Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor
Abstract
1. Introduction
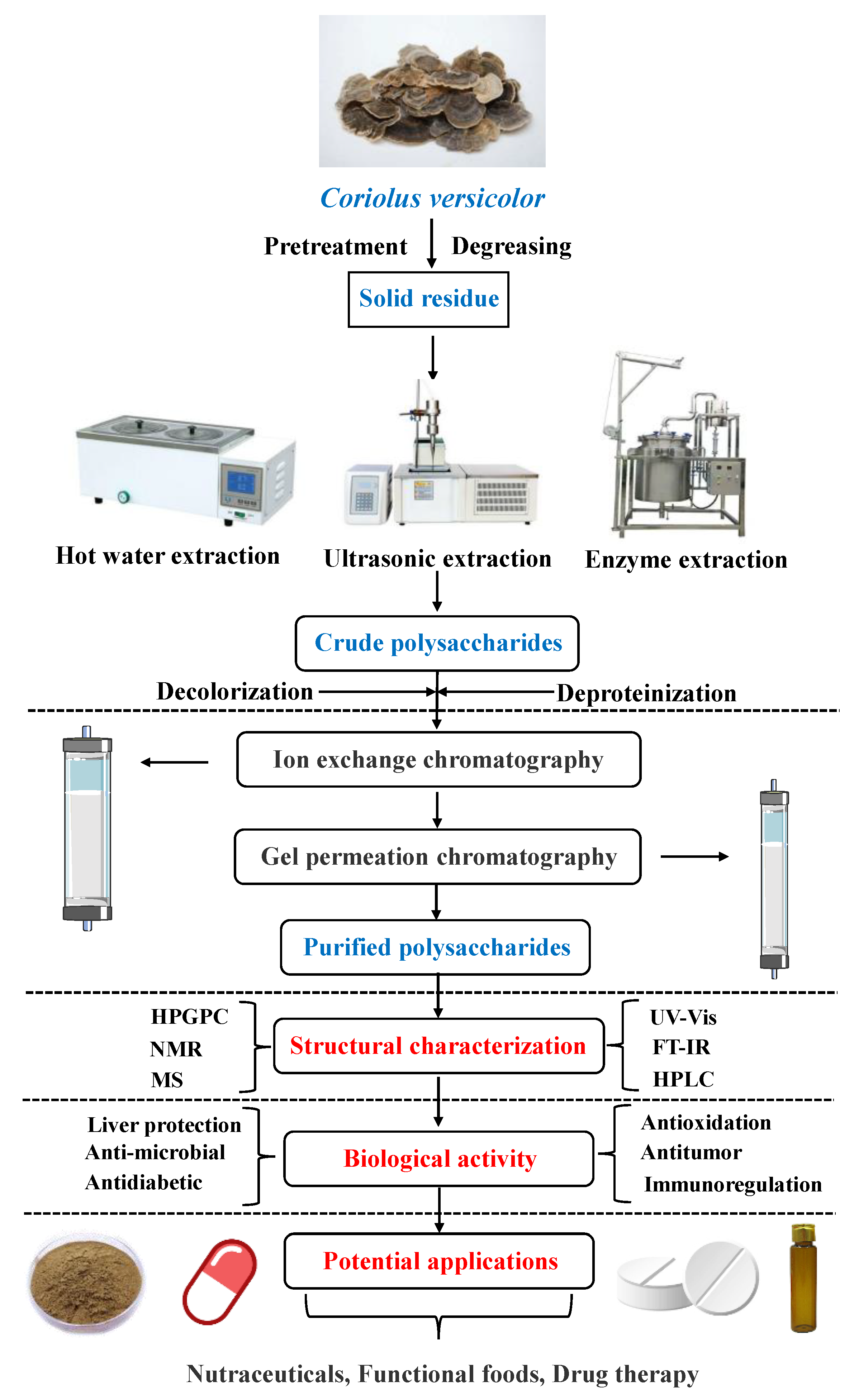
2. Extraction of CVP
2.1. Hot Water Extraction Method
2.2. Enzyme Extraction Method
2.3. Ultrasonic Extraction Method
3. Isolation, Purification, and Structural Characterization of CVP
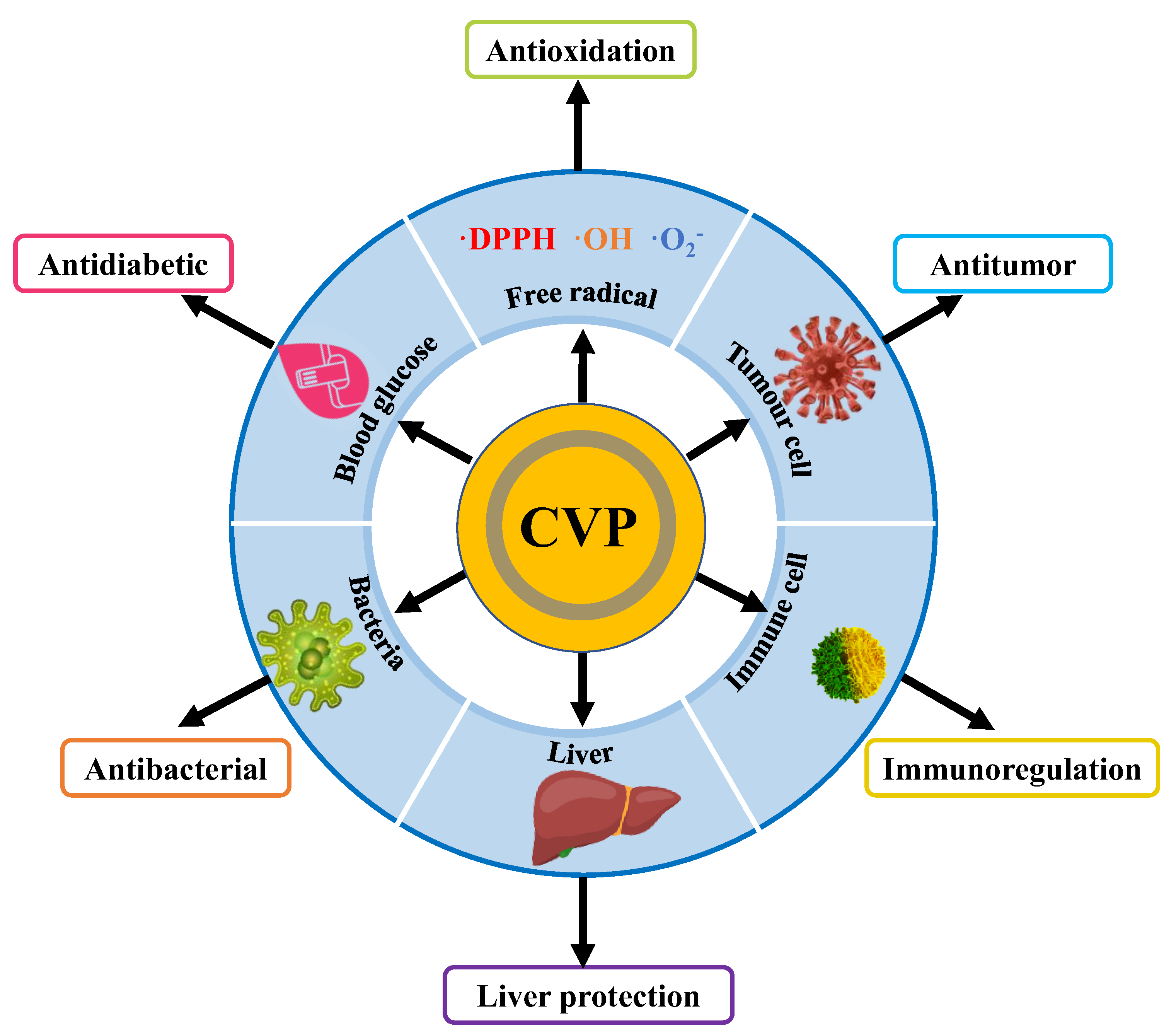
4. Bioactivities of CVP
4.1. Antioxidation
4.2. Antitumor Activity
| Cell/Animal | Effect | Speculative Mechanism | Ref. |
|---|---|---|---|
| Mouse melanoma B16 cells | ↓ | P53↓, Bcl-2↓ and Fas↓ | [56] |
| Human cervical cancer HeLa cells | ↓ | Bcl-2↓ | [57] |
| Human NK cells cultured in vitro | ↓ | NKG2Dreceptor↑ | [58] |
| H22 liver cancer transplanted mice | ↓ | Its mechanism may might be related to immune regulation and promoting tumor cell apoptosis | [59] |
| Human esophageal cancer cell line Eca-109 | ↓ | It acted on the inflammatory factor pathway CXCL12/CXCR4 | [60] |
| Colorectal cancer cells | ↑ | CVP could improve the long-term efficacy in the treatment of patients with advanced colorectal cancer | [61] |
| Mouse breast cancer 4T1 cells | ↓ | It has had antitumor and anti-metastatic effects on murine breast cancer 4T1 cells and 4T1 tumor mice | [62] |
| Human promyelocytic leukemia HL-60 cells | ↓ | AP-1↑, EGR1↑ | [63] |
| Leukemia HL-60 and U-937 cells | ↓ | PSP could disrupt the G1/S phase and G2/M phase during the cell cycle | [64] |
| Human promyelocytic leukemia HL-60 cells | ↓ | 3H thymidine↓ | [65] |
4.3. Immunoregulation Activity
| Animal Model | Regulating Effect | Ref. |
|---|---|---|
| Sprague-Dawley rat model of cerebral ischemia-reperfusion injury | P-P38MAPK and caspase-3↓ | [72] |
| Bone marrow cells from C57BL/6 mice | CD80 and CD86↑, IL-6, IL-12p40 and TFN-α↑ | [73] |
| BALB/C mice infected with N. gonorrhoeae | TNF-α and IFN-γ↑, NO↓ | [74] |
| Sarcoma-180 tumor bearing C57BLkunmingmice | IL-2, -4, -6, -10, -17A, and IFN-α, -γ↑ | [75] |
| RAW264. 7 mouse monocyte/macrophage line | eNOS, iNOS, and TNF-α↑ | [76] |
| ConA- or LPS-induced Kunming mice | Immune cells and cytokines↑ | [77] |
| RAW264. 7 mouse monocyte/macrophage line | SR-B1↑, Dectin-1↑, CK2↑ | [78] |
| Female BALB/c, C3H/HeJ, C3H/HeN mice | Membrane Ig and TLR4↑ | [79] |
4.4. Other Activities
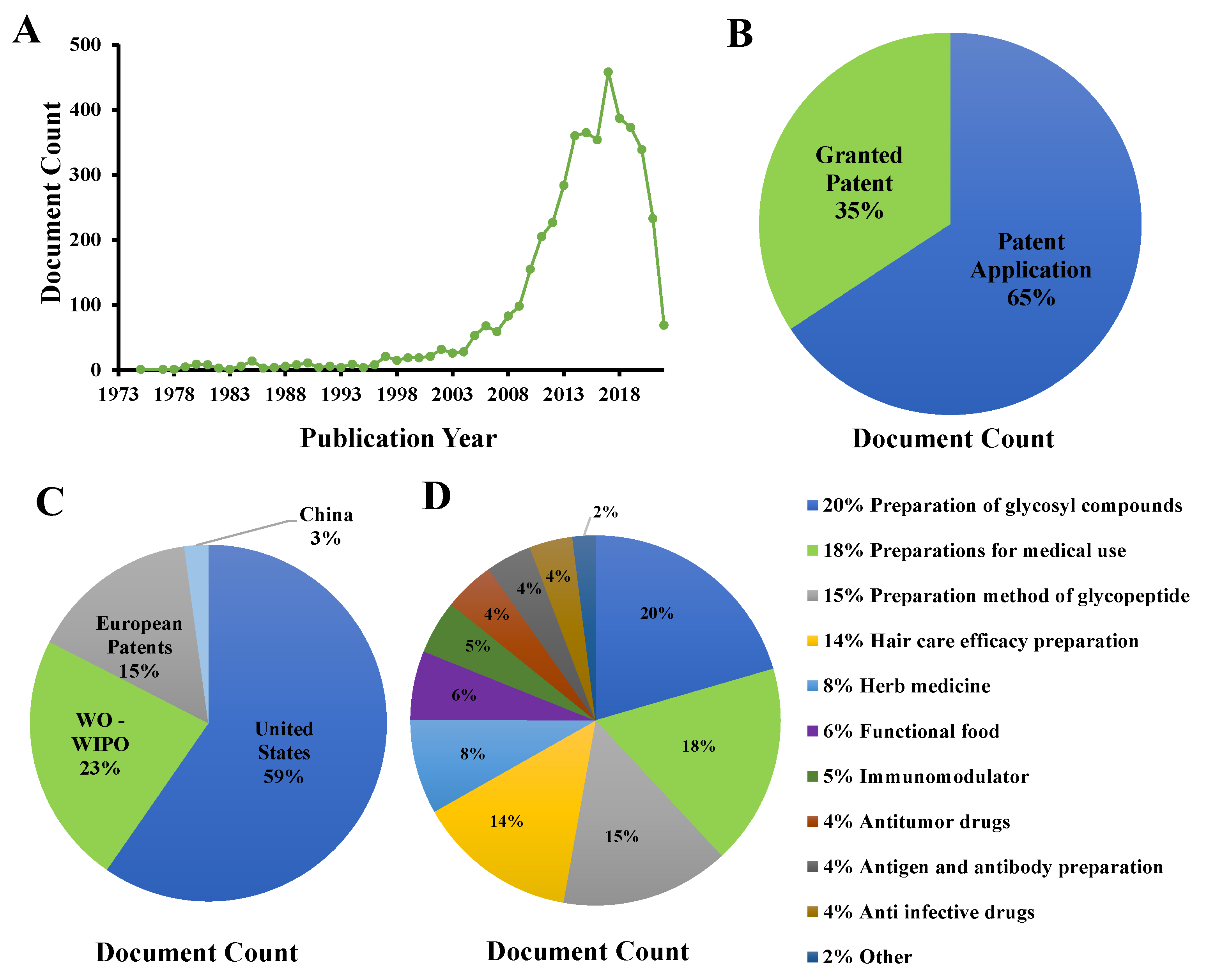
5. Product Development of CVP
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (2020 Edition), 11th ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 61–62.
- Aarti, B.; Prince, C.; Sawinder, K.; Agnieszka, N.; Melinda, F.; Szabolcs, F. Bioactives from Mushroom: Health attributes and food industry applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef]
- Xu, T.T.; Beelman, R.B.; Lambert, J.D. The cancer preventive effects of edible mushrooms. Anti-Cancer Agents Med. Chem. 2012, 12, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Barreira, J.; Barros, L.; Ferreira, I.C.F.R. Insights in the antioxidant synergistic effects of combined edible mushrooms: Phenolic and polysaccharidic extracts of Boletus edulis and Marasmius oreades. Food Nutr. Res. 2012, 51, 109–116. [Google Scholar] [CrossRef]
- Hsu, W.K.; Hsu, T.H.; Lin, F.Y.; Cheng, Y.K. Yang, J.P.W. Separation, purification, and α-glucosidase inhibition of polysaccharides from Coriolus versicolor LH1 mycelia. Carbohyd. Polym. 2013, 92, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Prog. Mol. Biol. Transl. 2019, 163, 361–381. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.Y.; Liu, M.; Chen, F.X.; Yang, W.J.; Chang, X.N.; Liu, N.; Zhao, Y.Y.; Wang, J.; Chen, X.F. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohyd. Res. 2020, 494, 108037. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, G.L. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Sun, X.W.; Sun, Y.P.; Zhang, Q.B.; Zhang, H.W.; Yang, B.Y.; Wang, Z.B.; Zhu, W.G.; Li, B.; Wang, Q.H.; Kuang, H.X. Screening and comparison of antioxidant activities of polysaccharides from Coriolus versicolor. Int. J. Biol. Macromol. 2014, 69, 12–19. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Z.Y.; Mao, H.Y.; Hu, P.; Li, X.J. Isolation and structure elucidation of polysaccharides from fruiting bodies of mushroom Coriolus versicolor and evaluation of their immunomodulatory effects. Int. J. Biol. Macromol. 2021, 166, 1387–1395. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohyd. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Liu, X.Y.; Faridul Hasan, K.M.; Wei, S.F. Immunological regulation, effects, extraction mechanisms, healthy utilization, and bioactivity of edible fungi: A comprehensive review. J. Food Process Eng. 2022, 45, e13970. [Google Scholar] [CrossRef]
- Sun, Y.N.; Zhang, M.; Fang, Z.X. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: A review. Trends Food Sci. Tech. 2020, 105, 468–482. [Google Scholar] [CrossRef]
- Huang, Z.F.; Zhang, M.L.; Jiang, X.W.; Zhang, S. Optimum extracting technology of intracellular polysaccharides from two edible-medicinal fungi mycelium. In Proceedings of the Seventh National Member Congress and 2017 Academic Annual Meeting of Chinese Mycological Society, Yichang, China, 11 August 2017. [Google Scholar]
- Chen, J.H.; Huang, D.P.; Huang, Y.; Su, L. Preliminary study on polysaccharide of Coriolus versicolor produced in Guangxi. J. Anhui Agri. Sci. 2012, 14, 2. [Google Scholar]
- Hu, C.X.; Hou, X.T.; Feng, Y.N.; Ma, T.Y.; Teng, L.R.; Lu, J.H. Optimization of extraction conditions of polysaccharide from Coriolus versicolor by response surface methodology. Sci. Technol. Food Ind. 2007, 28, 4. [Google Scholar] [CrossRef]
- Su, C.H.; Lai, M.N.; Lin, C.C.; Ng, L.T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl. Microbiol. Biotech. 2016, 100, 4385–4393. [Google Scholar] [CrossRef]
- Wang, Z.T.; Lin, H. Effects of liquid nitrogen grinding and mechanical crushing on the extraction of Polysaccharide from Coriolus versicolor. Sci. Technol. Inform. 2018, 16, 2. [Google Scholar]
- Yang, J.Q.; Yu, J.; Ji, C.Y.; Zhu, S.Z.; Wang, S.J.; Wang, Y.M.; Lin, S. Extraction of polysaccharide from Coriolus versicolor by complex enzyme method and its antioxidant activity. Sci. Technol. Food Ind. 2017, 38, 6. [Google Scholar] [CrossRef]
- Wang, J.; Quan, W.F.; Liu, G.J.; Ji, H.G.; Xue, J.; Xue, J. Comparative study on extraction technology of polysaccharide from Coriolus versicolor. Edible Fungi 2010, 3, 3. [Google Scholar] [CrossRef]
- Liu, Y.W.; Xiong, Y.K.; Yao, Z.S.; Cai, W. Study on ultrasonic extraction of polysaccharide from Coriolus versicolor. Jiangxi Sci. 2006, 24, 3. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, B.; Wang, M.M.; Wang, Q.Q.; Tian, G.H. Optimization of extraction process and properties of Coriolus Versicolor Polysaccharide. Food Sci. Technol. 2011, 3, 65. [Google Scholar]
- Ji, C.Y.; Yu, J.; Shao, W.J. The optimization of ultrasonic extraction in Coriolus Versicolor Polysaccharide. In Proceedings of the 13th Annual Meeting of Chinese Society of CIFST, Beijing, China, 9 November 2016. [Google Scholar]
- Li, Y.; Yang, F.Q.; Cui, S.L. Study on extraction technology of polysaccharide from Coriolus versicolor. Strait Pharm. 2016, 28, 3. [Google Scholar] [CrossRef]
- Guo, Q.; Liang, S.M.; Xiao, Z.C.; Ge, C.R. Research progress on extraction technology and biological activity of polysaccharides from Edible Fungi: A review. Food Rev. Int. 2022, 3, 1–32. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Pelt, S.V. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Gao, L.; Xue, C.H.; Mao, X.Z. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796. [Google Scholar] [CrossRef]
- You, Q.H.; Yin, X.L.; Zhao, Y.P. Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohyd. Polym. 2013, 98, 607–610. [Google Scholar] [CrossRef]
- Li, J.; Huang, G. Extraction, purification, separation, structure, derivatization and activities of polysaccharide from Chinese date. Process Biochem. 2021, 110, 231–242. [Google Scholar] [CrossRef]
- Ke, L.Q. Optimization of ultrasonic extraction of polysaccharides from lentinus edodes based on enzymatic treatment. J. Food Process Pres. 2015, 39, 254–259. [Google Scholar] [CrossRef]
- Golmohamadi, A.; Mller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef]
- Chemat, F.; Huma, Z.E.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2010, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.P.; Zhang, Z.D.; Cai, W.L.; Flores, A.D.R. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 4, 3122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Zhang, D.N.; Zhu, Q.; Yang, Q.H.Z.; Han, Y.B. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Wang, J.H.; Nie, J.; Wang, D.; Liu, H.; Liu, M.; Yang, Y.J.; Zhong, S.A. The structural characterization and anticancer activity of a polysaccharide from Coriolus versicolor. New J. Chem. 2022, 46, 9830–9840. [Google Scholar] [CrossRef]
- Xie, J.Z.; Zou, L.H.; Xie, Y.F.; Wu, X.D.; Wang, L.S. Analysis of the Monosaccharide Composition of Water-Soluble Coriolus versicolor Polysaccharides by Ultra-Performance Liquid Chromatography/Photodiode Array Detector. Chromatographia 2021, 84, 615–622. [Google Scholar] [CrossRef]
- Sknepnek, A.; Tomić, S.; Miletić, D.; Lević, S.; Čolić, M.; Nedović, V.; Nikšić, M. Fermentation characteristics of novel Coriolus versicolor and Lentinus edodes kombucha beverages and immunomodulatory potential of their polysaccharide extracts. Food Chem. 2020, 342, 128344. [Google Scholar] [CrossRef]
- Wang, K.L.; Lu, Z.M.; Mao, X.J.Z.; Chen, L.; Gong, J.S.; Ren, Y.L.; Geng, Y.; Li, H.; Xu, H.Y.; Xu, G.; et al. Structural characterization and anti-alcoholic liver injury activity of a polysaccharide from Coriolus versicolor mycelia. Int. J. Biol. Macromol. 2019, 137, 1102–1111. [Google Scholar] [CrossRef]
- Ren, Y.L.; Geng, Y.; Chen, H.D.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Polysaccharide peptides from Coriolus versicolor: A multi-targeted approach for the protection or prevention of alcoholic liver disease. J. Funct. Foods. 2018, 40, 769–777. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.K.; Ming, K.; Wang, D.Y.; Hu, Y.L.; Liu, J.G. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef]
- Ye, R.; Chen, Y.H.; Cai, J.; Li, J.Z.; Sun, W.; Pan, Y. Purification and Antioxidant Activity of Polysaccharide from Coriolus versicolor in Dabie Mountain of Xinyang City. Food Ind. 2020, 41, 4. [Google Scholar]
- Wang, F.F.; Hao, L.M.; Wang, Z.Z.; Rem, Z.Y.; Niu, S.; Wang, Q.Z.; Lu, J.K.; Jia, S.R. Comparative study on antioxidant activity of total fermented products of Coriolus versicolor and Its Polysaccharides. Food Ferment. Ind. 2013, 34, 4. [Google Scholar]
- Sun, X.W.; Chai, G.F.; Liang, S.S.; Wang, Q.H.; Kuang, H.X.; Zhang, Q.B. Study on in vitro antioxidant activity of Polysaccharides from Coriolus versicolor from different habitats. Inform. Tradi. Chin. Med. 2014, 6, 3. [Google Scholar]
- Chai, X.Y.; Lu, Y.Y.; Yu, S.J. Determination of polysaccharide content of Coriolus versicolor and comparison of its antioxidant activity in vitro. Edible Fungi 2017, 1, 3. [Google Scholar] [CrossRef]
- Chen, J.Y.; Jin, X.Y.; Zhang, L.T.; Yang, L.J. A study on the antioxidant effect of Coriolus versicolor polysaccharide in rat brain tissues. Afr. J. Tradit. Complement Altern. Med. 2013, 10, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Y.; Jiang, Y.; Ji, H.; Zhao, L.G.; Xiao, W.; Wang, Z.Z.; Ding, G. The Synergistic Beneficial Effects of Ginkgo Flavonoid and Coriolus versicolor Polysaccharide for Memory Improvements in a Mouse Model of Dementia. Evid-Based Compl. Alt. 2015, 2015, 128394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pang, Z.J.; Chen, Y.; Zhou, M. Polysaccharide Krestin Enhances Manganese Superoxide Dismutase Activity and mRNA Expression in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2000, 28, 331–341. [Google Scholar] [CrossRef]
- Pattanayak, M.; Samanta, S.; Maity, P.; Sen, I.K.; Nandi, A.K.; Manna, D.K. Heteroglycan of an edible mushroom Termitomyces clypeatus: Structure elucidation and antioxidant properties. Carbohydr. Res. 2015, 413, 30–36. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 4, 172. [Google Scholar] [CrossRef]
- Chu, K.K.W.; Ho, S.S.S.; Chow, A.H.L. Coriolus versicolor: A Medicinal Mushroom with Promising Immunotherapeutic Values. J. Clin. Pharmacol. 2002, 42, 976. [Google Scholar] [CrossRef]
- Liang, Y.S.; Zou, H.W.; Tan, Y.G. Research Progress on antitumor effect of Coriolus Versicolor Polysaccharide. China Prac. Med. 2014, 9, 4. [Google Scholar]
- Li, S.C.; Yang, X.M.; Ma, H.L.; Yan, J.K.; Guo, D.Z. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydr. Polym. 2015, 133, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ge, M.D.; Zhu, Y.J.; Song, Y.; Cheng, P.C.K.; Zhang, B.B.; Liu, L.M. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef]
- Wei, S.J.; Chen, W.Q. Effects of Coriolus versicolor Polysaccharide on proliferation and apoptosis of murine melanoma B16 Cell in vitro and its mechanism of Action. Chin. Pharm. 2016, 27, 4. [Google Scholar] [CrossRef]
- Wei, S.J.; Chen, W.Q. Effects of Coriolus versicolor fruiting body polysaccharide on growth and apoptosis of human cervical cancer HeLa cells. J. Sichuan Univ. (Nat. Sci. Ed.) 2016, 53, 7. [Google Scholar]
- Sun, F.; Tao, Z.Z.; Zhou, Z.H.; Lv, X.T.; Chen, N.Y.; Chen, L.; Yao, R.N. Enhance effect of polysaccharide K(PSK) on killing activity of human nature killing cells in vitro, BME. Clin. Med. 2017, 21, 6. [Google Scholar] [CrossRef]
- Ge, W.J.; Zhao, Y.; Tu, L.G.E.; Li, W.; Gao, Y.G.; Zhu, H.Y.; Li, Y.; Bao, H.Y. Antitumor effect of acid soluble polysaccharide of Coriolus versicolor on H22 Tumor bearing mice. J. Northwest Univ. A F Univ. (Nat. Sci. Ed.) 2018, 46, 7. [Google Scholar] [CrossRef]
- Wang, D.F.; Lou, N.; Li, X.D. Effect of Coriolus Versicolor Polysaccharide-B on the Biological Characteristics of Human Esophageal Carcinoma Cell Line Eca109. Cancer Biol. Med. 2012, 9, 164–167. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, B.B.; Zhang, M.K.; Zhang, M.K.; Song, J.W.; Wang, X.L. Efficacy of Coriolus versicolor Polysaccharide combined with XELOX regimen in the treatment of advanced colorectal cancer. World Latest Med. Inform (Electron. Version) 2019, 19, 2. [Google Scholar]
- Luo, K.W.; Yue, G.G.L.; Ko, C.H.; Lee, J.K.M.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef]
- Zeng, F.; Hon, C.; Sit, W.F.Y.; Zeng, C.C.; Hon, W.H.; Sit, K.Y.C.; Chow, R.K.H.; Hui, I.K.M.; Law, V.W.L.; Ng, X.T.; et al. Molecular characterization of Coriolus versicolor PSP-induced apoptosis in human promyelotic leukemic HL-60 cells using cDNA microarray. Int. J. Oncol. 2005, 27, 513–523. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, P.; Park, S.; Wu, J.M. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I’m-Yunity™ (PSP). BMC Complement. Med. Ther. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wan, J.M.F.; Sit1, W.H.; Yang, X.T.; Jiang, P.P.; Wong, L.L.Y. Polysaccharopeptides derived from Coriolus versicolor potentiate the S-phase specific cytotoxicity of Camptothecin (CPT) on human leukemia HL-60 cells. Chin. Med. 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.T.; Zhang, W.X.; Wang, Q.J.; Zhang, A.; Mu, H.B.; Bai, H.J.; Duan, J.Y. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohyd. Polym. 2014, 111, 245–255. [Google Scholar] [CrossRef]
- Jeong, S.C.; Yang, B.K.; Kim, G.N.; Jeong, H.; Wilson, M.A.; Cho, Y.; Rao, K.S.; Song, C.H. Macrophage-stimulating activity of polysaccharides extracted from fruiting bodies of Coriolus versicolor (Turkey Tail Mushroom). J. Med. Food. 2006, 9, 175–181. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Kozak, W. Protein-bound polysaccharides from Coriolus versicolor attenuate LPS-induced synthesis of pro-inflammatory cytokines and stimulate PBMCs proliferation. Immunol. Lett. 2016, 178, 140–147. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatuac, S.; Devi, K.S.P. Antioxidant and immunostimulant β-glucan from edible mushroom Russula albonigra(Krombh.). Carbohydr. Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Rout, D.; Mondal, S.; Chakraborty, I.; Pramanik, M.; Islam, S.S. Structrual characterization of an immunomodulating polysaccharide isolated from aquous extract of Pleurotus florida fruit-bodies. Med. Chem. Res. 2004, 13, 509–517. [Google Scholar] [CrossRef]
- Zhang, P.; Cheung, P.C.K. Evaluation of sulfated Lentinus edodes α-(1→3)-d-glucan as a potential antitumor agent. Biosci. Biotechnol. Biochem. 2002, 66, 1052–1056. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Miao, C.; Liu, Y.; Liu, R. Coriolus versicolor polysaccharides (CVP) regulates neuronal apoptosis in cerebral ischemia-reperfusion injury via the p38MAPK signaling pathway. Ann. Transl. Med. 2020, 8, 1168. [Google Scholar] [CrossRef]
- Dong, X.X.; Li, B.Y.; Zhang, J.Y.; Chen, Y.; Hu, Q.; Peng, B. In vitro screening of mouse bone marrow-derived dendritic cells induced by five plant polysaccharides. Chin. J. Comp. Med. 2020, 30, 5. [Google Scholar] [CrossRef]
- Pramudya, M.; Wahyuningsih, S.P.A. Immunomodulatory potential of polysaccharides from Coriolus versicolor against intracellular bacteria Neisseria gonorrhoeae. Vet. World. 2019, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Eugene, K.; Jamal, M.; Hou, J.; Hago, A.M.; Gamallat, Y.; Meyiah, A.; Bamba, D.; Gift, C.; Abdalla, M.; et al. Effect of Coriolus versicolor glucan on the stimulation of cytokine production in sarcoma-180-bearing mice. Biomed. Rep. 2017, 7, 567–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, S.C.; Koo, H.J.; Park, S.; Lim, J.D.; Kimb, Y.J.; Kimb, T.; Namkoong, S.; Jang, K.H.; Pyoe, S.; Jang, S.A.; et al. Effects of β-glucans from Coriolus versicolor on macrophage phagocytosis are related to the Akt and CK2/Ikaros. Int. J. Biol. Macromol. 2013, 57, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, Y.G.; Meng, X.S.; Wang, S.; Li, T.J.; Zhang, X.J.; Song, Y.R. Activation of spleen cells by Coriolus versicolor polysaccharide. Cent. South Pharm. 2017, 15, 4. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sohn, E.H. Effects of Beta-glucan from Coriolus versicolor on Scavenger Receptor B1 Expression and their Involvement of Dectin-1 and Casein Kinase 2. Korean J. Plant Res. 2012, 25, 664–669. [Google Scholar] [CrossRef][Green Version]
- Yang, F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Z.M.; Chen, L.; Geng, Y.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, H.Z. Hepatoprotective test of polysaccharide from Coriolus versicolor on alcoholic liver injury mice. Edible Med. Mushrooms 2018, 26, 5. [Google Scholar]
- Wang, Y. The Characteristics of Coriolus versicolor Polysaccharide and Its Effect on Sports Endurance. Edible Fungi China 2020, 39, 3. [Google Scholar]
- Wang, Y.Q.; Li, H.; Li, Y.; Zhao, Y.H.; Xiong, F.F.; Liu, Y.N.; Xue, H.R.; Yang, Z.Y.; Ni, S.; Sahil, A.; et al. Coriolus versicolor alleviates diabetic cardiomyopathy by inhibiting cardiac fibrosis and NLRP3 inflammasome activation. Phytother. Res. 2019, 33, 12. [Google Scholar] [CrossRef]
- Qu, J.L.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 20, 161. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Oliveira, W.F.D.; Silva, P.M.D.S.; Correia, M.T.D.S.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides-A review. Carbohyd. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Gul, I.; Basharat, A.; Qamar, S.A. Polysaccharides-based bio-nanostructures and their potential food applications. Int. J. Biol. Macromol. 2021, 76, 540–557. [Google Scholar] [CrossRef] [PubMed]

| Experimental Method | Temperature | Time | Other Conditions | Yields | Ref. |
|---|---|---|---|---|---|
| Hot water extraction | 90 °C | 120 min | Material-to-liquid ratio of 1:30 (g:mL) | 5.38% | [14] |
| 100 °C | 120 min | Ethanol concentration 95% | 6.98% | [15] | |
| 85 °C | 105 min | Feed-to-liquid ratio of 1:27 | 7.27% | [16] | |
| 100 °C | 180 min | Feed-to-liquid ratio of 1:50, 3 h | 4.39% | [17] | |
| 80 °C | 180 min | Liquid nitrogen grinding | 16.1% | [18] | |
| Enzyme extraction | 52 °C | 37 min | pH 5.5, enzyme concentration: 2.50% | 9.58% | [19] |
| 55 °C | 20 min | pH 6.0, enzyme concentration: 1.50% | 3.26% | [20] | |
| Ultrasonic extraction | Room temperature | 15 min | Material-to-liquid ratio of 1:20 (g:mL), ultrasonic extraction twice | 3.84% | [21] |
| 45 °C | 50 min | pH 8.5, 2% Na2CO3 | 13.87% | [22] | |
| Room temperature | 30 min | Material-to-liquid ratio of 1:45 (g:mL), ultrasonic power 450 W | 13.6% | [23] |
| Vitro/Vivo | Effect | Ref. |
|---|---|---|
| In vitro | The scavenging activity of DPPH radical (64.9% at 0.8 mg/mL), O2•− radical (78.4% at 1.2 mg/mL) and OH radical (71.2% at 2.0 mg/mL) | [9] |
| IC50 (DPPH radical) = 0.388 mg/mL, IC50 (ABTS radical) = 0.419 mg/mL, IC50 (OH radical) = 4.423 mg/mL | [43] | |
| The best scavenging activity of O2•− (60% at 5 mg/mL), OH radical (81% at 5 mg/mL) | [44] | |
| IC50 = 0.832 g/mL | [45] | |
| The best scavenging activity of ABTS radical (53% at 10 mg/mL), OH radical (72% at 5 mg/mL) | [46] | |
| In vivo | SOD↑, GSH-Px↑, MDA↓ | [47] |
| SOD↑, CAT↑ | [48] | |
| Ox-LDL↓, SOD↑ | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Zhang, S.; Li, M.; Ma, Y.; Zheng, Y.; Zhang, D.; Wu, L. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods 2022, 11, 2126. https://doi.org/10.3390/foods11142126
Jing Y, Zhang S, Li M, Ma Y, Zheng Y, Zhang D, Wu L. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods. 2022; 11(14):2126. https://doi.org/10.3390/foods11142126
Chicago/Turabian StyleJing, Yongshuai, Shilin Zhang, Mingsong Li, Yunfeng Ma, Yuguang Zheng, Danshen Zhang, and Lanfang Wu. 2022. "Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor" Foods 11, no. 14: 2126. https://doi.org/10.3390/foods11142126
APA StyleJing, Y., Zhang, S., Li, M., Ma, Y., Zheng, Y., Zhang, D., & Wu, L. (2022). Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods, 11(14), 2126. https://doi.org/10.3390/foods11142126






