:2) | 711.4970 | 12.68 | C40H72O8N0P1 | 1.1750 | −6.06 |
| 29 | PS (18:2_18:2) | 782.4978 | 13.39 | C42H73O10N1P1 | 1.2079 | −33.06 |
| 30 | SQDG (49:0) | 1031.7802 | 19.00 | C58H111O12S1 | 1.1724 | −3.94 |
| 31 | StE (18:3) | 673.5918 | 18.25 | C47H77O2 | 1.1318 | −3.12 |
| 32 | TG (10:0_10:1_10:1) | 568.4572 | 12.19 | C33H62O6N1 | 1.1319 | 33.00 |
| 33 | TG (10:0_10:2_11:4) | 579.3656 | 7.50 | C34H52O6Na1 | 1.1318 | 32.91 |
| 34 | TG (12:0e_9:0_18:2) | 680.6188 | 16.21 | C42H82O5N1 | 1.1318 | −5.83 |
| 35 | TG (27:1_18:1_20:3) | 1052.9580 | 22.10 | C68H126O6N1 | 1.1319 | 36.72 |
| 36 | TG (29:4) | 555.3656 | 6.93 | C32H52O6Na1 | 1.1320 | 32.36 |
| 37 | TG (31:5) | 576.4259 | 6.32 | C34H58O6N1 | 1.1320 | 31.83 |
| 38 | TG (4:0_6:0_10:3) | 426.2850 | 3.62 | C23H40O6N1 | 1.1319 | 26.70 |
| 39 | TG (41:1) | 729.6004 | 18.87 | C44H82O6Na1 | 1.1319 | 29.71 |
| 40 | TG (6:0_10:2_18:1) | 622.5041 | 13.90 | C37H68O6N1 | 1.1320 | 34.02 |
| 41 | TG (6:0_6:0_24:0) | 661.5378 | 12.66 | C39H74O6Na1 | 1.1319 | 1.46 |
| 42 | TG (70:5) | 1123.0362 | 22.64 | C73H136O6N1 | 1.1320 | 35.23 |
| 43 | TG (72:6) | 1154.0073 | 21.61 | C75H134O6Na1 | 1.1319 | 35.68 |
| 44 | TG (8:0_12:2_18:1) | 661.5402 | 12.65 | C41H73O6 | 1.1319 | 1.46 |
| 45 | TG (9:0_11:1_18:1) | 680.5824 | 17.04 | C41H78O6N1 | 1.1319 | −2.52 |
| 46 | TG (9:0_9:0_14:2) | 596.4885 | 13.40 | C35H66O6N1 | 1.1319 | 32.97 |
| No. | Compound | m/z | RT/Min | Chemical Formula | VIP | log2(FC) |
|---|---|---|---|---|---|---|
| 1 | AcHexChE (16:0) | 804.6712 | 16.76 | C49H90O7N1 | 1.2766 | 1.45 |
| 2 | Cer(t17:1_24:1) | 708.6148 | 16.63 | C43H82O6N1 | 1.2782 | −27.33 |
| 3 | Cer(t17:1_25:0) | 724.6461 | 18.31 | C44H86O6N1 | 1.2795 | −26.66 |
| 4 | DG (14:0_18:2) | 587.4646 | 13.83 | C35H64O5Na1 | 1.2783 | −1.94 |
| 5 | DG (16:0_10:1) | 505.3863 | 7.65 | C29H54O5Na1 | 1.2779 | 2.15 |
| 6 | DG (18:1_11:1) | 545.4176 | 8.57 | C32H58O5Na1 | 1.2777 | 1.26 |
| 7 | DG (18:1_14:0) | 589.4802 | 14.99 | C35H66O5Na1 | 1.2783 | −1.88 |
| 8 | DG (18:1_18:3) | 639.4959 | 14.37 | C39H68O5Na1 | 1.2756 | −1.60 |
| 9 | DG (18:2_18:2) | 639.4959 | 14.15 | C39H68O5Na1 | 1.2757 | −1.55 |
| 10 | DG (18:3_18:2) | 637.4802 | 13.16 | C39H66O5Na1 | 1.2788 | −3.34 |
| 11 | DG (19:0) | 409.2924 | 18.77 | C22H42O5Na1 | 1.2800 | −33.36 |
| 12 | DG (19:3) | 398.2901 | 4.89 | C22H40O5N1 | 1.2754 | 2.10 |
| 13 | DG (20:1_18:2) | 664.5875 | 16.49 | C41H78O5N1 | 1.2755 | −1.41 |
| 14 | DG (22:1_18:2) | 692.6188 | 17.55 | C43H82O5N1 | 1.2755 | −1.24 |
| 15 | DG (27:3e) | 496.4360 | 9.47 | C30H58O4N1 | 1.2763 | 28.80 |
| 16 | DG (28:7) | 521.3237 | 6.75 | C31H46O5Na1 | 1.2794 | 1.91 |
| 17 | DG (30:5e) | 517.4251 | 6.91 | C33H57O4 | 1.2756 | 1.85 |
| 18 | DG (31:8) | 561.3550 | 7.72 | C34H50O5Na1 | 1.2787 | 1.83 |
| 19 | DG (38:5) | 660.5562 | 14.22 | C41H74O5N1 | 1.2791 | −31.14 |
| 20 | DG (44:2) | 755.6524 | 16.29 | C47H88O5Na1 | 1.2793 | 2.23 |
| 21 | DG (9:0_12:3) | 409.2949 | 18.83 | C24H41O5 | 1.2800 | −33.48 |
| 22 | dMePE (55:2) | 1036.8679 | 19.97 | C62H119O8N1P1 | 1.5310 | 3.47 |
| 23 | MGDG (16:0_16:0) | 729.5522 | 14.24 | C41H77O10 | 1.5991 | −4.49 |
| 24 | MGDG (26:2) | 641.4270 | 7.23 | C35H61O10 | 1.4987 | −1.13 |
| 25 | MGDG (31:4) | 707.4740 | 7.19 | C40H67O10 | 1.5067 | 2.69 |
| 26 | MGDG (48:6) | 941.7087 | 16.48 | C57H97O10 | 1.4505 | 2.54 |
| 27 | OAHFA (16:0_24:0) | 621.5827 | 19.73 | C40H77O4 | 1.6580 | 22.65 |
| 28 | OAHFA (18:1_27:0) | 689.6453 | 19.43 | C45H85O4 | 1.5752 | 2.97 |
| 29 | PA (18:1_18:2) | 716.5225 | 12.66 | C39H75O8N1P1 | 1.2790 | −28.81 |
| 30 | PE (35:2) | 730.5381 | 12.60 | C40H77O8N1P1 | 1.2796 | −28.11 |
| 31 | PI (16:0_18:1) | 835.5342 | 12.80 | C43H80O13N0P1 | 1.6472 | 20.12 |
| 32 | TG (11:0_10:1_10:1) | 582.4728 | 8.15 | C34H64O6N1 | 1.2773 | 1.29 |
| 33 | TG (12:0_18:2_18:2) | 816.7076 | 19.32 | C51H94O6N1 | 1.2774 | −3.73 |
| 34 | TG (12:0e_8:0_11:1) | 570.5092 | 12.60 | C34H68O5N1 | 1.2798 | −31.75 |
| 35 | TG (12:1e_6:0_10:4) | 503.3731 | 6.33 | C31H51O5 | 1.2787 | 2.81 |
| 36 | TG (12:1e_6:0_11:3) | 519.4044 | 8.15 | C32H55O5 | 1.2761 | 1.93 |
| 37 | TG (12:1e_6:0_20:5) | 641.5140 | 15.35 | C41H69O5 | 1.2775 | −1.34 |
| 38 | TG (14:0_18:2_18:2) | 844.7389 | 19.88 | C53H98O6N1 | 1.2796 | −1.50 |
| 39 | TG (14:1e_10:1_10:2) | 589.4827 | 14.95 | C37H65O5 | 1.2783 | −1.88 |
| 40 | TG (16:0_14:2_18:1) | 818.7232 | 19.74 | C51H96O6N1 | 1.2792 | −2.10 |
| 41 | TG (16:0e_10:4_18:1) | 727.6235 | 18.12 | C47H83O5 | 1.2766 | −28.55 |
| 42 | TG (16:2e_10:3_18:2) | 740.6188 | 14.23 | C47H82O5N1 | 1.2791 | 5.12 |
| 43 | TG (18:1_10:1_11:2) | 695.5221 | 9.09 | C42H72O6Na1 | 1.2758 | 1.60 |
| 44 | TG (18:1_10:3_14:4) | 707.5245 | 8.98 | C45H71O6 | 1.2767 | 2.29 |
| 45 | TG (18:1_11:2_12:4) | 695.5245 | 9.08 | C44H71O6 | 1.2762 | 1.59 |
| 46 | TG (18:3e_11:1_18:2) | 767.6548 | 16.22 | C50H87O5 | 1.2797 | 2.09 |
| 47 | TG (18:3e_12:2_18:2) | 779.6548 | 15.42 | C51H87O5 | 1.2769 | 1.76 |
| 48 | TG (18:3e_16:0_18:1) | 863.7463 | 19.69 | C55H100O5Na1 | 1.2757 | −1.61 |
| 49 | TG (18:4_16:0_16:0) | 849.6943 | 16.11 | C53H94O6Na1 | 1.2755 | 1.45 |
| 50 | TG (18:4_6:0_18:3) | 709.5402 | 9.45 | C45H73O6 | 1.2768 | 1.21 |
| 51 | TG (20:0e_16:0_18:2) | 895.8089 | 20.20 | C57H108O5Na1 | 1.2766 | 2.48 |
| 52 | TG (20:2e_10:2_18:1) | 805.6680 | 16.75 | C51H90O5Na1 | 1.2756 | 1.45 |
| 53 | TG (20:4e_10:2_20:2) | 805.6705 | 16.76 | C53H89O5 | 1.2756 | 1.45 |
| 54 | TG (20:4e_10:4_10:4) | 679.4333 | 7.59 | C43H60O5Na1 | 1.2761 | −1.12 |
| 55 | TG (29:0_10:3_10:3) | 809.6654 | 16.28 | C52H89O6 | 1.2777 | 3.66 |
| 56 | TG (31:4) | 578.4415 | 7.55 | C34H60O6N1 | 1.2762 | 1.06 |
| 57 | TG (32:1e) | 589.4802 | 15.04 | C35H66O5Na1 | 1.2785 | −1.87 |
| 58 | TG (36:4e) | 639.4959 | 14.27 | C39H68O5Na1 | 1.2765 | −1.49 |
| 59 | TG (36:5e) | 637.4802 | 13.15 | C39H66O5Na1 | 1.2779 | −3.26 |
| 60 | TG (37:5e) | 629.5140 | 15.69 | C40H69O5 | 1.2783 | −3.93 |
| 61 | TG (38:8e) | 637.4827 | 13.13 | C41H65O5 | 1.2779 | −3.26 |
| 62 | TG (4:0_6:0_11:2) | 425.2898 | 18.21 | C24H41O6 | 1.2798 | −31.62 |
| 63 | TG (52:14e) | 838.6344 | 9.96 | C55H84O5N1 | 1.2794 | 4.90 |
| 64 | TG (54:8e) | 861.7331 | 19.52 | C57H97O5 | 1.2782 | −3.68 |
| 65 | TG (55:10) | 902.7232 | 19.92 | C58H96O6N1 | 1.2751 | −6.08 |
| 66 | TG (56:8e) | 906.7909 | 18.53 | C59H104O5N1 | 1.2764 | −1.19 |
| 67 | TG (6:0_12:2_12:2) | 564.4259 | 8.98 | C33H58O6N1 | 1.2784 | 2.14 |
| 68 | TG (6:0_9:0_18:1) | 612.5198 | 14.69 | C36H70O6N1 | 1.2755 | 1.02 |
| 69 | TG (8:0_10:0_11:1) | 556.4572 | 8.66 | C32H62O6N1 | 1.2755 | 1.04 |
| 70 | TG (8:0_10:0_11:2) | 559.3969 | 8.14 | C32H56O6Na1 | 1.2770 | 1.25 |
| 71 | TG (8:0_11:1_11:2) | 566.4415 | 10.11 | C33H60O6N1 | 1.2759 | 1.78 |
| 72 | TG (8:0_11:2_12:3) | 559.3993 | 8.15 | C34H55O6 | 1.2780 | 1.25 |
| 73 | TG (8:0_8:0_10:1) | 519.3656 | 7.35 | C29H52O6Na1 | 1.2765 | 2.74 |
| 74 | TG (8:0_8:0_12:4) | 519.3680 | 7.35 | C31H51O6 | 1.2765 | 2.74 |
| 75 | TG (9:0_10:1_18:1) | 666.5667 | 16.79 | C40H76O6N1 | 1.2754 | −1.08 |
| 76 | TG (9:0_9:0_11:3) | 552.4259 | 6.91 | C32H58O6N1 | 1.2756 | 2.02 |
| 77 | TG (9:0_9:0_18:1) | 654.5667 | 17.01 | C39H76O6N1 | 1.2791 | −1.47 |
| 78 | WE (22:5_21:2) | 621.5605 | 17.95 | H73C43O2 | 1.2769 | −1.28 |
| 79 | ZyE (16:1) | 621.5605 | 17.96 | C43H73O2 | 1.2769 | −1.28 |
| 80 | ZyE (21:2) | 706.6497 | 19.65 | C48H84O2N1 | 1.2773 | −2.07 |
| 81 | ZyE (35:6) | 894.8062 | 20.19 | C62H104O2N1 | 1.2752 | 1.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wan, Y.; Liu, T.; Zeng, X.; Liang, X.; Wu, X.; Fu, G. Effect of Refining Degree on the Quality Changes and Lipid Oxidation of Camellia (Camellia oleifera) Oil during Heating. Foods 2022, 11, 2232. https://doi.org/10.3390/foods11152232
Wang M, Wan Y, Liu T, Zeng X, Liang X, Wu X, Fu G. Effect of Refining Degree on the Quality Changes and Lipid Oxidation of Camellia (Camellia oleifera) Oil during Heating. Foods. 2022; 11(15):2232. https://doi.org/10.3390/foods11152232
Chicago/Turabian StyleWang, Mei, Yin Wan, Ting Liu, Xiuying Zeng, Xinmei Liang, Xiaojiang Wu, and Guiming Fu. 2022. "Effect of Refining Degree on the Quality Changes and Lipid Oxidation of Camellia (Camellia oleifera) Oil during Heating" Foods 11, no. 15: 2232. https://doi.org/10.3390/foods11152232
APA StyleWang, M., Wan, Y., Liu, T., Zeng, X., Liang, X., Wu, X., & Fu, G. (2022). Effect of Refining Degree on the Quality Changes and Lipid Oxidation of Camellia (Camellia oleifera) Oil during Heating. Foods, 11(15), 2232. https://doi.org/10.3390/foods11152232






