Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation, High-Hydrostatic Pressure and Cold Plasma
Abstract
1. Introduction
2. Nonthermal Decontamination Technologies
2.1. Ozonation
2.2. High Hydrostatic Pressure (HHP)
2.3. Cold Plasma (CP) Technology
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerveny, J.; Meyer, J.D.; Hall, P.A. Compendium of the Microbiological Spoilage of Foods and Beverages. Compend. Microbiol. Spoilage Foods Beverages 2009, 69–86. [Google Scholar] [CrossRef]
- Alum, E.A.; Urom, S.M.O.C.; Ben, C.M.A. Microbiological Contamination of Food the Mechanisms Impacts and Prevention. Int. J. Sci. Technol. Res. 2015, 4, 65–78. [Google Scholar]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential Application of Essential Oils for Mitigation of Listeria Monocytogenes in Meat and Poultry Products. Front. Nutr. 2020, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M.; Stoean, S.; Alexe, P. Overview of Biological Hazards Associated with the Consumption of the Meat Products. J. Agroaliment. Process. Technol. 2014, 20, 192–197. [Google Scholar]
- ur Rahman, U.; Sahar, A.; Ishaq, A.; Aadil, R.M.; Zahoor, T.; Ahmad, M.H. Advanced Meat Preservation Methods: A Mini Review. J. Food Saf. 2018, 38, e12467. [Google Scholar] [CrossRef]
- Khalafalla, F.A.; Ali, H.M.; El-Fouley, A. Microbiological Evaluation of Chicken Meat Products. J. Vet. Med. Res. 2019, 26, 151–163. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Stefani, L.M.; Lucheis, S.B.; Okano, W.; Cruz, J.C.M.; Souza, G.V.; Casagrande, T.A.C.; Bastos, P.A.S.; Pinheiro, R.R.; Arruda, M.M.; et al. Methicillin-Resistant Staphylococcus Aureus in Poultry and Poultry Meat: A Meta-Analysis. J. Food Prot. 2018, 81, 1055–1062. [Google Scholar] [CrossRef]
- Hu, K.L.; Yu, X.Q.; Chen, J.; Tang, J.N.; Wang, L.Z.; Li, Y.M.; Tang, C. Production of Characteristic Volatile Markers and Their Relation to Staphylococcus Aureus Growth Status in Pork. Meat Sci. 2020, 160, 107956. [Google Scholar] [CrossRef]
- Whyte, P.; McGill, K.; Collins, J.D. An Assessment of Steam Pasteurization and Hot Water Immersion Treatments for the Microbiological Decontamination of Broiler Carcasses. Food Microbiol. 2003, 20, 111–117. [Google Scholar] [CrossRef]
- Gu, J.-G.; Park, J.-M.; Yoon, S.-J.; Ahn, B.-K.; Kang, C.-W.; Song, J.-C.; Kim, J.-M. Assessment of Dipping Treatment with Various Lactic Acid or Sodium Benzoate Concentrations to Extend the Shelf-Life of Spent Hen Breast Meats. Korean J. Food Sci. Anim. Resour. 2011, 31, 428–435. [Google Scholar] [CrossRef][Green Version]
- Sarjit, A.; Dykes, G.A. Trisodium Phosphate and Sodium Hypochlorite Are More Effective as Antimicrobials against Campylobacter and Salmonella on Duck as Compared to Chicken Meat. Int. J. Food Microbiol. 2015, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Valenzuela-Melendres, M.; Heperkan, D.; Bautista, D.; Anderson, D.; Hwang, C.A.; Peña-Ramos, A.; Camou, J.P.; Torrentera-Olivera, N. Development of a Predictive Model for Salmonella Spp. Reduction in Meat Jerky Product with Temperature, Potassium Sorbate, PH, and Water Activity as Controlling Factors. Int. J. Food Microbiol. 2016, 236, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kocharunchitt, C.; Mellefont, L.; Bowman, J.P.; Ross, T. Application of Chlorine Dioxide and Peroxyacetic Acid during Spray Chilling as a Potential Antimicrobial Intervention for Beef Carcasses. Food Microbiol. 2020, 87, 103355. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Jaspal, M.H.; Yaqub, T.; Haq, A.U.; Nasir, J.; Avais, M.; Asghar, B.; Badar, I.H.; Ahmad, S.; Yar, M.K. Effect of Lactic Acid Spray on Microbial and Quality Parameters of Buffalo Meat. Meat Sci. 2020, 159, 107923. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, P.; Chen, D.; Jadeja, R.; Hung, Y.C.; Doyle, M.P. Reductions of Shiga Toxin–Producing Escherichia Coli and Salmonella Typhimurium on Beef Trim by Lactic Acid, Levulinic Acid, and Sodium Dodecyl Sulfate Treatments. J. Food Prot. 2014, 77, 528–537. [Google Scholar] [CrossRef]
- Burfoot, D.; Allen, V.; Mulvey, E.; Jewell, K.; Harrison, D.; Morris, V. Reducing Campylobacter Numbers on Chicken Carcasses Using Lactic Acid in Processing Plants. Int. J. Food Sci. Technol. 2015, 50, 2451–2457. [Google Scholar] [CrossRef]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge Gaps in Control of Campylobacter for Prevention of Campylobacteriosis. Transbound. Emerg. Dis. 2018, 65, 30–48. [Google Scholar] [CrossRef]
- Killinger, K.M.; Kannan, A.; Bary, A.I.; Cogger, C.G. Validation of a 2 Percent Lactic Acid Antimicrobial Rinse for Mobile Poultry Slaughter Operations. J. Food Prot. 2010, 73, 2079–2083. [Google Scholar] [CrossRef]
- Yoon, K.S. Effect of Gamma Irradiation on the Texture and Microstructure of Chicken Breast Meat. Meat Sci. 2003, 63, 273–277. [Google Scholar] [CrossRef]
- Anang, D.M.; Rusul, G.; Bakar, J.; Ling, F.H. Effects of Lactic Acid and Lauricidin on the Survival of Listeria Monocytogenes, Salmonella Enteritidis and Escherichia Coli O157:H7 in Chicken Breast Stored at 4 °C. Food Control 2007, 18, 961–969. [Google Scholar] [CrossRef]
- Ko, J.-K.; Ma, Y.-H.; Song, K.-B. Effect of Chlorine Dioxide Treatment on Microbial Growth and Qualities of Chicken Breast. Prev. Nutr. Food Sci. 2005, 10, 122–129. [Google Scholar] [CrossRef]
- Cantalejo, M.J.; Zouaghi, F.; Pérez-Arnedo, I. Combined Effects of Ozone and Freeze-Drying on the Shelf-Life of Broiler Chicken Meat. LWT 2016, 68, 400–407. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Roobab, U.; Fidalgo, L.G.; Arshad, R.N.; Khan, A.W.; Zeng, X.A.; Bhat, Z.F.; Bekhit, A.E.D.A.; Batool, Z.; Aadil, R.M. High-Pressure Processing of Fish and Shellfish Products: Safety, Quality, and Research Prospects. Compr. Rev. Food Sci. Food Saf. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Ayub, Z.; Muhammad Aadil, R.; Abid, M.; Zhang, J.; Liqing, Z. Recent Advances in Plasma Technology: Influence of Atmospheric Cold Plasma on Spore Inactivation. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Lira Santos, T.C. Improving Quality and Shelf-Life of Whole Chilled Pacific White Shrimp (Litopenaeus Vannamei) by Ozone Technology Combined with Modified Atmosphere Packaging. LWT 2019, 99, 568–575. [Google Scholar] [CrossRef]
- Roobab, U.; Afzal, R.; Ranjha, M.M.A.N.; Zeng, X.A.; Ahmed, Z.; Aadil, R.M. High Pressure-Based Hurdle Interventions for Raw and Processed Meat: A Clean-Label Prospective. Int. J. Food Sci. Technol. 2022, 57, 816–826. [Google Scholar] [CrossRef]
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The Effects of Atmospheric Cold Plasma on Inactivation of Listeria Monocytogenes and Staphylococcus Aureus and Some Quality Characteristics of Pastırma—A Dry-Cured Beef Product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. [Google Scholar] [CrossRef]
- Gertzou, I.N.; Karabagias, I.K.; Drosos, P.E.; Riganakos, K.A. Effect of Combination of Ozonation and Vacuum Packaging on Shelf Life Extension of Fresh Chicken Legs during Storage under Refrigeration. J. Food Eng. 2017, 213, 18–26. [Google Scholar] [CrossRef]
- Zouaghi, F.; Cantalejo, M.J. Study of Modified Atmosphere Packaging on the Quality of Ozonated Freeze-Dried Chicken Meat. Meat Sci. 2016, 119, 123–131. [Google Scholar] [CrossRef]
- Muhlisin, M.; Utama, D.T.; Lee, J.H.; Choi, J.H.; Lee, S.K. Effects of Gaseous Ozone Exposure on Bacterial Counts and Oxidative Properties in Chicken and Duck Breast Meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, U.G.; Ozunlu, O.; Ergezer, H.; Karaca, H. Effects of Ozone Treatment on Microbiological Quality and Physicochemical Properties of Turkey Breast Meat. Ozone Sci. Eng. 2020, 42, 95–103. [Google Scholar] [CrossRef]
- Giménez, B.; Graiver, N.; Giannuzzi, L.; Zaritzky, N. Treatment of Beef with Gaseous Ozone: Physicochemical Aspects and Antimicrobial Effects on Heterotrophic Microflora and Listeria Monocytogenes. Food Control 2021, 121, 1–9. [Google Scholar] [CrossRef]
- Cooke, E.M. Principles and Practice of Disinfection, Preservation and Sterilization. J. Clin. Pathol. 1983, 36, 364. [Google Scholar] [CrossRef][Green Version]
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging Non-Thermal Technologies for Decontamination of Salmonella in Food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- USDA-FSIS. Safe and Suitable Ingredients Used in the Production of Meat and Poultry, and Egg Products; USDA-FSIS: Washington, DC, USA, 2016. Available online: https://www.google.com/search?q=USDA-FSIS.+Safe+and+Suitable+Ingredients+Used+in+the+Production+of+Meat+and+Poultry%2C+and+Egg+Products%3B+USDA-FSIS%3A+Washington%2C+DC%2C+USA%2C+2016&oq=USDA-FSIS.+Safe+and+Suitable+Ingredients+Used+in+the+Production+of+M (accessed on 16 May 2022).
- USDA-FSIS USDA FSIS, 2001, Letter from Robert C. Post (FSIS, Washington, DC) to Mark D. Dopp (Am. Meat Institute, Arlington, VA) Dated 21 December 2001. Available online: https://www.fsis.usda.gov/ (accessed on 16 May 2022).
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone Based Food Preservation: A Promising Green Technology for Enhanced Food Safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Pyatkovskyy, T.; Mohamed, H.M.; Yousef, A.E.; Sastry, S.K. Physics of Fresh Produce Safety: Role of Diffusion and Tissue Reaction in Sanitization of Leafy Green Vegetables with Liquid and Gaseous Ozone-Based Sanitizers. J. Food Prot. 2015, 78, 2108–2116. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a Novel Emerging Technology for the Dissipation of Pesticide Residues in Foods—A Review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Roobab, U.; Khan, A.W.; Lorenzo, J.M.; Arshad, R.N.; Chen, B.R.; Zeng, X.A.; Bekhit, A.E.D.; Suleman, R.; Aadil, R.M. A Systematic Review of Clean-Label Alternatives to Synthetic Additives in Raw and Processed Meat with a Special Emphasis on High-Pressure Processing (2018–2021). Food Res. Int. 2021, 150, 110792. [Google Scholar] [CrossRef]
- Roobab, U.; Inam-Ur-Raheem, M.; Khan, A.W.; Arshad, R.N.; Zeng, X.; Aadil, R.M. Innovations in High-Pressure Technologies for the Development of Clean Label Dairy Products: A Review. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Global High Pressure Processing (HPP) Food Market—Analysis by Product Type, Distribution Channel, by Region, by Country (2020 Edition): Market Insights, Outlook Post COVID-19 Pandemic (2020–2025); Research and Markets: Dublin, Ireland, 2020.
- Argyri, A.A.; Papadopoulou, O.S.; Nisiotou, A.; Tassou, C.C.; Chorianopoulos, N. Effect of High Pressure Processing on the Survival of Salmonella Enteritidis and Shelf-Life of Chicken Fillets. Food Microbiol. 2018, 70, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cap, M.; Paredes, P.F.; Fernández, D.; Mozgovoj, M.; Vaudagna, S.R.; Rodriguez, A. Effect of High Hydrostatic Pressure on Salmonella Spp Inactivation and Meat-Quality of Frozen Chicken Breast. LWT 2020, 118, 108873. [Google Scholar] [CrossRef]
- Chuang, S.; Sheen, S.; Sommers, C.H.; Zhou, S.; Sheen, L.Y. Survival Evaluation of Salmonella and Listeria Monocytogenes on Selective and Nonselective Media in Ground Chicken Meat Subjected to High Hydrostatic Pressure and Carvacrol. J. Food Prot. 2020, 83, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Karwe, M.V.; Matthews, K.R. Differences in Inactivation of Escherichia Coli O157: H7 Strains in Ground Beef Following Repeated High Pressure Processing Treatments and Cold Storage. Food Microbiol. 2016, 58, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kong, X.; Jiang, Y. Recovery of High Pressure Processing (HPP) Induced Injured Escherichia Coli O157:H7 Inhibited by Lactobacillus Sakei on Vacuum-Packed Ground Beef. Food Biosci. 2021, 41, 100928. [Google Scholar] [CrossRef]
- Lee, H.; Shahbaz, H.M.; Yang, J.; Jo, M.H.; Kim, J.U.; Yoo, S.; Kim, S.H.; Lee, D.U.; Park, J. Effect of High Pressure Processing Combined with Lactic Acid Bacteria on the Microbial Counts and Physicochemical Properties of Uncooked Beef Patties during Refrigerated Storage. J. Food Process. Preserv. 2021, 45, e15345. [Google Scholar] [CrossRef]
- Eccoña Sota, A.; Cap, M.; Rodriguez, A.; Szerman, N.; Speroni, F.; Vaudagna, S.R. Effects of High Hydrostatic Pressure and Beef Patty Formulations on the Inactivation of Native Strains of Shiga Toxin-Producing Escherichia Coli O157:H7. Food Bioprocess Technol. 2021, 14, 1194–1198. [Google Scholar] [CrossRef]
- Banerjee, R.; Jayathilakan, K.; Chauhan, O.P.; Naveena, B.M.; Devatkal, S.; Kulkarni, V.V. Vacuum Packaged Mutton Patties: Comparative Effects of High Pressure Processing and Irradiation. J. Food Process. Preserv. 2017, 41, e12880. [Google Scholar] [CrossRef]
- Sun, S.; Sullivan, G.; Stratton, J.; Bower, C.; Cavender, G. Effect of HPP Treatment on the Safety and Quality of Beef Steak Intended for Sous Vide Cooking. LWT-Food Sci. Technol. 2017, 86, 185–192. [Google Scholar] [CrossRef]
- Evelyn; Milani, E.; Silva, F.V.M. Comparing High Pressure Thermal Processing and Thermosonication with Thermal Processing for the Inactivation of Bacteria, Moulds, and Yeasts Spores in Foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. High Pressure Thermal Processing for the Inactivation of Clostridium Perfringens Spores in Beef Slurry. Innov. Food Sci. Emerg. Technol. 2016, 33, 26–31. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Modeling the Inactivation of Psychrotrophic Bacillus Cereus Spores in Beef Slurry by 600 MPa HPP Combined with 38–70 °C: Comparing with Thermal Processing and Estimating the Energy Requirements. Food Bioprod. Process. 2016, 99, 179–187. [Google Scholar] [CrossRef]
- Rodrigues, I.; Trindade, M.A.; Caramit, F.R.; Candoğan, K.; Pokhrel, P.R.; Barbosa-Cánovas, G.V. Effect of High Pressure Processing on Physicochemical and Microbiological Properties of Marinated Beef with Reduced Sodium Content. Innov. Food Sci. Emerg. Technol. 2016, 38, 328–333. [Google Scholar] [CrossRef]
- Mizi, L.; Cofrades, S.; Bou, R.; Pintado, T.; López-Caballero, M.E.; Zaidi, F.; Jiménez-Colmenero, F. Antimicrobial and Antioxidant Effects of Combined High Pressure Processing and Sage in Beef Burgers during Prolonged Chilled Storage. Innov. Food Sci. Emerg. Technol. 2019, 51, 32–40. [Google Scholar] [CrossRef]
- Porto-fett, A.C.S.; Jackson-davis, A.; Kassama, L.S.; Daniel, M.; Oliver, M.; Jung, Y.; Luchansky, J.B. Inactivation of Shiga Toxin-Producing Escherichia Coli in Refrigerated and Frozen Meatballs Using High Pressure Processing. Microorganisms 2020, 8, 360. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Yu, Z.; Wu, T.; Bennett, L.E. Effects of High Pressure Processing on Microbial, Textural and Sensory Properties of Low-Salt Emulsified Beef Sausage. Food Control 2022, 133, 108596. [Google Scholar] [CrossRef]
- Balamurugan, S.; Gemmell, C.; Lau, A.T.Y.; Arvaj, L.; Strange, P.; Gao, A.; Barbut, S. High Pressure Processing during Drying of Fermented Sausages Can Enhance Safety and Reduce Time Required to Produce a Dry Fermented Product. Food Control 2020, 113, 107224. [Google Scholar] [CrossRef]
- Balamurugan, S.; Inmanee, P.; De Souza, J.E.; Strange, P.; Pirak, T.; Barbut, S. Effects of High Pressure Processing and Hot Water Pasteurization of Cooked Sausages on Inactivation of Inoculated Listeria monocytogenes, Natural Populations of Lactic Acid Bacteria, Pseudomonas spp., and Coliforms and Their Recovery during Storage at 4 A. J. Food Prot. 2018, 81, 1245–1251. [Google Scholar] [CrossRef]
- Bonilauri, P.; Grisenti, M.S.; Daminelli, P.; Merialdi, G.; Ramini, M.; Bardasi, L.; Taddei, R.; Cosciani-Cunico, E.; Dalzini, E.; Frustoli, M.A.; et al. Reduction of Salmonella Spp. Populations in Italian Salami during Production Process and High Pressure Processing Treatment: Validation of Processes to Export to the U.S. Meat Sci. 2019, 157, 107869. [Google Scholar] [CrossRef]
- Bonilauri, P.; Merialdi, G.; Ramini, M.; Bardasi, L.; Taddei, R.; Grisenti, M.S.; Daminelli, P.; Cosciani-Cunico, E.; Dalzini, E.; Frustoli, M.A.; et al. Modeling the Behavior of Listeria Innocua in Italian Salami during the Production and High-Pressure Validation of Processes for Exportation to the U.S. Meat Sci. 2021, 172, 108315. [Google Scholar] [CrossRef]
- Lee, S.H.; Choe, J.; Shin, D.J.; Yong, H.I.; Choi, Y.; Yoon, Y.; Jo, C. Combined Effect of High Pressure and Vinegar Addition on the Control of Clostridium Perfringens and Quality in Nitrite-Free Emulsion-Type Sausage. Innov. Food Sci. Emerg. Technol. 2019, 52, 429–437. [Google Scholar] [CrossRef]
- Castro, S.M.; Kolomeytseva, M.; Casquete, R.; Silva, J.; Teixeira, P.; Castro, S.M.; Queirós, R.; Saraiva, J.A. Biopreservation Strategies in Combination with Mild High Pressure Treatments in Traditional Portuguese Ready-to-Eat Meat Sausage. Food Biosci. 2017, 19, 65–72. [Google Scholar] [CrossRef]
- Pérez-Baltar, A.; Alía, A.; Rodríguez, A.; Córdoba, J.J.; Medina, M.; Montiel, R. Impact of Water Activity on the Inactivation and Gene Expression of Listeria Monocytogenes during Refrigerated Storage of Pressurized Dry-Cured Ham. Foods 2020, 9, 1092. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-Pressure Processing and Antimicrobial Biodegradable Packaging to Control Listeria Monocytogenes during Storage of Cooked Ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Y.L. Technologies and Mechanisms for Safety Control of Ready-to-Eat Muscle Foods: An Updated Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1886–1901. [Google Scholar] [CrossRef]
- Nikparvar, B.; Subires, A.; Capellas, M.; Hernandez, M.; Bar, N. A Dynamic Model of Membrane Recovery Mechanisms in Bacteria Following High Pressure Processing. IFAC-PapersOnLine 2019, 52, 243–250. [Google Scholar]
- Marušić Radovčić, N.; Ježek, D.; Markov, K.; Frece, J.; Ćurić, D.; Medić, H. The Effect of High Pressure Treatment on the Quality of Chicken Breast Meat. Hrvat. Časopis za Prehrambenu Tehnol. Biotehnol. i Nutr. 2020, 14, 76–81. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold Plasma Treated Thyme Essential Oil/Silk Fibroin Nanofibers against Salmonella Typhimurium in Poultry Meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A Review on Microbiological Decontamination of Fresh Produce with Nonthermal Plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar]
- Chaplot, S.; Yadav, B.; Jeon, B.; Roopesh, M.S. Atmospheric Cold Plasma and Peracetic Acid-Based Hurdle Intervention to Reduce Salmonella on Raw Poultry Meat. J. Food Prot. 2019, 82, 878–888. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Corona Discharge Plasma Jet for Inactivation of Escherichia Coli O157:H7 and Listeria Monocytogenes on Inoculated Pork and Its Impact on Meat Quality Attributes. Ann. Microbiol. 2016, 66, 685–694. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Grant, I.R. Evaluation of the Efficacy of Multiple Physical, Biological and Natural Antimicrobial Interventions for Control of Pathogenic Escherichia Coli on Beef. Food Microbiol. 2018, 76, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rossow, M.; Ludewig, M.; Braun, P.G. Effect of Cold Atmospheric Pressure Plasma Treatment on Inactivation of Campylobacter Jejuni on Chicken Skin and Breast Fillet. LWT 2018, 91, 265–270. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between Different D-Dimer Cutoff Values to Assess the Individual Risk of Recurrent Venous Thromboembolism: Analysis of Results Obtained in the DULCIS Study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Hinton, A.; Zhang, J. Influence of In-Package Cold Plasma Treatment on Microbiological Shelf Life and Appearance of Fresh Chicken Breast Fillets. Food Microbiol. 2016, 60, 142–146. [Google Scholar] [CrossRef]
- Sahebkar, A.; Hosseini, M.; Sharifan, A. Plasma-Assisted Preservation of Breast Chicken Fillets in Essential Oils-Containing Marinades. LWT 2020, 131, 109759. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-Package Decontamination of Chicken Breast Using Cold Plasma Technology: Microbial, Quality and Storage Studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Zhuang, H.; Rothrock, M.J.; Hiett, K.L.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M.; Šerá, B. In-Package Air Cold Plasma Treatment of Chicken Breast Meat: Treatment Time Effect. J. Food Qual. 2019, 2019, 1837351. [Google Scholar] [CrossRef]
- Zhuang, H.; Rothrock, M.J.; Line, J.E.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M. Optimization of In-Package Cold Plasma Treatment Conditions for Raw Chicken Breast Meat with Response Surface Methodology. Innov. Food Sci. Emerg. Technol. 2020, 66, 102477. [Google Scholar] [CrossRef]
- Roh, S.H.; Oh, Y.J.; Lee, S.Y.; Kang, J.H.; Min, S.C. Inactivation of Escherichia Coli O157:H7, Salmonella, Listeria Monocytogenes, and Tulane Virus in Processed Chicken Breast via Atmospheric in-Package Cold Plasma Treatment. LWT 2020, 127, 109429. [Google Scholar] [CrossRef]
- Roh, S.H.; Lee, S.Y.; Park, H.H.; Lee, E.S.; Min, S.C. Effects of the Treatment Parameters on the Efficacy of the Inactivation of Salmonella Contaminating Boiled Chicken Breast by In-Package Atmospheric Cold Plasma Treatment. Int. J. Food Microbiol. 2019, 293, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Cheigh, C.I.; Kang, J.H.; Lee, S.Y.; Min, S.C. Evaluation of In-Package Atmospheric Dielectric Barrier Discharge Cold Plasma Treatment as an Intervention Technology for Decontaminating Bulk Ready-to-Eat Chicken Breast Cubes in Plastic Containers. Appl. Sci. 2020, 10, 6301. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, H.; Yeh, H.Y.; Bowker, B.; Zhang, J. Effect of Rosemary Extract on Microbial Growth, PH, Color, and Lipid Oxidation in Cold Plasma-Processed Ground Chicken Patties. Innov. Food Sci. Emerg. Technol. 2019, 57, 102168. [Google Scholar] [CrossRef]
- Yong, H.I.; Han, M.; Suh, J.; Yoo, S.J.; Lee, H.; Jo, C. Effect of Dielectric Barrier Discharge Plasma on the Discoloration of Myoglobin. Meat Sci. Technol. 2016, 60–63. Available online: http://icomst-proceedings.helsinki.fi/papers/2016_07_28.pdf (accessed on 16 May 2022).
- Lis, K.A.; Boulaaba, A.; Binder, S.; Li, Y.; Kehrenberg, C.; Zimmermann, J.L.; Klein, G.; Ahlfeld, B. Inactivation of Salmonella Typhimurium and Listeria Monocytogenes on Ham with Nonthermal Atmospheric Pressure Plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Misra, N.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Effect of In-Package Atmospheric Cold Plasma Discharge on Microbial Safety and Quality of Ready-to-Eat Ham in Modified Atmospheric Packaging during Storage. J. Food Sci. 2020, 85, 1203–1212. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Govindan, B.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Cold Plasma Treatment of Ready-to-Eat Ham: Influence of Process Conditions and Storage on Inactivation of Listeria Innocua. Food Res. Int. 2019, 123, 276–285. [Google Scholar] [CrossRef]
- Benecke, P.; Ahlfeld, B.; Boulaaba, A.; Zimmermann, J.L.; Klein, G. Effect of Atmospheric Cold Plasma (ACP) on Escherichia Coli, Listeria Monocytogenes and Salmonella Enterica Serovar Typhimurium on Ready-to-Eat Mortadella-Type Sausage. Meat Sci. Technol. 2016, 1–4. Available online: http://icomst-proceedings.helsinki.fi/papers/2016_06_09.pdf (accessed on 16 May 2022).
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Sites, J.E.; Boyd, G.; Lacombe, A. Dielectric Barrier Discharge Atmospheric Cold Plasma Inhibits Escherichia Coli O157:H7, Salmonella, Listeria Monocytogenes, and Tulane Virus in Romaine Lettuce. Int. J. Food Microbiol. 2016, 237, 114–120. [Google Scholar] [CrossRef]
- Fernández, A.; Shearer, N.; Wilson, D.R.; Thompson, A. Effect of Microbial Loading on the Efficiency of Cold Atmospheric Gas Plasma Inactivation of Salmonella Enterica Serovar Typhimurium. Int. J. Food Microbiol. 2012, 152, 175–180. [Google Scholar] [CrossRef]
- Bauer, A.; Ni, Y.; Bauer, S.; Paulsen, P.; Modic, M.; Walsh, J.L.; Smulders, F.J.M. The Effects of Atmospheric Pressure Cold Plasma Treatment on Microbiological, Physical-Chemical and Sensory Characteristics of Vacuum Packaged Beef Loin. Meat Sci. 2017, 128, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xiang, Q.; Cullen, P.J.; Su, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Plasma-Activated Water (PAW) and Slightly Acidic Electrolyzed Water (SAEW) as Beef Thawing Media for Enhancing Microbiological Safety. LWT 2020, 117, 108649. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of Cold Plasma Technology for Microbiological Safety in Meat Industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Bourke, P.; Cullen, P.J. Effects of Cold Plasma on Surface, Thermal and Antimicrobial Release Properties of Chitosan Film. J. Renew. Mater. 2017, 5, 14–20. [Google Scholar] [CrossRef]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-Package Cold Plasma Technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar]
- Rothrock, M.J.; Zhuang, H.; Lawrence, K.C.; Bowker, B.C.; Gamble, G.R.; Hiett, K.L. In-Package Inactivation of Pathogenic and Spoilage Bacteria Associated with Poultry Using Dielectric Barrier Discharge-Cold Plasma Treatments. Curr. Microbiol. 2017, 74, 149–158. [Google Scholar] [CrossRef]
- Lee, H.; Yong, H.I.; Kim, H.J.; Choe, W.; Yoo, S.J.; Jang, E.J.; Jo, C. Evaluation of the Microbiological Safety, Quality Changes, and Genotoxicity of Chicken Breast Treated with Flexible Thin-Layer Dielectric Barrier Discharge Plasma. Food Sci. Biotechnol. 2016, 25, 1189–1195. [Google Scholar] [CrossRef]
- Kronn, T.G.; Lawrence, K.C.; Zhuang, H.; Hiett, K.L.; Rothrock, M.J.; Huang, Y.W.; Keener, K.M.; Abdo, Z. Nonthermal Plasma System for Extending Shelf Life of Raw Broiler Breast Fillets. Trans. ASABE 2015, 58, 493–500. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuang, H.; Lawrence, K.; Zhang, J.H. Disinfection of Chicken Fillets in Packages with Atmospheric Cold Plasma: Effects of Treatment Voltage and Time. J. Appl. Microbiol. 2018, 124, 1212–1219. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Line, J.E.; Hinton, A.; Gao, Y.; Zhuang, H. The Effect of Rosemary Extract and Cold Plasma Treatments on Bacterial Community Diversity in Poultry Ground Meats. Heliyon 2019, 5, e02719. [Google Scholar] [CrossRef]
- German Research Community, D. German Research Community (Deutsche Forschungsgemeinschaft, DFG) Stellungnahme Zum Einsatz von Plasma Verfahren Zur Behandlung von Lebensmitteln [Opinion on the Application of Plasma-Technology for Treatment of Foods]. 2012. Available online: https://www.google.com/search?sxsrf=APq-WBvttleHJnVhXUJXXX4mkX8r-npK9g:1649245379719&q=German+Research+Community+(Deutsche+Forschungsgemeinschaft,+DFG)+Stellungnahme+zum+Einsatz+von+Plasma+Verfahren+zur+Behandlung+von+Lebensmitteln+%5BOpinion+on+the+Appli (accessed on 16 May 2022).
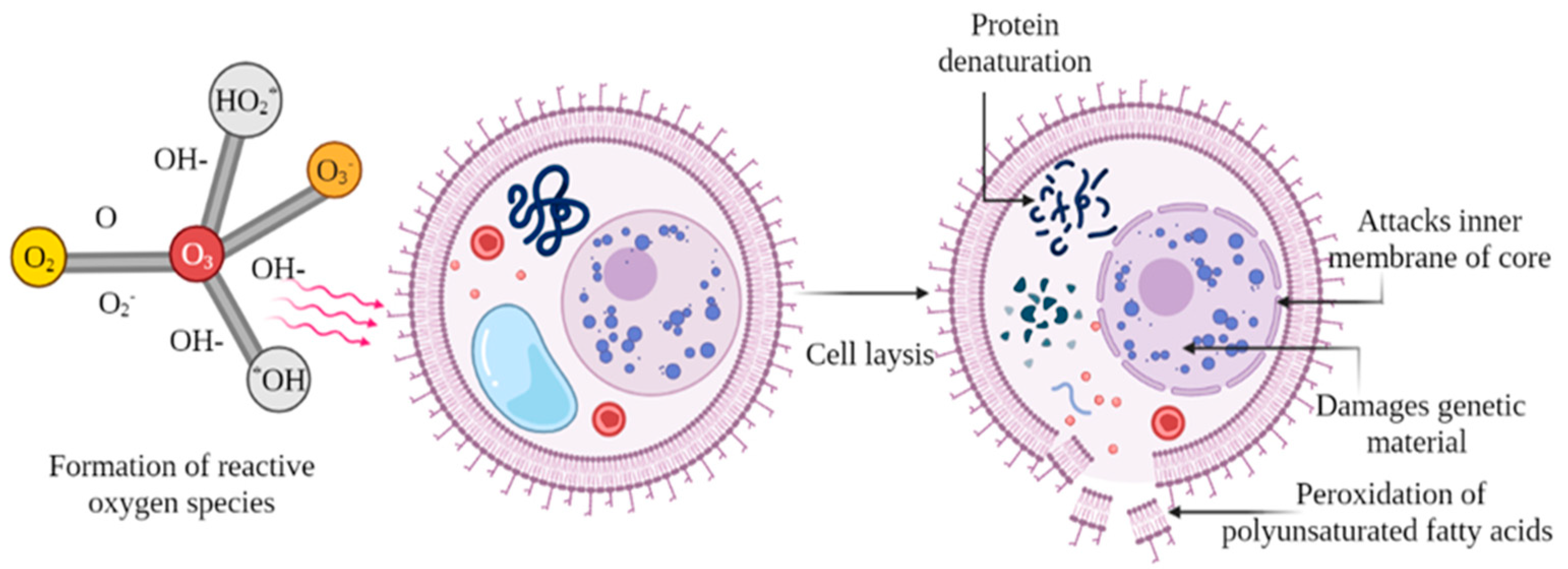
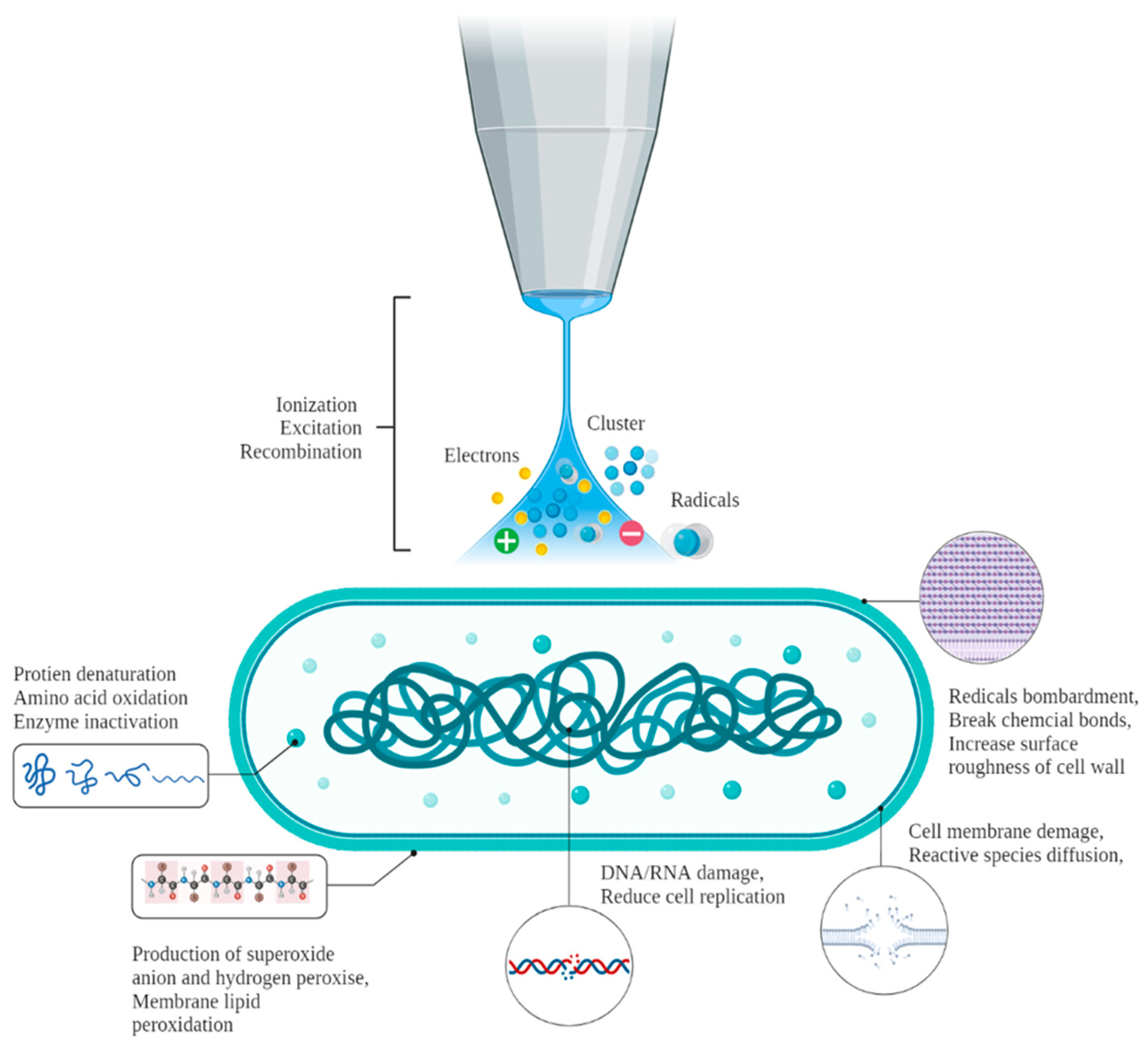
| Sample | Specification | Microbes | Highlights | Reference |
|---|---|---|---|---|
| Chicken legs | 2–10 mg/L for 1 h combined with vacuum packaging (polyamide/polyethylene bags) stored at 4 °C for 16 days. | TVC, Pseudomonas spp., LAB, Yeast-molds, & Enterobacteriaceae | 6-day shelf-life extension compared to vacuum packaging alone (4-day extension). Positively affected odor, texture, and taste retained an acceptable score for 14–16 days. | [29] |
| Chicken meat (freeze-dried) | 0.6 ppm at 4 °C (90% RH) for 10 min. | TAMB, LAB, E. coli. & Salmonella spp. | 1.1 log CFU/g was observed in TAMB and LAB. E. coli. and Salmonella spp. was not detected. Combination with MAP (20% CO2, 80% N2) improved the texture and sensory proprieties. | [30] |
| Chicken meat (freeze-dried) | 0.4–0.7 ppm at 4 °C (90% RH) for 10–120 min. | LAB & TAMB | Reduced 4.77 and 6.8 log CFU/g, respectively. The combined use of ozone and lyophilization would be useful for extending shelf-life to 8 months. | [22] |
| Chicken breast meat | 10 × 10−6 kg O3/m3/h for 3 days. | Coliform, aerobic, and anaerobic bacteria | Aerobic: 2.96 log CFU/g (untreated = 5.35 log CFU/g) Anaerobic: 2.18 log CFU/g (untreated = 4.63 log CFU/g) Coliform: 1.74 log CFU/g (untreated = 3.35 log CFU/g) | [31] |
| Duck breast meat | Aerobic: 2.52 log CFU/g (untreated = 4.11 log CFU/g) Anaerobic: 3.46 log CFU/g (untreated = 3.95 log CFU/g) Coliform: 1.39 (untreated = 3.28) | |||
| Turkey breast meat | 1 × 10−2 kg/m3 at 22 °C (21.6% RH) for 8 h. | TAMB, Enterobacteriaceae & yeast-mold | Reduced 2.9, 2.3 and 1.9 log CFU/g, respectively. | [32] |
| Beef (sliced) | 218–286 mg/m3, 5–20 pulses for 2–40 min with intervals of 30 min. | Heterotrophic microflora & L. monocytogenes | Decreased 1.5 log CFU/g heterotrophic counts. Decreased inoculated L. monocytogenes counts by more than 1 log CFU/g. Exposure times of more than 10 min negatively affected red color and rancidity. | [33] |
| Meat Type | Treatment Conditions | Storage Conditions | Findings | Reference |
|---|---|---|---|---|
| Chicken fillets | 500 MPa for 10 min. | 4 and 12 °C | HHP resulted in the reduction of the pathogen population below the detection limit of the enumeration method (0.48 log CFU/g), irrespective of the inoculum. HHP extended the shelf life of chicken fillets by 6 and 2 days, at 4 and 12 °C, respectively. | [44] |
| Frozen chicken breast | 500 MPa for 1 min and 400 MPa for 5 min. | _ | HHP showed inactivation of Salmonella at 400 MPa for 5 min and 500 MPa for 1 min. | [45] |
| Ground chicken meat | 350 MPa for 10 min + 0.75% carvacrol. | HHP with 0.60% carvacrol treatment resulted in a >5-log pathogen reduction. | [46] | |
| Ground beef | 400 MPa for 15 min at 25, 35, and 45 °C. | 4 and −20 °C for up to 5 days | At 25 °C, 5 log reduction in E. coli O157:H7 was observed further low-temperature storage serves as the hurdle in its survival and recovery after treatment. HHP showed no effect on the chromatic profile of grounded beef. | [47] |
| Vacuum-packed ground beef | 200 and 400 MPa for 5 min at 25 °C. | _ | L. sakei is good pressure-resistant lactic acid bacteria used in combination with HHP at 400 MPa and is efficient in controlling pathogenic E. coli strains. | [48] |
| Uncooked ground beef patties | 300, 400, and 500 MPa for 5 min. | 4 °C for 10 days | HHP combine with Lactobacillus acidophilus showed less total aerobic count (3.35 log CFU/g) than untreated (6.74 log CFU/g) beef patties with 0.80 log CFU/mL yeast and mold count. The combined treatment showed a delayed decrease in pH value, inhibited lipid oxidation with better color retention and the highest sensory score. | [49] |
| Beef patty | 400 and 600 MPa for 5 min. | Refrigerated storage for 18 h | An amount of 2 and 4 log CF/mL reductions after 400 and 600 MPa in Shiga toxin-producing E. coli O157:H7, respectively. Variations in fat concentration of 10 and 20% did not affect. In contrast, 1% NaCl evident more reduction than 2%, indicating bar protective effect of salt. | [50] |
| Vacuum-pack ripened mutton patties | 200 and 400 MPa for 10 min. | 4 °C for 28 days | Significant reduction in total plate count after HHP at both levels, with a significant increase in lightness (L*). Redness (a*), yellowness (b*); hardness, gumminess, and chewiness of patties reduced significantly. | [51] |
| Beef steak | 450 MPa, 600 MPa 1, 3, 6, 10, 15 min. | _ | HHP have the potential to allow the production of a convenient and safe product by achieving 5 log definition of pasteurization of beef steak inoculated with E. coli 0157:H7. | [52] |
| Beef slurry | 600 MPa for 20 min at 75 °C. | _ | Best inactivation of spores of Clostridium perfringens in beef slurry was a 2.2 log reduction. | [53] |
| Beef slurry | 600 MPa for 20 min at 75 °C. | _ | After HHP, a greater reduction (2.2 log) in C. perfringens spores was observed as compared to thermal treatment (no reduction) after 20 min. | [54] |
| Beef slurry | 600 MPa at 70 °C for 20 min. | _ | A 4.9 log reduction in Bacillus cereus spores after treatment at 70 °C but same temperature thermal processing led to 0.5 log reduction in spore. Increasing HHP temperature from 38 to 70 °C increases the spore inactivation for up to 3 logs. | [55] |
| Marinated beef (Longissimus lumborum) | 300, 400, and 600 MPa for 5 min. | Refrigerated storage for 14 days | HHP was proven to provide safe meat along with a sodium reduction in it. Meat marinated with salt and citric acid has no sufficient inactivation of L. innocua and Enterococcus faecium, while when combine with HHP, a 6 log cycle reduction was observed. | [56] |
| Beef burgers | 300 MPa for 10 min at 9.9 °C and 600 MPa 10 min, 10.2 °C. | _ | Mesophilic and psychotropic count remain at the detection limit after HHP at 600 MPa, with no effect on lipid oxidation for at least 6 days. | [57] |
| Raw meatballs (beef, veal, beef + veal + pork) | 400 and 600 MPa for 0 and 18 min. | 4 and −12 °C for 18 h | No difference in the extent of inactivation in different species of meat used for meatballs preparation in refrigerated storage (0.9 to 2.9 log CFU/g) as compared to frozen samples (1.0 to 3.0 log CFU/g). A total of 600 MPa requires 1–3 min and 400 MPa requires 9 min for a ≥2.0 log CFU/g reduction. | [58] |
| Emulsified beef sausages | 100–400 MPa for 15 min at 10 °C. | _ | HHP proved to be an effective technique to produce microbial safe beef sausages (reduce total viable count equivalent to the sausages having higher salt concentration) with lower salt concentration. | [59] |
| Dry fermented sausages | 600 MPa for 3 min. | 4 °C for 4 weeks | Inactivation of E. coli O157:H7 in dried fermented sausages was observed to be affected by aw. At aw ≤ 0.90, or moisture protein ratio in the range of 1.9–2.3, led to 6.4 log reduction. Further drying reduced to 2.2 log reduction. Recovery of E. coli O157:H7 was observed for 1 week of storage but in 2-, 3-, and 4-week storage, no further recovery was observed. | [60] |
| Pork cooked sausages | 600 MPa for 3 min. | 4 and 10 °C for 35 days | Cooking of sausages leads to a >6 log reduction in inoculated L. monocytogenes. During storage at 4 °C, no significant growth was observed after HHP. But at 10 °C storage, growth remains below the detection limit up to 21 days after the 4.5 log CFU/mL increase in population was observed. No lactic acid bacterial growth was observed till the end of storage. | [61] |
| Italian salami | 600 MPa for 300 s. | _ | HHP related microbial inactivation depicts an inverse relation with aw. All 20 salami samples showed a 5 log reduction in Salmonella after treatment. | [62] |
| Italian salami | 600 MPa for 300 s. | _ | An amount of 0.34–4.32 log CFU/g reduction during processing in L. innocua was observed which was reduced to 0.48–3.4 log CFU/g after HHP. The efficacy of HHP was associated with aw and higher pH after acidification, drying and seasoning phase. | [63] |
| Nitrite-free emulsion-type sausage | 0.1, 500 MPa for 12 min + 0, 1, 2% vinegar | 4 °C for two weeks followed by at 20 °C for three weeks | HHP (500 MPa; four cycles and each for 3 min) + vinegar (1%) reduced vegetative cells and spores of C. perfringens by 4.8 and 2.8 log CFU/g, respectively. | [64]. |
| Traditional Portuguese ready-to-eat meat sausage (Chouriço de carne) | 300 MPa for 5 min at 10 °C + lactic acid bacteria (Pediococcus acidilactici, HA-6111-2) and its bacteriocin (bacHA-6111-2). | Refregrated storage for 60 days. | The hurdle technology (bacteriocin and pressurization) showed a 0.5 log CFU/g decrease in L. innocua cells compared to non-treated cells. | [65] |
| Dry-cured ham | 450 MPa for 10 min and 600 MPa for 5 min. | 4 °C for 30 days | The efficacy of HHP against L. monocytogenes was reduced by low aw values. The changes in HHP-surviving bacteria gene transcription patterns were strain-dependent. | [66] |
| Cooked ham | 400 MPa for 10 min at 17 °C + alginate films containing enterocins. | 1 or 6 °C for 2 months | Both antimicrobial packaging and pressurization delayed the growth of L. monocytogenes levels below the detection limit (day 90) during 6 °C storage. | [67] |
| Sample | Experimental Conditions | Target Microbes | Remarks | Citation |
|---|---|---|---|---|
| Chicken breast meat | In-package DBD-CP: 55–80 kV for 3 min, stored at 4 °C for 24 h or 3 days. | Mesophiles or Psychrophiles | Significant decreases in microbial populations after storage of treated sample for 3 days at 4 °C. | [77] |
| In-package CP: 80 kV for 180 s at 25 °C and stored at 4 °C. | Mesophiles, Psychrophiles & Pseudomonas spp. | High microbial counts in air packed sample (>6 log CFU/g) than in MAP (<4 log CFU/g) stored for 7 days and 14 days (<6 log CFU/g). | [78] | |
| 32 kHz for 10 min + Crocus sativus L., Allium sativum L., and Zataria multiflora Boiss | E. coli & Staph. aureus | CP with essential oils reached a satisfactory load below 3.5 log CFU/g. Negative effect on odor, flavor, and overall acceptability. | [79] | |
| In-package DBD-CP: 100 kV for 1–5 min. | Natural microflora | 2 log CFU/g reduction within 5 min in Mesophiles, Psychrotrophic & Enterobacteriaceae. | [80] | |
| DBD-CP: 70 kV for 0–300 s, stored at 4 °C for 5 days. | Psychrophiles Campylobacter jejuni & S. typhimurium | 90% reductions in Psychrophile; and 0.5, 0.4, and 0.7 log reductions in psychrophiles, Salmonella, and Campylobacter. | [81] | |
| In-package CP: 60–80 kV for 60–300 s, stored at 4 °C for 5 days. | Campylobacter & Salmonella | 1.0 log reduction in psychrophiles at 60 kV with 35% O2. Also, 60 kV for 60 s treatment with 35% O2/60% CO2/5% N2 reduces microbes and appearance of meat. | [82] | |
| Chicken breast (boiled) | In-package CP: 39 kV for 3.5 min. | E. coli O157:H7, Salmonella, L. monocytogenes & Tulane virus | 3.7 log CFU/cube Salmonella, 3.9 log 28 CFU/cube E. coli O157:H7, 3.5 log CFU/cube L. monocytogenes, and 2.2 PFU/cube TV reduction after treatment. | [83] |
| In-package DBD-CP: 38.7 kV for 0.3–2.5 min | Salmonella | Whey protein coating increased treatment efficacy. An increase in initial inoculum concentration from 3.8 to 5.7 log CFU/sample lead to an increase in D-value increased from 0.2 to 1.3 min with1.7 log CFU/sample (highest) Salmonella reduction. | [84] | |
| RTE chicken breast cubes | In package CP: 24 kV for 3 min, stored at 4 °C for 21 days. | Mesophilic aerobic bacteria, Salmonella, & Tulane virus | 0.7, 1.4 and 1.1 log PFU/cube reduction in mesophilic aerobic bacteria, Salmonella, and Tulane virus, respectively. | [85] |
| Chicken breast patties (ground) | DBD-CP: 70 kV for 180 s at 22 °C, packaged in operating gas: 65% O2, 30% CO2. | Total plate count | 0.9 log reduction after 5-day storage as compared to non-CP treated samples. Rosemary extract prevents lipids oxidation and inhibits microbial growth in CP-processed meat under refrigerated conditions. | [86] |
| Chicken skin & breast fillet | Plasma jet: Feed gases (argon or air) for exposure times (30–180 s), distances from plasma jet nozzle to sample surface (5–12 mm). | Campylobacter jejuni | 0.78 to 2.55 and 0.65 to 1.42 log CFU/cm2 reductions were observed using argon or air as feed gases, respectively. Argon as a feed gas for a longer time (≥120 s) resulted in the highest reductions. | [76] |
| Chicken meat | DBD-CP + Paraacetic Acid (PPA 100–200 ppm) 3.5 kHz, 0–30 kV, 0–200 W. for 1–6 min, 2 mm distance. | S. typhimurium | 2.3 to 5.3 log CFU/cm2 reductions with combined treatment in contrast to PAA or CP treatments alone. 4.7 and 5.3 log CFU/cm2 was the highest reduction obtained after PAA + CP and CP + PAA, respectively. | [73] |
| Beef | 6 kV and 20 kHz for 30 s–10 min. | E. coli | 0.9 and 1.82 log CFU/cm2 reduction after 2- and 5 min treatments, respectively. | [75] |
| Pastırma (a dry-cured beef product) | Oxygen (100%), argon (100%) and two oxygen/argon mixtures (25%O2/75%Ar and 50%O2/50%Ar) for 180 and 300 s. | L. Monocytogenes, Staph. aureus, total mesophilic aerobic bacteria & yeast–mold | Maximum 0.85 log CFU/cm2 and 0.83 log CFU/cm2 reduction in S. aureus and L. monocytogenes counts, respectively. 1.41- and 1.66-log CFU/cm2 reduction in total mesophilic aerobic bacteria and yeast–mold counts, respectively. | [28] |
| Pork loin | DBD-CP: 80 kV for 60–180 s. | Total Aerobic bacteria | 53% reduction in total aerobic bacteria showed a significant effect on O2 concentration (60%) and time (180 s). | [87] |
| Pork (fresh & frozen) | Plasma jet: Air 20 kV, 58 kHz, 1.5 A for 0–120 s | L. monocytogenes & E. coli O157: H7 | 1.5 log and >1.0 log reduction in E. coli O157: H7 and Listeria monocytogenes, respectively. | [74] |
| Ham | 2 and 10 kHz, 6.4 or 10 kV for 10–20 min at 22 °C. | S. typhimurium & L. monocytogenes | 1.14 log and 1.02 log reduction in S. Typhimurium and L. monocytogenes log after 20 min, respectively. CP combined with cold storage for 7–14 days at 8 °C packed under sealed high nitrogen gas flush (70% N2, 30% CO2) effectively inactivating S. Typhimurium (1.84 log) and L. monocytogenes (2.55 log). | [88] |
| RTE ham | In-package DBD-CP: 3.5 kHz, 0–30 kV for 23 °C and stored at 4 °C for 18 h. | L. monocytogenes | 2 log (CFU/cm2) reduction after CP combined with MAP (20% O2 + 40% N2 + 40% CO2) and after 7 days storage at 4 °C cell counts reduced below the detection limit (>6 log reduction). | [89] |
| In-package DBD-CP: 3.5 kHz, 0–28 kV for 180 s, and stored for 6 and 24 h at 4 °C. | L. innocua | At 4 °C, 1.75 and 1.51 log CFU/cm2 reduction on 1% and 3% NaCl ham surface, respectively. At 23 °C, 1.78 and 1.43 log CFU/cm2 reduction, respectively. | [90] | |
| RTE mortadella-type sausage | 18 kV, 12.5 kHz for 0–120 s, 6 mm distance. Samples were sealed under high nitrogen gas flush (70% N2, 30% CO2) and stored at 4 °C for 1–21 days. | E. coli, L. monocytogenes & S. enterica serovar Typhimurium | The maximum inactivation for Salmonella was 0.3 logs. After 120 s CP and storage over 21 days counts for Listeria as well as E. coli were lower compared to a 30 s treatment (6.58 to 6.25 log and 5.63 to 5 log CFU/g, respectively). | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roobab, U.; Chacha, J.S.; Abida, A.; Rashid, S.; Muhammad Madni, G.; Lorenzo, J.M.; Zeng, X.-A.; Aadil, R.M. Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation, High-Hydrostatic Pressure and Cold Plasma. Foods 2022, 11, 2173. https://doi.org/10.3390/foods11152173
Roobab U, Chacha JS, Abida A, Rashid S, Muhammad Madni G, Lorenzo JM, Zeng X-A, Aadil RM. Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation, High-Hydrostatic Pressure and Cold Plasma. Foods. 2022; 11(15):2173. https://doi.org/10.3390/foods11152173
Chicago/Turabian StyleRoobab, Ume, James S. Chacha, Afeera Abida, Sidra Rashid, Ghulam Muhammad Madni, Jose Manuel Lorenzo, Xin-An Zeng, and Rana Muhammad Aadil. 2022. "Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation, High-Hydrostatic Pressure and Cold Plasma" Foods 11, no. 15: 2173. https://doi.org/10.3390/foods11152173
APA StyleRoobab, U., Chacha, J. S., Abida, A., Rashid, S., Muhammad Madni, G., Lorenzo, J. M., Zeng, X.-A., & Aadil, R. M. (2022). Emerging Trends for Nonthermal Decontamination of Raw and Processed Meat: Ozonation, High-Hydrostatic Pressure and Cold Plasma. Foods, 11(15), 2173. https://doi.org/10.3390/foods11152173











