A Comparative Metabolomics Approach for Egyptian Mango Fruits Classification Based on UV and UPLC/MS and in Relation to Its Antioxidant Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manog Fruit Samples
2.2. Experiment and Data Processing
2.2.1. Fruits Extraction for LC/MS and UV/Vis Analyses
2.2.2. Multivariate Data Analysis (MVA) of Mango Fruit Data Sets
2.3. Determination of Total Phenolics and Antioxidant Activity
2.3.1. Total Phenolics Content (TPC)
2.3.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3. Results
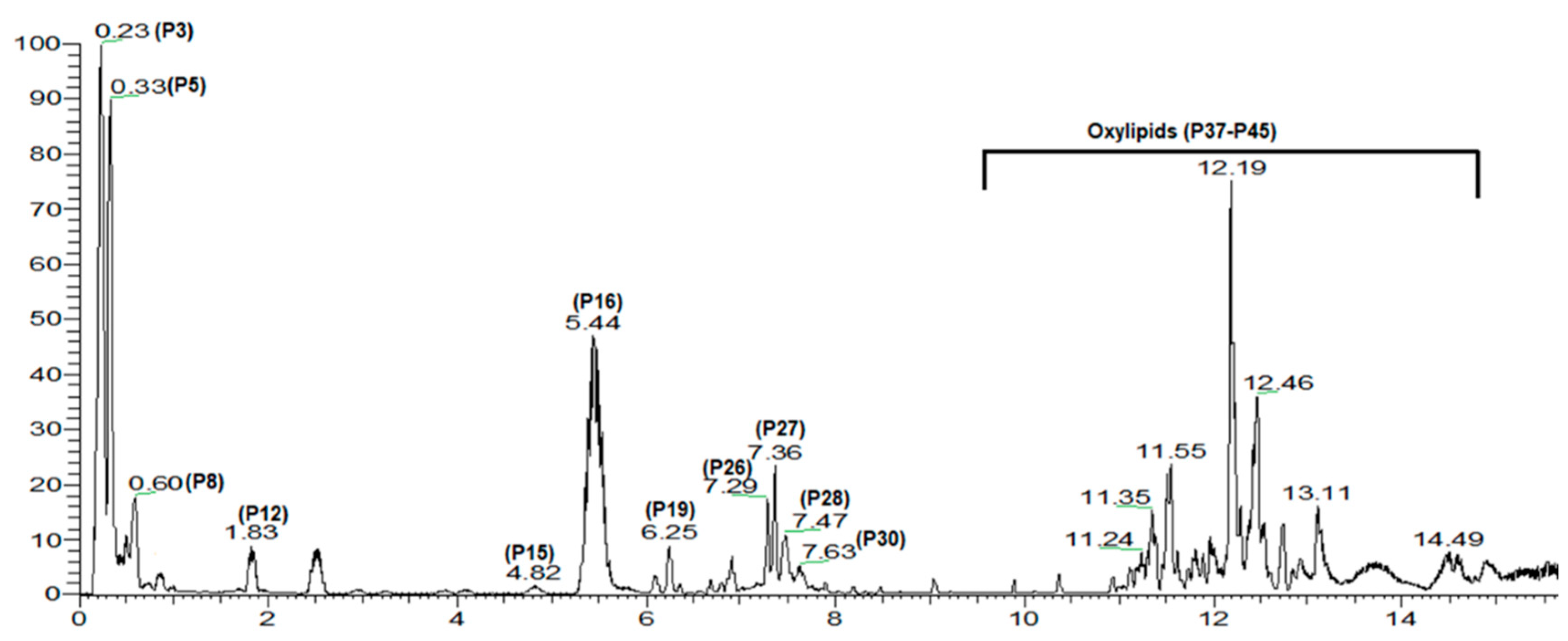
3.1. Metabolites Identification Using UPLC/MS
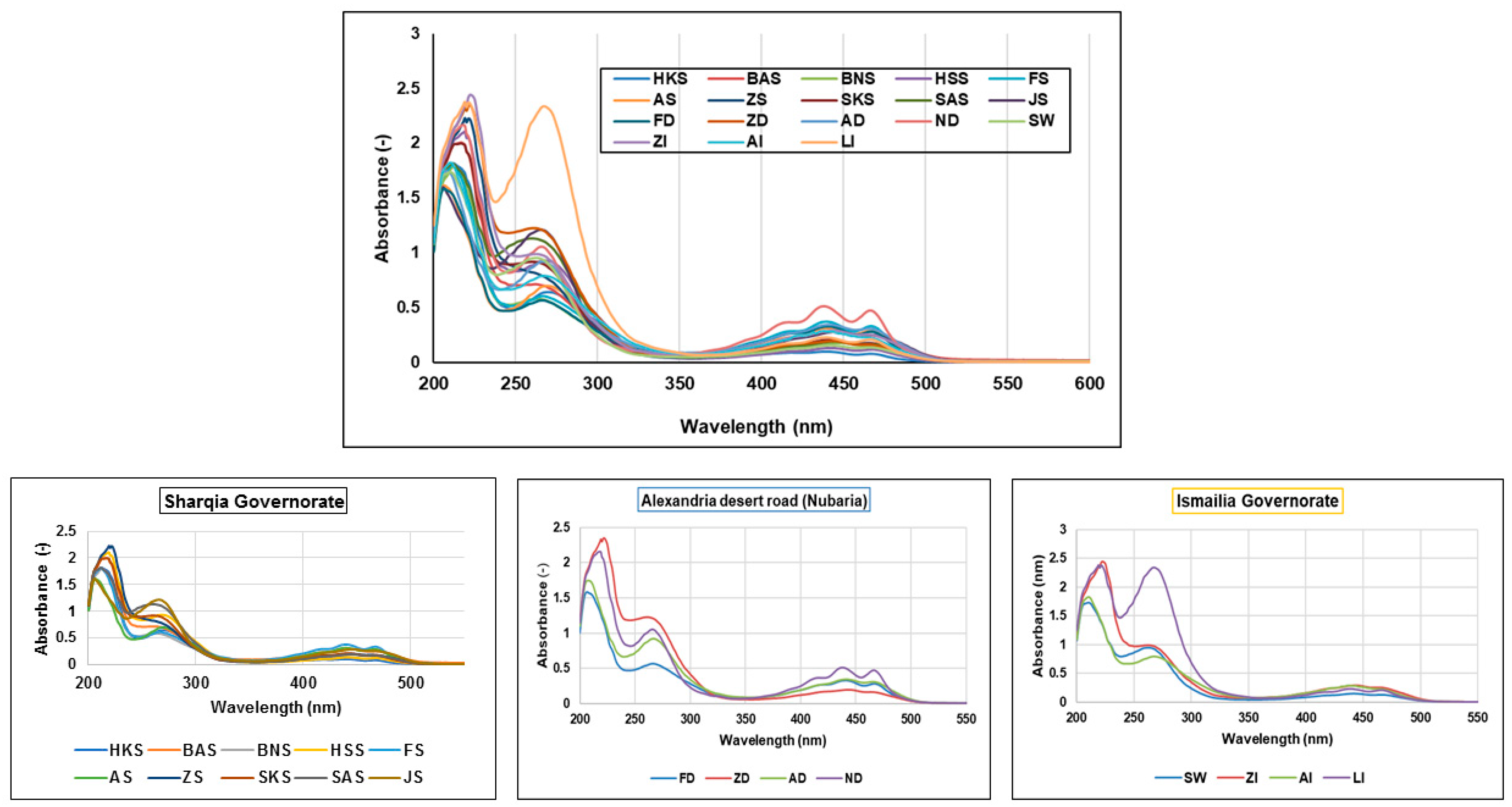
3.2. UV/Vis Spectral Analysis
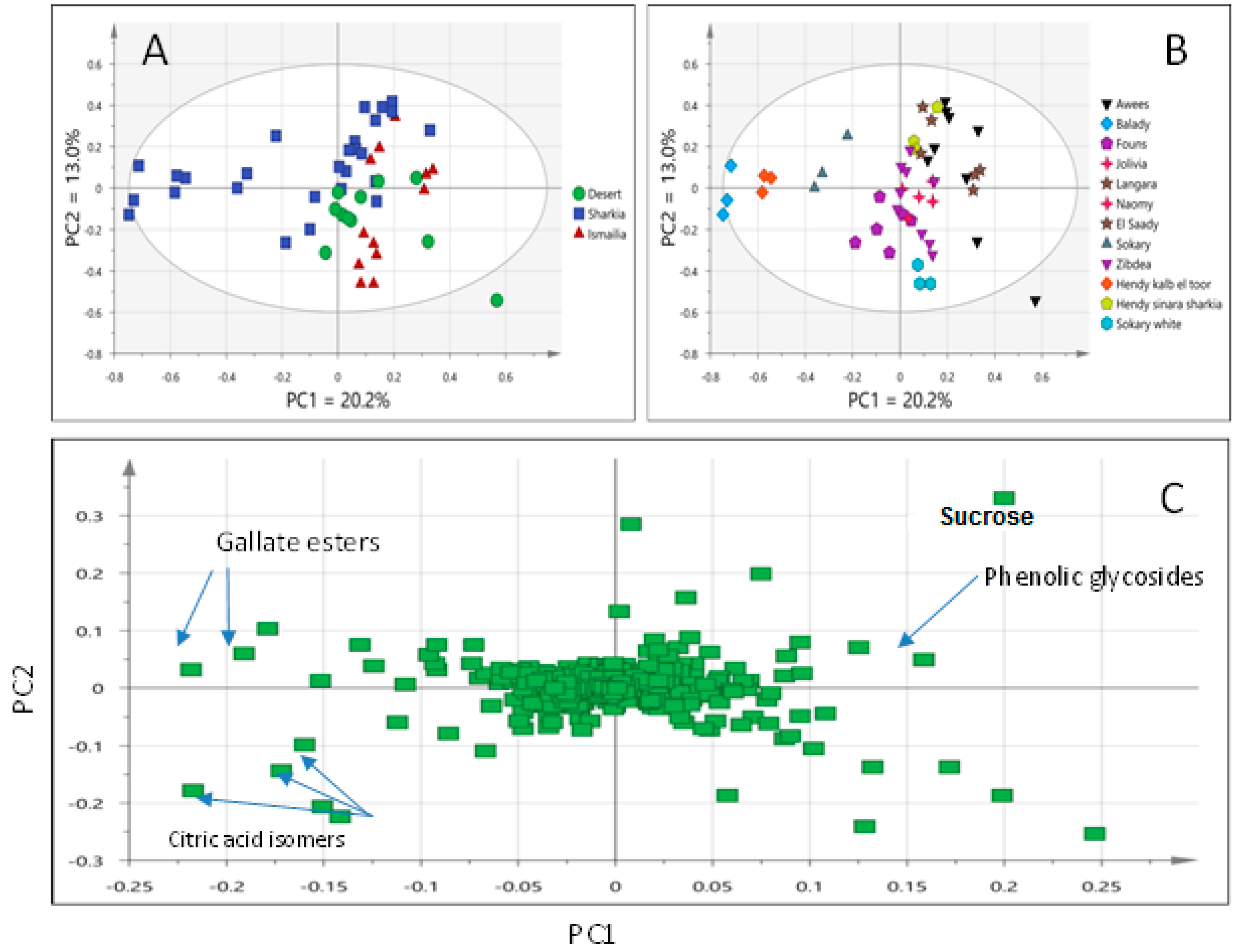
3.3. UPLC/MS and UV/Vis-Based MVA of All Mango Fruit Data Sets
3.4. UPLC/MS and UV-Based Multivariate Analysis of Mango Data Subsets
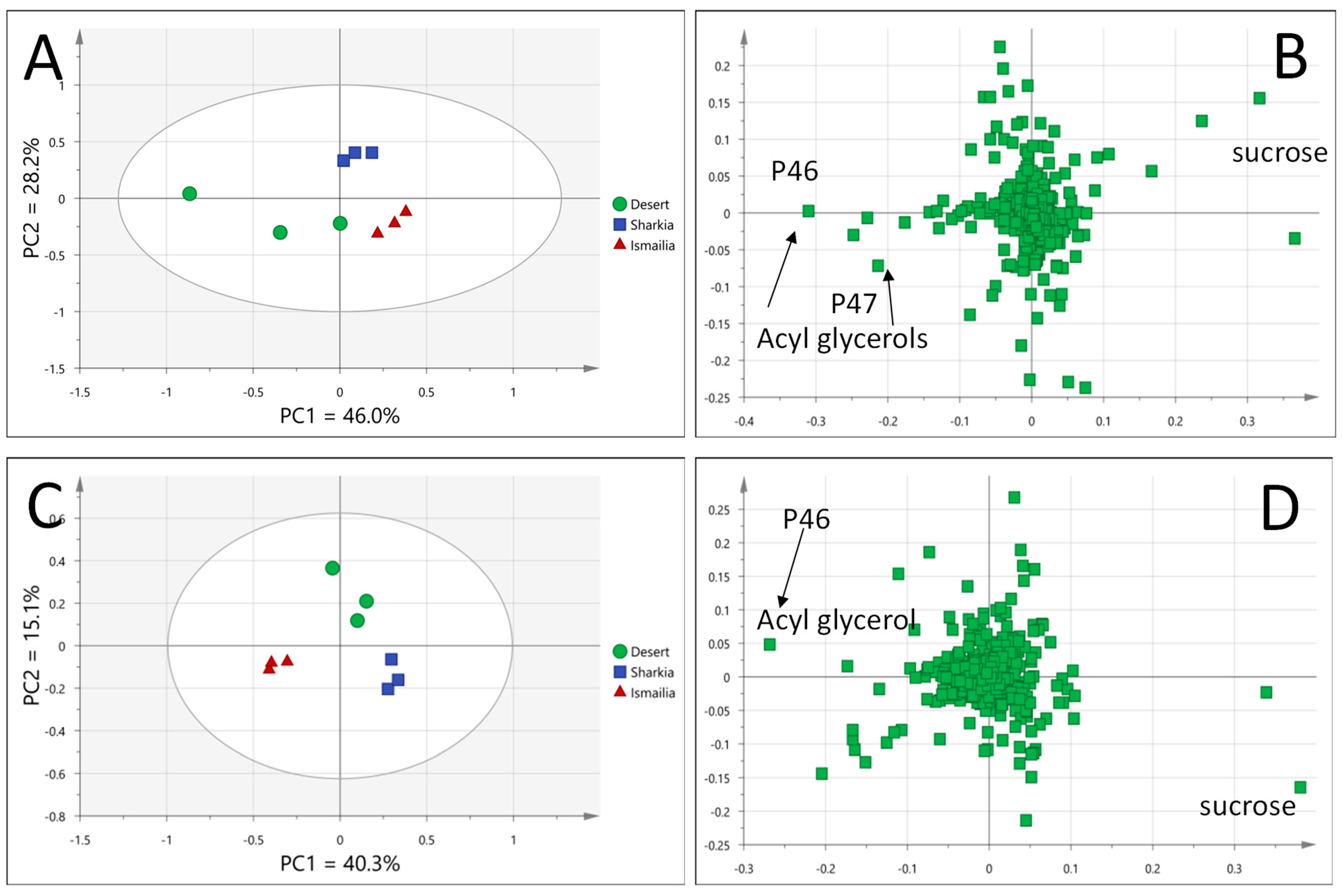
3.4.1. Awees Cultivar Classified Based on Geographical Origin
3.4.2. Zibdea cv. Classified Based on Geographical Origin
3.5. Phenolic Content in Relation to Antioxidant Activity among Mango Fruit cvs.
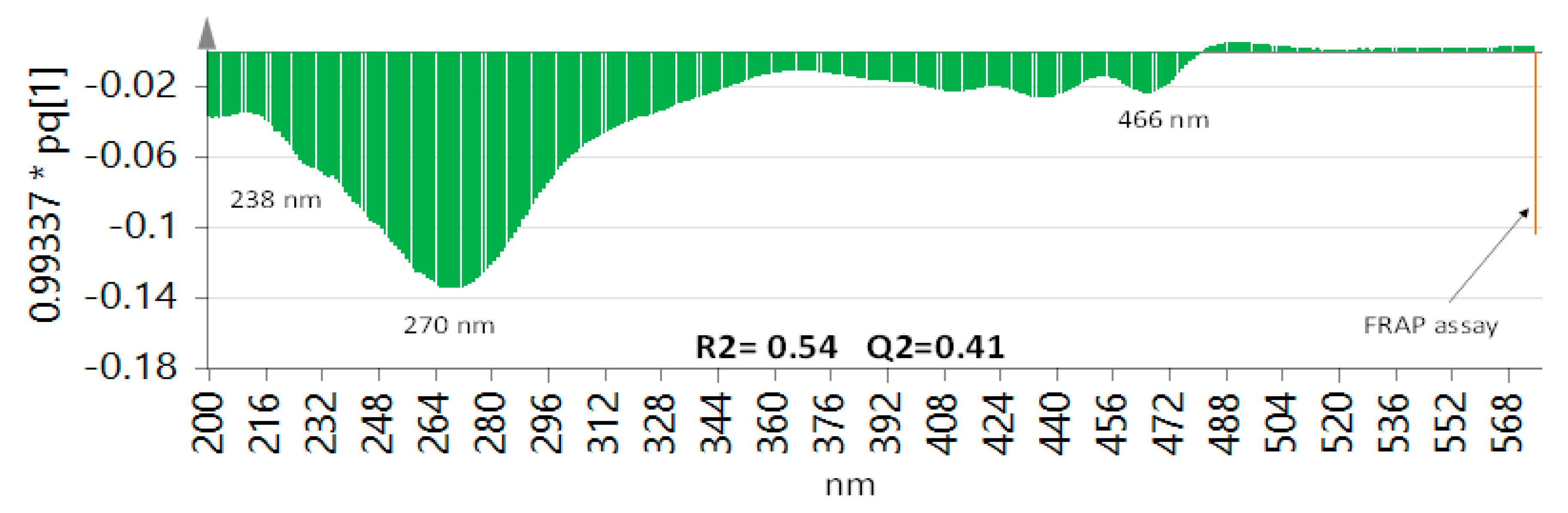
3.6. Correlation between FRAP Assay and UV-Vis and UPLC–MS Data Sets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid. Based Complementary Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenucci, M.S.; Tornese, R.; Mita, G.; Durante, M. Bioactive compounds and antioxidant activities in different fractions of mango fruits (Mangifera indica L., cultivar Tommy Atkins and Keitt). Antioxidants 2022, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- FAO. Country Showcase. Available online: https://www.fao.org/country-showcase/selected-product-detail/en/c/1287916/ (accessed on 3 June 2022).
- Farag, M.A.; Dokalahy, E.U.; Eissa, T.F.; Kamal, I.M.; Zayed, A. Chemometrics-based aroma discrimination of 14 Egyptian mango fruits of different cultivars and origins, and their response to probiotics analyzed via SPME coupled to GC-MS. ACS Omega 2022, 7, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef] [Green Version]
- Vithana, M.D.; Singh, Z.; Johnson, S.K. Dynamics in the concentrations of health-promoting compounds: Lupeol, mangiferin and different phenolic acids during postharvest ripening of mango fruit. J. Sci. Food Agric. 2018, 98, 1460–1468. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols: Anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Blandón-Naranjo, L.; Vázquez, M.; Contreras-Calderón, J. Antioxidant capacity of mango fruit (Mangifera indica). An electrochemical study as an approach to the spectrophotometric methods. Food Chem. 2018, 266, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, M.; Chaimongkol, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Chaiyaso, T.; Ruksiriwanich, W.; Jantrawut, P.; et al. Mango peel pectin: Recovery, functionality and sustainable Uses. Polymers 2021, 13, 3898. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R.; et al. Fruit characteristics, peel nutritional compositions, and their relationships with mango peel pectin quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef]
- Farag, M.A.; Zayed, A.; Sallam, I.E.; Abdelwareth, A.; Wessjohann, L.A. Metabolomics-based approach for coffee beverage improvement in the context of processing, brewing methods, and quality attributes. Foods 2022, 11, 864. [Google Scholar] [CrossRef]
- Otify, A.M.; El-Sayed, A.M.; Michel, C.G.; Farag, M.A. Metabolites profiling of date palm (Phoenix dactylifera L.) commercial by-products (pits and pollen) in relation to its antioxidant effect: A multiplex approach of MS and NMR metabolomics. Metab. Off. J. Metab. Soc. 2019, 15, 119. [Google Scholar] [CrossRef]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Metabolome classification of commercial Hypericum perforatum (St. John’s Wort) preparations via UPLC-qTOF-MS and chemometrics. Planta Med. 2012, 78, 488–496. [Google Scholar] [CrossRef]
- Farag, M.A.; Mahrous, E.A.; Lübken, T.; Porzel, A.; Wessjohann, L. Classification of commercial cultivars of Humulus lupulus L. (hop) by chemometric pixel analysis of two dimensional nuclear magnetic resonance spectra. Metabolomics 2014, 10, 21–32. [Google Scholar] [CrossRef]
- Farag, M.A.; Hegazi, N.; Dokhalahy, E.; Khattab, A.R. Chemometrics based GC-MS aroma profiling for revealing freshness, origin and roasting indices in saffron spice and its adulteration. Food Chem. 2020, 331, 127358. [Google Scholar] [CrossRef]
- Ganneru, S.; Shaik, H.; Peddi, K.; Mudiam, M.K.R. Evaluating the metabolic perturbations in Mangifera indica (mango) ripened with various ripening agents/practices through gas chromatography-mass spectrometry based metabolomics. J. Sep. Sci. 2019, 42, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-W.; Su, M.-Q.; Wu, H.-X.; Zhou, Y.-G.; Wang, S.-B. Analysis of the volatile profile of core Chinese mango germplasm by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Molecules 2018, 23, 1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, A.E.M.; Darwish, S.M.; Ayad, E.H.E.; El-Hamahmy, R.M. Egyptian mango by-product 2: Antioxidant and antimicrobial activities of extract and oil from mango seed kernel. Food Chem. 2007, 103, 1141–1152. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic characterization, antioxidant activity, and enzyme inhibitory properties of Berberis thunbergii DC. leaves: A valuable source of phenolic acids. Molecules 2019, 24, 4174. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, B.G.; Costa, H.B.; Ventura, J.A.; Kondratyuk, T.P.; Barroso, M.E.S.; Correia, R.M.; Pimentel, E.F.; Pinto, F.E.; Endringer, D.C.; Romão, W. Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI-MS). Food Chem. 2016, 204, 37–45. [Google Scholar] [CrossRef]
- Alañón, M.E.; Oliver-Simancas, R.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Evolution of bioactive compounds of three mango cultivars (Mangifera indica L.) at different maturation stages analyzed by HPLC-DAD-q-TOF-MS. Food Res. Int. 2019, 125, 108526. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Morantes, S.J.; Sánchez-Camargo, A.d.P.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. An integrated approach for the valorization of mango seed kernel: Efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res. Int. 2019, 126, 108616. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic characterization, antioxidant, and cytotoxic activities of Mangifera indica cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Ammar, N.M.; Kholeif, T.E.; Metwally, N.S.; El-Sheikh, N.M.; Wessjohann, L.A.; Abdel-Hamid, A.Z. Rats’ urinary metabolomes reveal the potential roles of functional foods and exercise in obesity management. Food Funct. 2017, 8, 985–996. [Google Scholar] [CrossRef]
- Barry, K.M.; Davies, N.W.; Mohammed, C.L. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography–electrospray ionisation mass spectrometry. Phytochem. Anal. 2001, 12, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anouar, E.; Košinová, P.; Kozlowski, D.; Mokrini, R.; Duroux, J.L.; Trouillas, P. New aspects of the antioxidant properties of phenolic acids: A combined theoretical and experimental approach. Phys. Chem. Chem. Phys. 2009, 11, 7659–7668. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—A review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Gorjanović, S.; Komes, D.; Laličić-Petronijević, J.; Pastor, F.T.; Belščak-Cvitanović, A.; Veljović, M.; Pezo, L.; Sužnjević, D.Ž. Antioxidant efficiency of polyphenols from coffee and coffee substitutes-electrochemical versus spectrophotometric approach. J. Food Sci. Technol. 2017, 54, 2324–2331. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. piqueño). Molecules 2013, 19, 438–458. [Google Scholar] [CrossRef] [Green Version]

| Geographical Origin | Cultivar (cv.) | Code |
|---|---|---|
| Sharqia Governorate (muddy soil) | Hendy kalb el toor | HKS |
| Balady Arneba Sharqia | BNS | |
| Hendy Sinara Sharqia | HSS | |
| Founs | FS | |
| Awees | AS | |
| Zibdea | ZS | |
| Sokary | SKS | |
| El Saady | SAS | |
| Jolivia | JS | |
| Alexandria desert road (Nubaria, sand soil) | Founs | FD |
| Zibdea | ZD | |
| Awees | AD | |
| Naomy | ND | |
| Ismailia Governorate (muddy soil) | Sokary White | SW |
| Zibdea | ZI | |
| Awees | AI | |
| Langara | LI |
| Peak No. | Rt. (min) | [M-H]− | Molecular Formula | Error (ppm) | MS/MS | Identification | Class |
|---|---|---|---|---|---|---|---|
| 1. | 0.15 | 179.05582 | C6H11O6− | 4.5 | 129 | Glucose | Sugar |
| 2. | 0.2 | 683.2238 | C24H43O22− | 0.3 | 341, 179 | Unknown tetrasaccharide | Sugar |
| 3. | 0.23 | 191.0195 | C6H7O7− | 4.7 | 173, 111 | Citric/Isocitric acid | Organic acid |
| 4. | 0.3 | 683.2238 | C24H43O22− | 0.3 | 341, 179 | Unknown tetrasaccharide | Sugar |
| 5. | 0.33 | 191.0195 | C6H7O7− | 4.4 | 173, 111 | Citric/Isocitric acid isomer | Organic acid |
| 6. | 0.44 | 341.1079 | C12H21O11− | 0.4 | 179 | Sucrose | Sugar |
| 7. | 0.5 | 205.0349 | C7H9O7− | 2.8 | 173, 143, 111 | Citric acid Methyl ester | Organic acid |
| 8. | 0.6 | 331.0660 | C13H15O10− | 0.07 | 169, 125 | Galloylhexose | Gallotannin |
| 9. | 0.85 | 299.0763 | C13H15O8− | 0.39 | 239, 209, 179, 137 | p-Hydroxybenzoic acid -O-hexoside | Phenolic acid |
| 10. | 0.93 | 381.04489 | C16H13O11− | 321, 263, 233 | Unknown | ||
| 11. | 0.96 | 341.1074 | C12H21O11− | 1.2 | 179 | Sucrose isomer | Sugar |
| 12. | 1.83 | 183.0295 | C8H7O5− | 3.83 | 168, 124 | Methyl gallate | Gallate |
| 13. | 1.98 | 355.1024 | C16H19O9− | 0.17 | 193 | Ferulic acid-O-hexoside | Phenolic acid |
| 14. | 2.55 | 443.1906 | C21H31O10− | 1.41 | 425, 281, 263, 237, 219, 189, 161, 143 | Unknown glycoside | |
| 15. | 4.86 | 355.1023 | C16H19O9− | 0.08 | 193 | Ferulic acid-O-hexoside isomer | Phenolic acid |
| 16. | 5.62 | 421.07544 | C19H17O11− | −2.04 | 281 | Mangiferin/isomangiferin | Xanthone |
| 17. | 6.1 | 385.1856 | C19H29O8− | 0.38 | 223, 205, 161, 153 | Unknown glycoside | |
| 18. | 6.2 | 335.0399 | C15H11O9− | 0.66 | 183 | Methyl digallate ester | Gallate |
| 19. | 6.26 | 517.2278 | C24H37O12− | 0.33 | 385, 205, 168 | Sinapic acid-O-hexoside-pentoside | Phenolic acid |
| 20. | 6.33 | 433 | C20H33O10− | 301 | Quercetin-O-pentoside | Flavonoid | |
| 21. | 6.36 | 519.2437 | C24H39O12− | 0.09 | 387, 371, 313, 218, 186, 148 | Dihydrosinapic acid-O-hexosyl-pentoside | Phenolic acid |
| 22. | 6.68 | 443.1906 | C21H31O10− | 1.2 | 425, 399, 281, 263, 237, 219, 161, 143 | Unknown glycoside | |
| 23. | 6.88 | 261 | C12H21O6− | 243, 201, 187 | Unknown | ||
| 24. | 6.91 | 403.1596 | C18H27O10− | 0.6 | 241, 197 | Unknown glycoside | |
| 25. | 7.25 | 335.0399 | C15H11O9− | 0.48 | 183, 124 | Methyl digallate ester isomer 1 | Gallate |
| 26. | 7.29 | 939.1080 | C41H31O26− | 1.9 | 769, 617, 469, 393, 169 | Pentagalloyl glucose | Gallotannin |
| 27. | 7.36 | 335.0397 | C15H11O9− | 0.17 | 183, 124 | Methyl digallate ester isomer 2 | Gallate |
| 28. | 7.47 | 1091.1172 | C48H35O30− | 4.3 | 769, 545, 469 | Hexagalloyl glucose | Gallotannin |
| 29. | 7.56 | 545 | C24H17O15− | 469 | Unknown malonate conjugate | ||
| 30. | 7.61 | 1243.1279 | C55H39O34− | 3.05 | 839, 621, 545, 469 | Heptagalloyl glucose | Gallotannin |
| 31. | 7.9 | 487.0502 | C22H15O13− | 1 | 335, 183 | Methyl trigallate ester | Gallate |
| 32. | 8.10 | 477.23242 | C22H37O11− | −1.29 | 315 | Rhamnetin-O-hexoside | Flavonoid |
| 33. | 8.22 | 263.12802 | C15H19O4− | 0.89 | 219, 153 | Unknown | |
| 34. | 8.40 | 463.21704 | C21H35O11− | −0.752 | 417, 301 | Quercetin-O-hexoside | Flavonoid |
| 35. | 8.64 | 245.04451 | C13H9O5− | −0.235 | 213, 185 | Unknown | |
| 36. | 8.96 | 327.21634 | C18H31O5− | −0.796 | 291, 229, 211, 171 | Unknown | |
| 37. | 9.07 | 242.17535 | C13H24O3N− | 1.158 | 225, 181 | Unknown nitrogenous lipid | Oxylipid |
| 38. | 9.09 | 259.06033 | C14H11O5− | 0.888 | 231, 187 | Unknown | |
| 39. | 9.77 | 315.18011 | C16H27O6− | −0.33 | - | Rhamnetin | Flavonoid |
| 40. | 10.10 | 191.10725 | C12H15O2− | 3.10 | 146, 111 | Unknown | |
| 41. | 11.29 | 569.27112 | C39H37O8− | 4.36 | 389, 315, 253 | Unknown | |
| 42. | 11.77 | 311.16782 | C11H27O8− | −7.14 | 183 | Unknown oxylipid | Oxylipid |
| 43. | 11.88 | 513.30542 | C27H45O9− | −0.75 | 277, 253 | Unknown oxylipid | Oxylipid |
| 44. | 12.06 | 407.21893 | C25H31O4− | −6.7 | 153 | Unknown | |
| 45. | 12.1 | 489.30533 | C25H45O9− | −0.98 | 253 | Unknown oxylipid | Oxylipid |
| 46. | 12.2 | 452.32419 | C25H40O7− | 0.42 | 255 | Acyl glycerol I | Oxylipid |
| 47. | 12.3 | 540.36285 | C29H48O9− | 0.24 | 480 | Acyl glycerol II | Oxylipid |
| Sample Code | TPC (mg GAE/g Extract) | FRAP (mg TE/g Extract) | FRAP (mg AA/g Extract) |
|---|---|---|---|
| LI | 173.9 ± 12.8 a | 0.41 ± 0.1 a | 0.08 ± 0.0 a |
| BNS | 26.7 ± 1.3 i | 0.05 ± 0.0 h | 0.02 ± 0.0 h |
| SS | 115.2 ± 8.8 b,c | 0.21 ± 0.0 c,d | 0.05 ± 0.0 c,d |
| ZD | 129.1 ± 7.9 b | 0.24 ± 0.1 c | 0.05 ± 0.0 c |
| BAS | 73.7 ± 5.9 e,f,g | 0.13 ± 0.0 e | 0.03 ± 0.0 e |
| HSS | 60.3± 3.6 f,g | 0.12 ± 0.0 e,f | 0.03 ± 0.0 e,f |
| ZS | 104.3 ± 7.0 c,d | 0.2 ± 0.0 d | 0.05 ±0.0 d |
| AI | 74.7 ± 5.1 e,f | 0.12 ± 0.0 e,f,g | 0.03 ± 0.0 e,f,g |
| AS | 179.1 ± 10.5 a | 0.2 ± 0.0 d | 0.05 ± 0.0 d |
| ZI | 102.1 ± 7.2 c,d | 0.13 ± 0.0 e | 0.03 ± 0.0 e |
| FS | 62.2 ± 2.1 f,g | 0.23 ± 0.1 c,d | 0.05 ± 0.0 c,d |
| ND | 75.0 ± 2.6 e,f | 0.14 ± 0.0 e | 0.04 ± 0.0 e |
| JS | 58.1 ± 2.1 f,g | 0.12 ± 0.0 e,f | 0.03 ± 0.0 e,f |
| SaS | 164.4 ± 9.8 a | 0.09 ± 0.0 f,g | 0.03 ± 0.0 f,g |
| FD | 54.3 ± 3.7 g | 0.28 ± 0.1 b | 0.06 ± 0.0 b |
| HKS | 43.9± 3.2 h,i | 0.08 ± 0.0 g,h | 0.03 ± 0.0 g,h |
| SW | 100.5 ± 7.1 c,d | 0.12 ± 0.0 e,f | 0.03 ± 0.0 e,f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Abdelwareth, A.; Zayed, A.; Eissa, T.F.; Dokalahy, E.; Frolov, A.; Wessjohann, L.A. A Comparative Metabolomics Approach for Egyptian Mango Fruits Classification Based on UV and UPLC/MS and in Relation to Its Antioxidant Effect. Foods 2022, 11, 2127. https://doi.org/10.3390/foods11142127
Farag MA, Abdelwareth A, Zayed A, Eissa TF, Dokalahy E, Frolov A, Wessjohann LA. A Comparative Metabolomics Approach for Egyptian Mango Fruits Classification Based on UV and UPLC/MS and in Relation to Its Antioxidant Effect. Foods. 2022; 11(14):2127. https://doi.org/10.3390/foods11142127
Chicago/Turabian StyleFarag, Mohamed A., Amr Abdelwareth, Ahmed Zayed, Tarek F. Eissa, Eric Dokalahy, Andrej Frolov, and Ludger A. Wessjohann. 2022. "A Comparative Metabolomics Approach for Egyptian Mango Fruits Classification Based on UV and UPLC/MS and in Relation to Its Antioxidant Effect" Foods 11, no. 14: 2127. https://doi.org/10.3390/foods11142127
APA StyleFarag, M. A., Abdelwareth, A., Zayed, A., Eissa, T. F., Dokalahy, E., Frolov, A., & Wessjohann, L. A. (2022). A Comparative Metabolomics Approach for Egyptian Mango Fruits Classification Based on UV and UPLC/MS and in Relation to Its Antioxidant Effect. Foods, 11(14), 2127. https://doi.org/10.3390/foods11142127










