Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan Microparticles by Spray Drying
2.2.1. Non-Cross-Linked Chitosan Microcapsules
2.2.2. Cross-Linked Chitosan Microcapsules
2.3. Particle Size and Morphology Characterization
2.4. Evaluation of Enzymatic Activity
2.5. Evaluation of Protein Encapsulation and Release
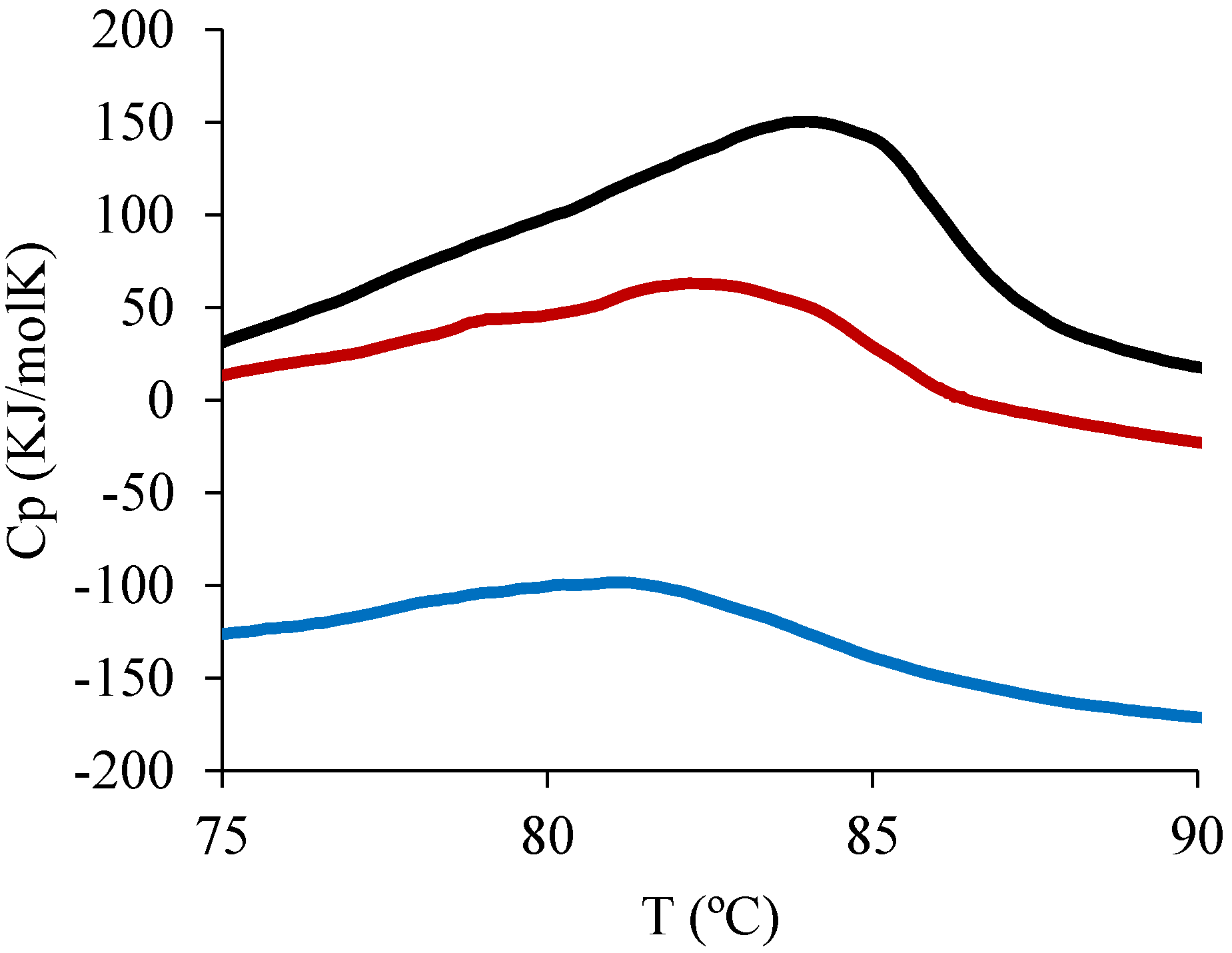
2.6. Differential Scanning Calorimetry (DSC)
2.7. Statistic
3. Results and Discussion
3.1. BSA-Loaded Chitosan Microparticles Prepared by Spray Drying
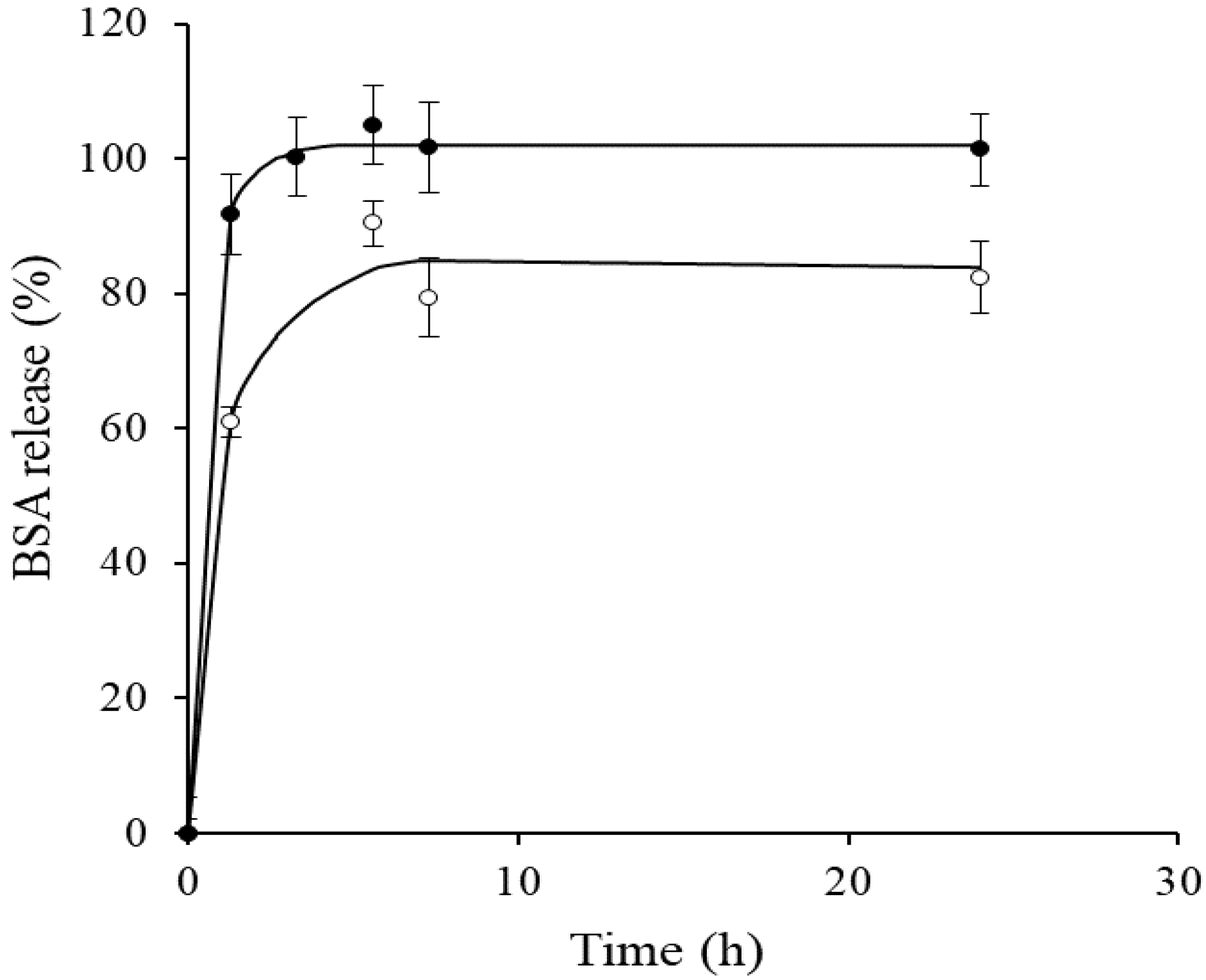
3.1.1. BSA Encapsulation, Protein Release and Stability
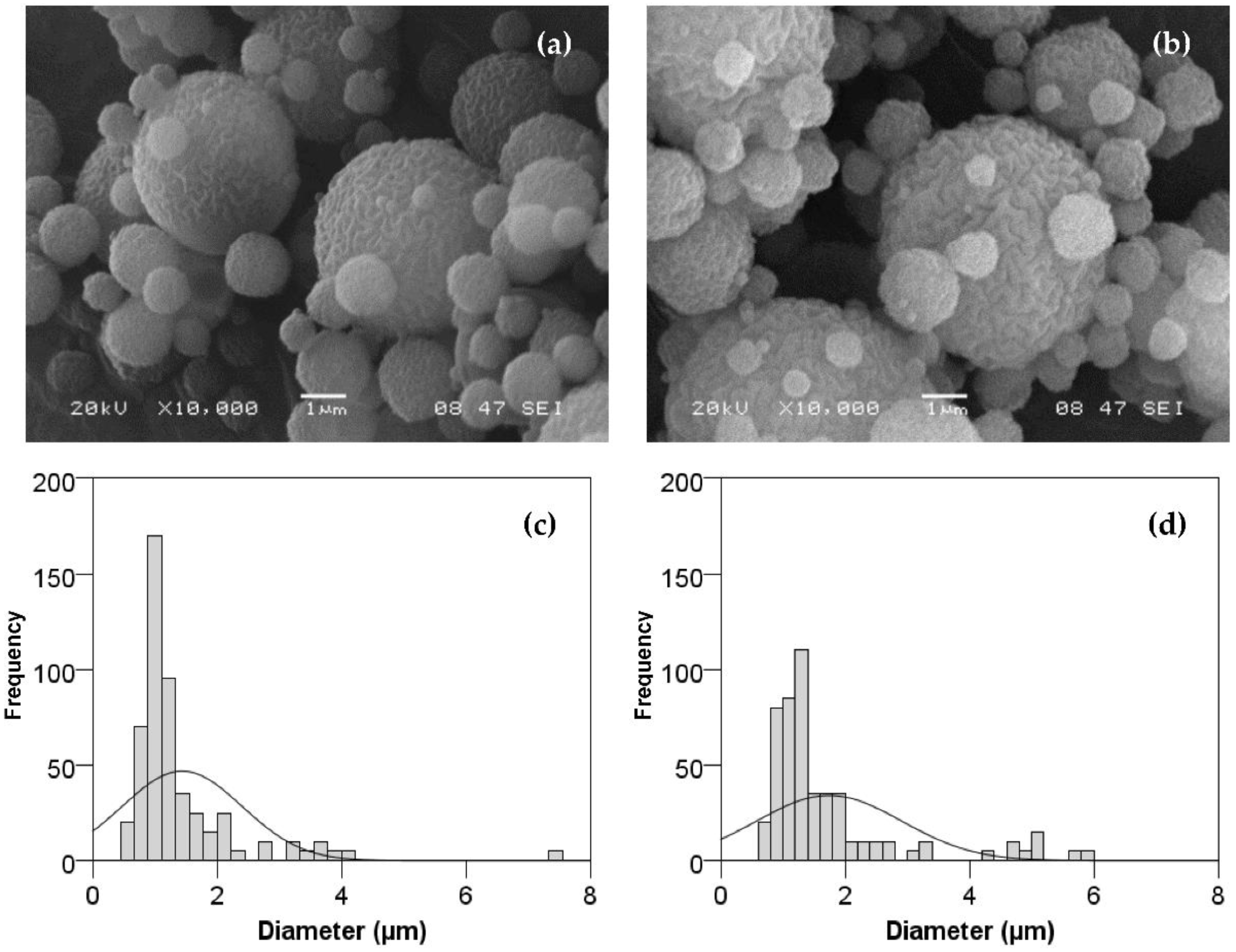
3.1.2. BSA–Chitosan Particles Morphology and Size
3.2. Flavourzyme®-Loaded Crosslinked Chitosan Microparticles Prepared by Spray Drying
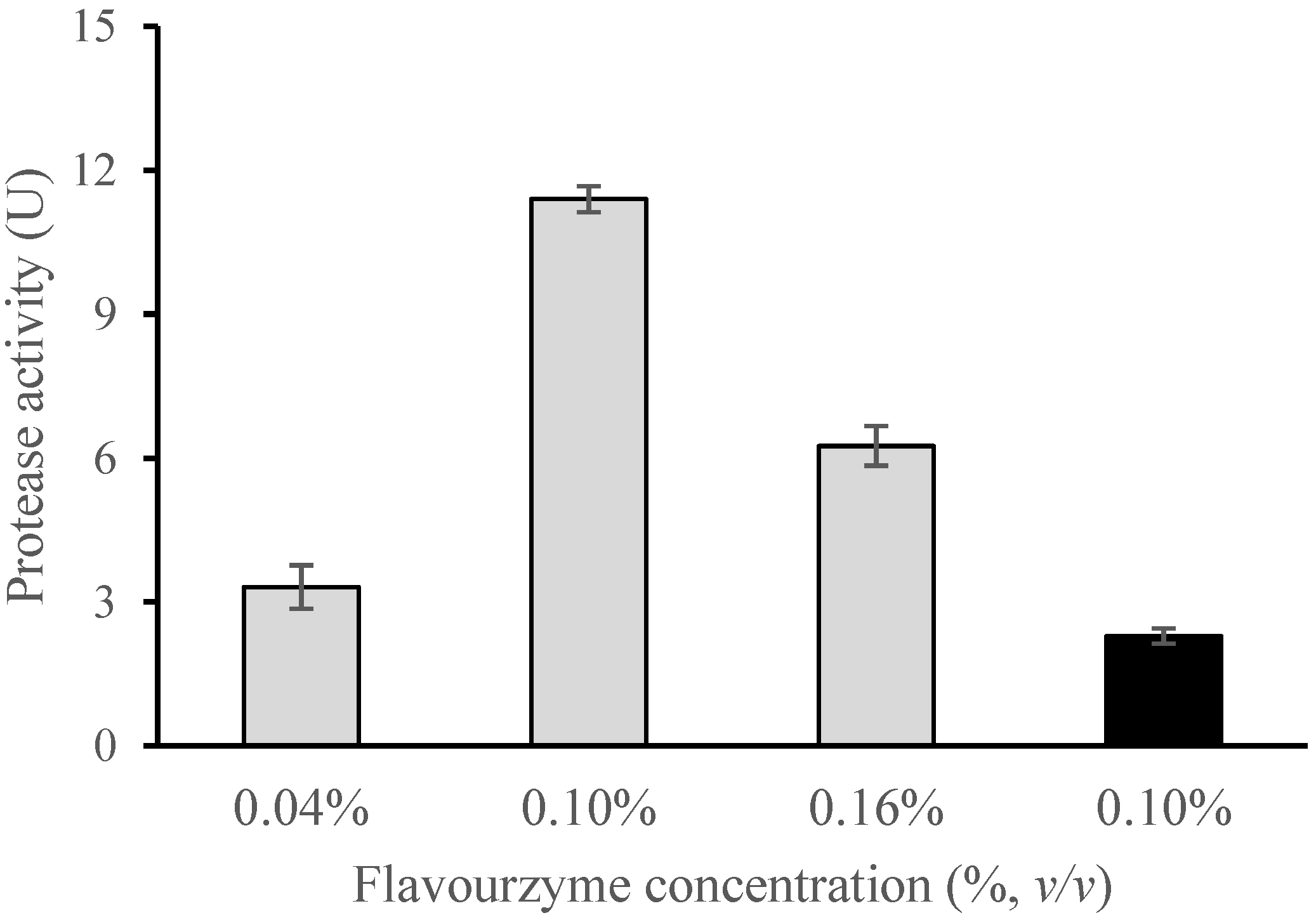
3.2.1. Effect of Enzyme Concentration and Inlet Temperature on Encapsulation Efficiency
3.2.2. Effect of Flavourzyme® Concentration and Inlet Temperature on Microparticle Size and Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fox, P.F.; Wallace, J.M.; Morgan, S.; Lynch, C.M.; Niland, E.J.; Tobin, J. Acceleration of cheese ripening. Antonie van Leeuwenhoek 1996, 70, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.B.; Güven, M. Effects of proteolytic and lipolytic enzyme supplementation on lipolysis and proteolysis characteristics of white cheeses. Foods 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjani, K.; Kailasapathy, K.; Phillips, M. Microencapsulation of enzyme for potential application in acceleration of cheese ripening. Int. Dairy J. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Lam, S.H. Application of encapsulated enzymes to accelerate cheese ripening. Int. Dairy J. 2005, 15, 929–939. [Google Scholar] [CrossRef]
- Upadhyaya, V.K.; Kelly, A.L.; McSweeney, P.L.H. Use of nattokinase, a subtilisin-like serine proteinase, to accelerate proteolysis in Cheddar cheese during ripening. Lait 2006, 86, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, A. Cheese ripening: A review on modern technologies towards flavour enhancement, process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Wilkinson, M.G.; Fox, P.F. Determination of key enzyme activities in commercial peptidase and lipase preparations from microbial or animal sources. Enzyme Microb. Technol. 2002, 31, 310–320. [Google Scholar] [CrossRef]
- Kheadr, E.E.; Vuillemard, J.C.; El-Deeb, S.A. Impact of liposome-encapsulated enzyme cocktails on Cheddar cheese ripening. Food Res. Int. 2003, 36, 241–252. [Google Scholar] [CrossRef]
- Jahadi, M.; Khosravi-Darani, K.; Ehsani, M.R.; Mozafari, M.R.; Saboury, A.A.; Pourhosseini, P.S. The encapsulation of Flavourzyme in nanoliposome by heating method. J. Food Sci. Technol. 2015, 52, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Raychaudhuri, U.; Chakrabrty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Amighi, F.; Emam-Djomeh, Z.; Madadlou, A. Optimised production and spray drying of ACE-inhibitory enzyme-modified cheese. J. Dairy Res. 2016, 83, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industrial applications. A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Farahmandi, K.; Rajab, S.; Tabandeh, F.; Shahraky, M.K.; Maghsoudi, A.; Ashengroph, M. Efficient spray-drying of Lactobacillus rhamnosus PTCC1637 using total CFU yield as the decision factor. Food Biosci. 2021, 40, 100816. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Malmo, C.L.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 cells coated in alginate beads with chitosan by spray drying to use as a probiotic cell in a chocolate soufflé. Food Bioprocess Technol. 2013, 6, 795–805. [Google Scholar] [CrossRef]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2022, 1–33, ahead of print. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Developing microencapsulated flaxseed oil containing shrimp (Litopenaeus setiferus) astaxanthin using a pilot scale spray dryer. Biosyst. Eng. 2011, 108, 121–132. [Google Scholar] [CrossRef]
- Bassani, A.; Carullo, D.; Rossi, F.; Fiorentini, C.; Garrido, G.D.; Reklaitis, G.V.R.; Bonadies, I.; Spigno, G. Modeling of a spray-drying process for the encapsulation of high-added value extracts from food by-products. Comput. Chem. Eng. 2022, 161, 107772. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, e14059, ahead of print. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Rafiee, S.; Madadlou, A. Energy and exergy analyses of the spray drying process of fish oil microencapsulation. Biosyst. Eng. 2012, 111, 229–241. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Bhainsa, K.C. Applicability of spray drying technique to prepare nano-micro carriers: A review. Nanoarchitectonics 2022, 3, 33–45. [Google Scholar] [CrossRef]
- Bernucci, B.S.P.; Loures, C.M.G.; Lopes, S.C.A.; Oliveira, M.C.; Sabino, A.P.; Vilela, J.M.C.; Andrade, M.S.; Lacerda, I.C.; Nicoli, J.R.; Oliveira, E.S. Effect of microencapsulation conditions on the viability and functionality of Bifidobacterium longum 51A. LWT-Food Sci. Technol. 2017, 80, 341–347. [Google Scholar] [CrossRef]
- Cabral, T.P.F.; Bellini, N.C.; Assis, K.R.; Teixeira, C.C.C.; Lanchote, A.D.; Cabral, H.; Freitas, L.A.P. Microencapsulate Aspergillus niger peptidases from agroindustrial waste wheat bran: Spray process evaluation and stability. J. Microencapsul. 2017, 34, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Lopes, A.R.; Sousa, V.; Rocha, F.; Nunes, O.C. Microencapsulation of Gulosibacter molinativorax ON4T cells by a spray-drying process using different biopolymers. J. Hazard. Mater. 2017, 338, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; McKnight, S.; Adhikari, B. Microencapsulation of α-amylase by carrying out complex coacervation and drying in a single step using a novel three-fluid nozzle spray drying. Dry. Technol. 2013, 31, 1901–1910. [Google Scholar] [CrossRef]
- Hartini, N.; Ponrasu, T.; Wu, J.-J.; Sriariyanun, M.; Cheng, Y.-S. Microencapsulation of curcumin in crosslinked jelly fig pectin using vacuum spray drying technique for effective drug delivery. Polymers 2021, 13, 2583. [Google Scholar] [CrossRef]
- Lopes, A.R.; Sousa, V.M.; Estevinho, B.N.; Leite, J.P.; Moreira, N.F.F.; Gales, L.; Rocha, F.; Nunes, O.C. Production of microparticles of molinate degrading biocatalyst using the spray drying technique. Chemosphere 2016, 161, 61–68. [Google Scholar] [CrossRef]
- Potin, F.; Goure, E.; Lubbers, S.; Husson, F.; Surel, R. Functional properties of hemp protein concentrate obtained by alkaline extraction and successive ultrafiltration and spray-drying. Int. J. Food Sci. Technol. 2022, 57, 436–446. [Google Scholar] [CrossRef]
- Rodklongtan, A.; Chitprasert, P. Combined effects of holy basil essential oil and inlet temperature on lipid peroxidation and survival of Lactobacillus reuteri KUB-AC5 during spray drying. Food Res. Int. 2017, 100, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 1–16, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lei, H.; Gao, X.; Xiong, X.; Wu, W.D.; Wu, Z.; Chen, X.D. Fabrication of uniform enzyme-immobilized carbohydrate microparticles with high enzymatic activity and stability via spray drying and spray freeze drying. Power Technol. 2018, 330, 40–49. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Heras, A. Encapsulation of an Agrobacterium radiobacter extract containing D-hydantoinase and D-carbamoylase activities into alginate-chitosan polyelectrolyte complexes. Preparation of the biocatalyst. J. Mol. Catal. B-Enzym. 2009, 58, 54–64. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- González Siso, M.I.; Lang, E.; Carreño-Gómez, B.; Becerra, M.; Otero Espinar, F.; Blanco Méndez, J. Enzyme encapsuation on chitosan microbeads. Process Biochem. 1997, 32, 211–216. [Google Scholar] [CrossRef]
- Kadri, T.; Cuprys, A.; Rouissi, T.; Brar, S.K.; Daghrir, R.; Lauzon, J.-M. Nanoencapsulation and release study of enzymes from Alkanivorax borkumensis in chitosan-tripolyphosphate formulation. Biochem. Eng. J. 2018, 137, 1–10. [Google Scholar] [CrossRef]
- Wolf, M.; Paulino, A.T. Full-factorial central composite rotational design for the immobilization of lactase in natural polysaccharide-based hydrogels and hydrolysis of lactose. Int. J. Biol. Macromol. 2019, 135, 986–997. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Immobilization of yeast inulinase on chitosan beads for the hydrolysis of inulin in a batch system. Int. J. Biol. Macromol. 2017, 95, 87–93. [Google Scholar] [CrossRef]
- Singh, T.A.; Jajoo, A.; Bhasin, S. Optimization of various encapsulation systems for efficient immobilization of actinobacterial glucose isomerase. Biocatal. Agric. Biotechnol. 2020, 29, 101766. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, M.; Fang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Xu, J. Recent advancements in encapsulation of chitosan-based enzymes and their applications in food industry. Crit. Rev. Food Sci. Nutr. 2022, 1–19, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloid Surface A 2019, 578, 123644. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Tokárová, V.; Kašpar, O.; Knejzlík, Z.; Ulbrich, P.; Štěpánek, F. Development of spray-dried chitosan microcarriers for nanoparticle delivery. Powder Technol. 2013, 235, 797–805. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, X.; Cao, W.; Ren, X.; Ren, G.; Liu, P.; Chen, J. Effects of different drying methods on the characterization, dissolution rate and antioxidant activity of ursolic acid-loaded chitosan nanoparticles. Foods 2021, 10, 2470. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release of drug delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef]
- Kašpar, O.; Tokárová, V.; Nyanhongo, G.S.; Gübitz, G.; Štěpánek, F. Effect of cross-linking method on the activity of spray-dried chitosan microparticles with immobilized laccase. Food Bioprod. Process. 2013, 91, 525–535. [Google Scholar] [CrossRef]
- Ortega, N.; Perez-Mateos, M.; Pilar, M.C.; Busto, M.D. Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J. Agric. Food Chem. 2009, 57, 109–115. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier-Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B-Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Neto, Y.A.A.H.; de Freitas, L.A.P.; Cabral, H. Multivariate analysis of the stability of spray-dried Eupenicillium javanicum peptidases. Dry. Technol. 2014, 32, 614–621. [Google Scholar] [CrossRef]
- Kusonwiriyawong, C.; Lipipun, V.; Vardhanabhuti, N.; Zhang, Q.; Rithidej, C. Spray-dried chitosan microparticles for cellular delivery of an antigenic protein: Physico-chemical properties and cellular uptake by dendritic cells and macrophages. Pharm. Res. 2013, 30, 1677–1697. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Inter. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Trows, S.; Scherließ, R. Carrier-based dry powder formulation for nasal delivery of vaccines utilizing BSA as model drug. Powder Technol. 2016, 292, 223–231. [Google Scholar] [CrossRef]
- Cao, X.; Tian, Y.; Wang, Z.; Liu, Y.; Wang, C. BSA denaturation in the absence and the presence of urea studied by the iso-conversional method and the master plots method. J. Therm. Anal. Calorim. 2010, 102, 75–81. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yano, H. Differential scanning calorimetric studies on bovine serum albumin: I. Effects of pH and ionic strength. Int. J. Biol. Macromol. 1990, 12, 263–268. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agent on the properties of spray dried microspheres. J. Microencapsul. 2005, 22, 377–395. [Google Scholar] [CrossRef]
- Vehring, V.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Katsarov, P.D.; Pilicheva, B.A.; Manev, H.M.; Lukova, P.K.; Kassarova, M.I. Optimization of chitosan microspheres spray drying via 32 full factorial design. Folia Med. 2017, 59, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, X.; Zhang, D.; Zhilong, X. Encapsulation efficiency and release behaviours of bovine serum albumin loaded in alginate microspheres prepared by spraying. J. Appl. Polym. Sci. 2008, 110, 2488–2497. [Google Scholar] [CrossRef]
- Shiga, H.; Joreau, H.; Neoh, T.L.; Furuta, T.; Yoshii, H. Encapsulation of alcohol dehydrogenase in mannitol by spray drying. Pharmaceutics 2014, 6, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafabakhsh, Z.; Khosravi-darani, K.; Khajeh, K.; Jahadi, M.; Komeilli, R.; Mortazavian, A.M. Stability and catalytic kinetics of protease loaded liposomes. Biochem. Eng. J. 2013, 72, 11–17. [Google Scholar] [CrossRef]
- Yust, M.D.; Millan-Linares, M.D.; Alcalde-Hidalgo, J.M.; Millan, F.; Pedroche, J. Hydrolysis of chickpea proteins with Flavourzyme immobilized on glyoxyl-agarose gels improves functional properties. Food Sci. Technol. Int. 2013, 19, 217–223. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Apple, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an enzyme preparation with industrial relevance: Automated nine-step purification and partial characterization of eight enzymes. J. Agri. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Littringer, E.M. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Finney, J.; Buffo, R.; Reineccius, G.A. Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J. Food Sci. 2002, 67, 1108–1114. [Google Scholar] [CrossRef]
- Moghadam, M.; Sani, A. Optimization of spray drying parameters in production of malt extract powder. Curr. Nutr. Food Sci. 2014, 10, 77–85. [Google Scholar] [CrossRef]
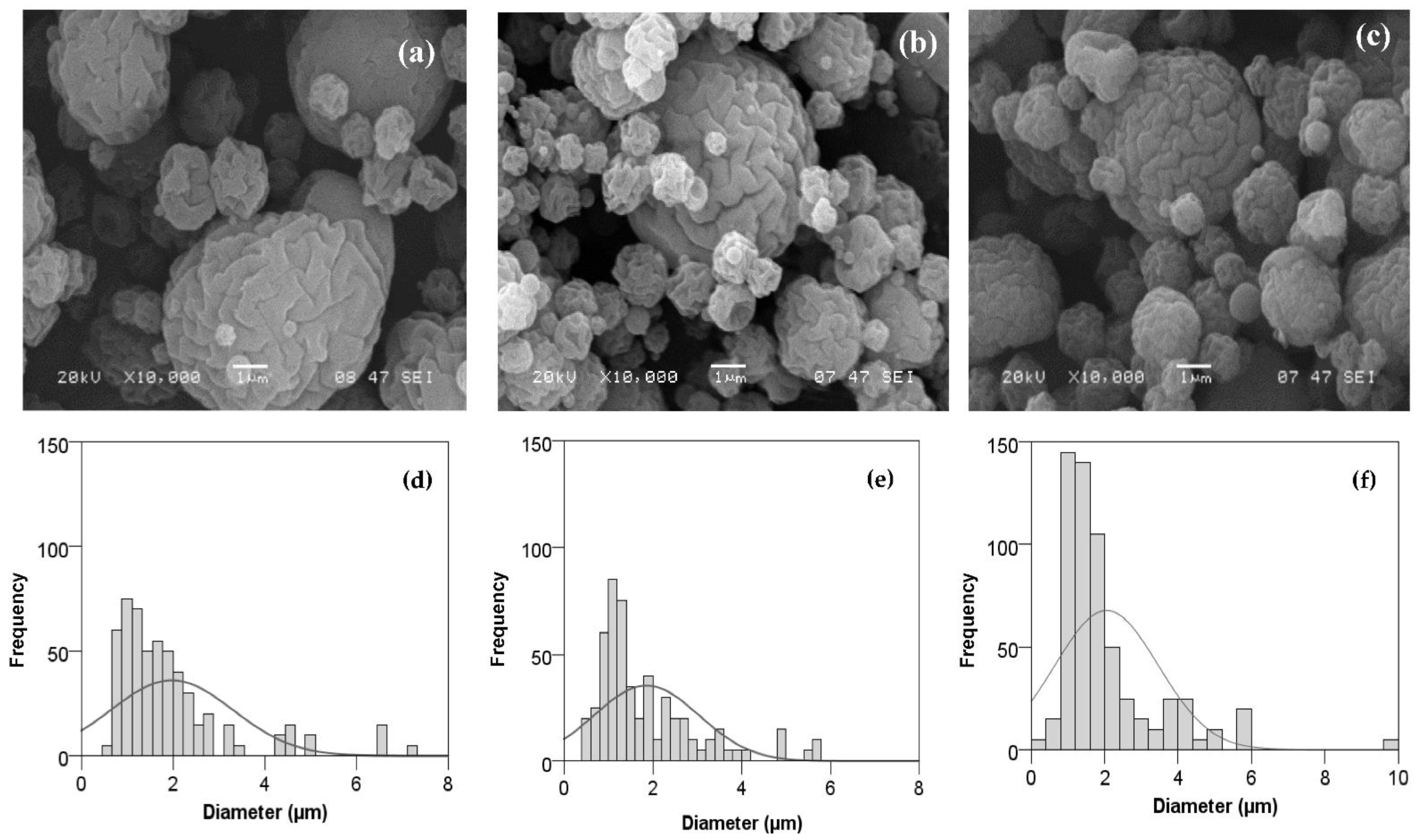
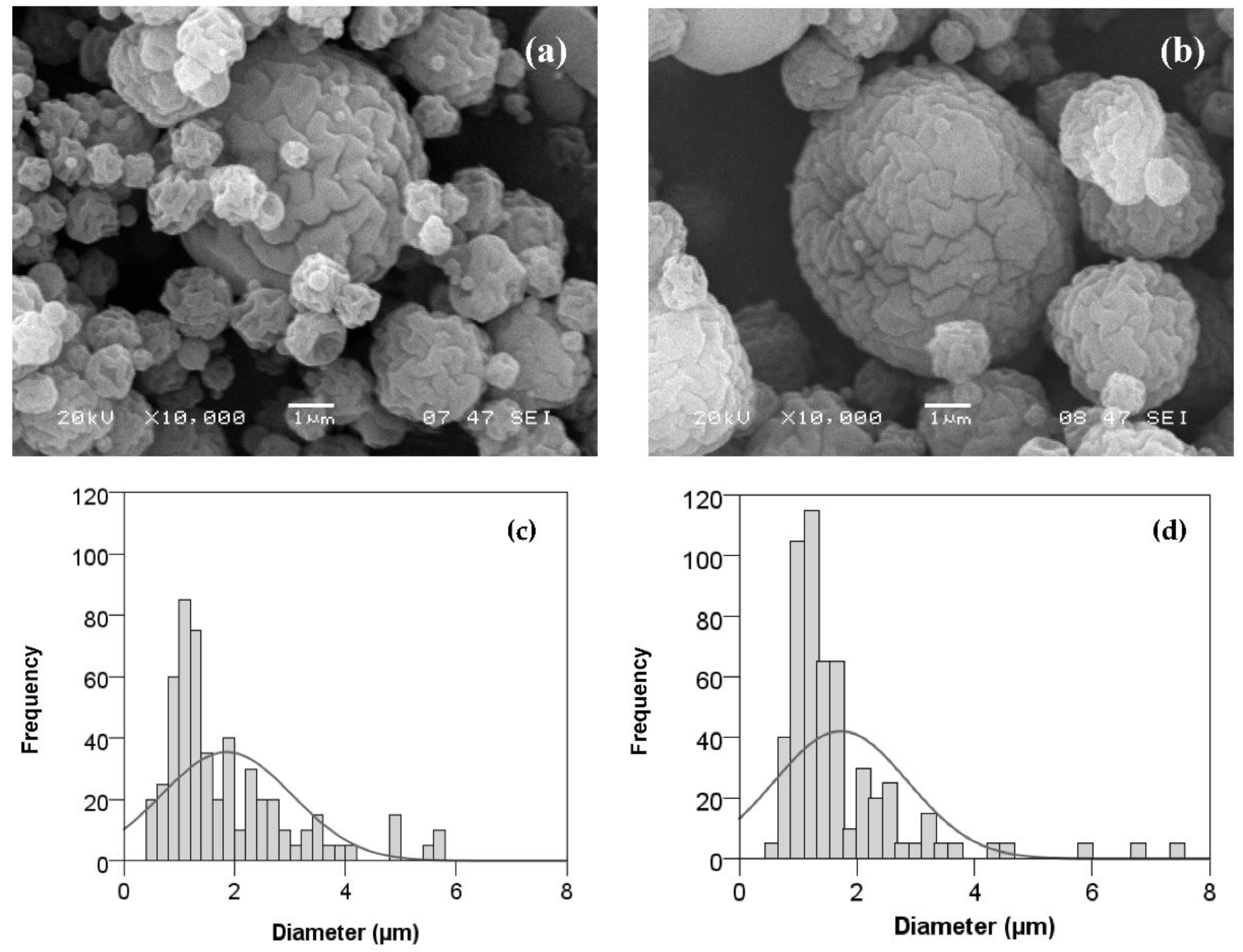
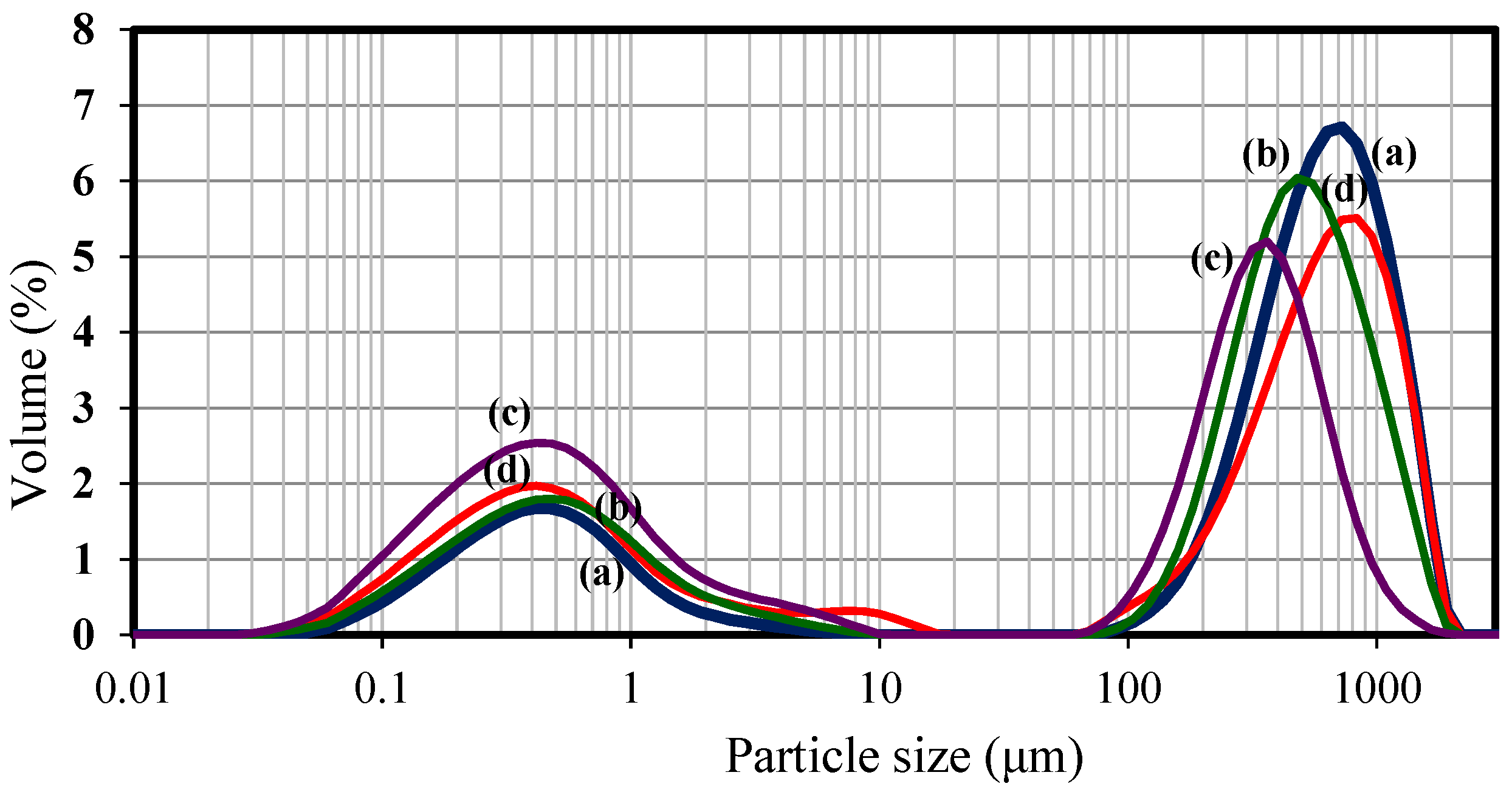
| Flavourzyme® Concentration (%, v/v) | Inlet Temperature (°C) | Outlet Temperature (°C) | N2 Flowrate (NL/h) |
|---|---|---|---|
| 0.04 | 130 | 57 ± 1 | 334.6 |
| 0.10 | 130 | 62 ± 1 | 334.6 |
| 0.16 | 130 | 54 ± 1 | 334.6 |
| 0.10 | 160 | 83 ± 1 | 345.8 |
| Flavourzyme® Concentration (%, v/v) | Inlet Temperature (°C) | Encapsulation Efficiency (EE, %) | Activity Yield 2 (AY, %) |
|---|---|---|---|
| 0.04 | 130 | 78.5 ± 11.0 β | 25.6 ± 3.5 α |
| 0.10 | 130 | 78.6 ± 1.7 β | 88.0 ± 2.1 δ |
| 0.16 | 130 | 62.4 ± 2.1 β | 60.8 ± 4.6 γ |
| 0.10 | 160 | 40.6 ± 5.1 α | 34.2 ± 0.4 β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busto, M.D.; González-Temiño, Y.; Albillos, S.M.; Ramos-Gómez, S.; Pilar-Izquierdo, M.C.; Palacios, D.; Ortega, N. Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles. Foods 2022, 11, 2077. https://doi.org/10.3390/foods11142077
Busto MD, González-Temiño Y, Albillos SM, Ramos-Gómez S, Pilar-Izquierdo MC, Palacios D, Ortega N. Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles. Foods. 2022; 11(14):2077. https://doi.org/10.3390/foods11142077
Chicago/Turabian StyleBusto, María D., Yaiza González-Temiño, Silvia M. Albillos, Sonia Ramos-Gómez, María C. Pilar-Izquierdo, David Palacios, and Natividad Ortega. 2022. "Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles" Foods 11, no. 14: 2077. https://doi.org/10.3390/foods11142077
APA StyleBusto, M. D., González-Temiño, Y., Albillos, S. M., Ramos-Gómez, S., Pilar-Izquierdo, M. C., Palacios, D., & Ortega, N. (2022). Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles. Foods, 11(14), 2077. https://doi.org/10.3390/foods11142077







