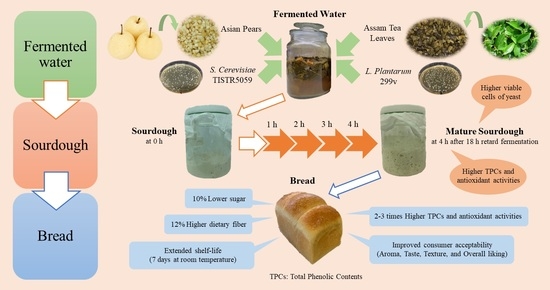
Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Water
2.2. Preparation of Sourdough
2.3. Preparation of Bread
2.4. Determination of Microbiological Quality
2.5. Determination of Chemical and Physical Quality
2.6. Determination of Total Phenolic Contents and Antioxidant Activities
2.7. Determination of Nutritional Quality
2.8. Sensory Evaluation
2.9. Shelf-Life Stability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fermented Water and Fermentation Time on Sourdough Quality
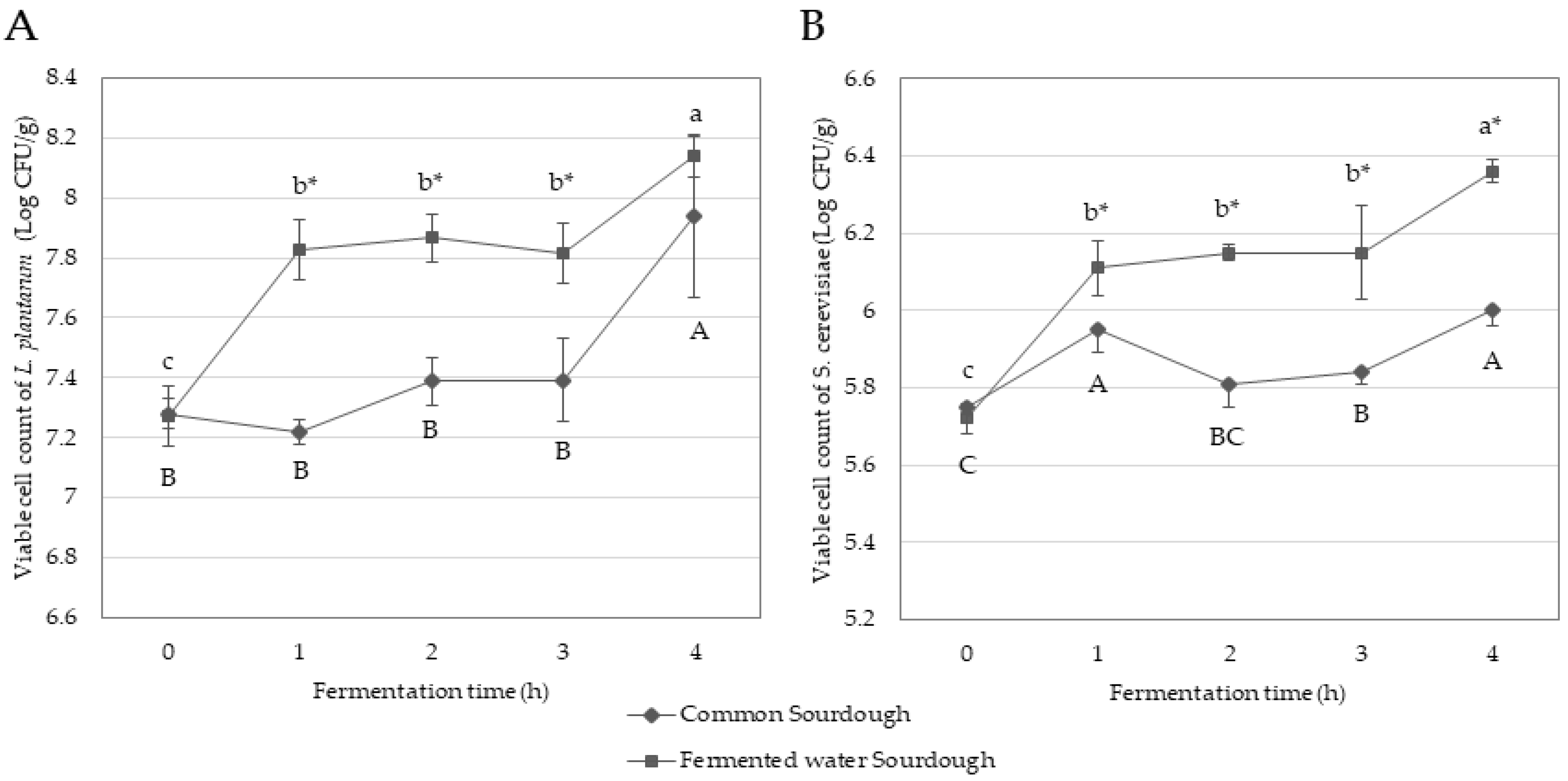
3.1.1. Growth of L. plantarum and S. cerevisiae
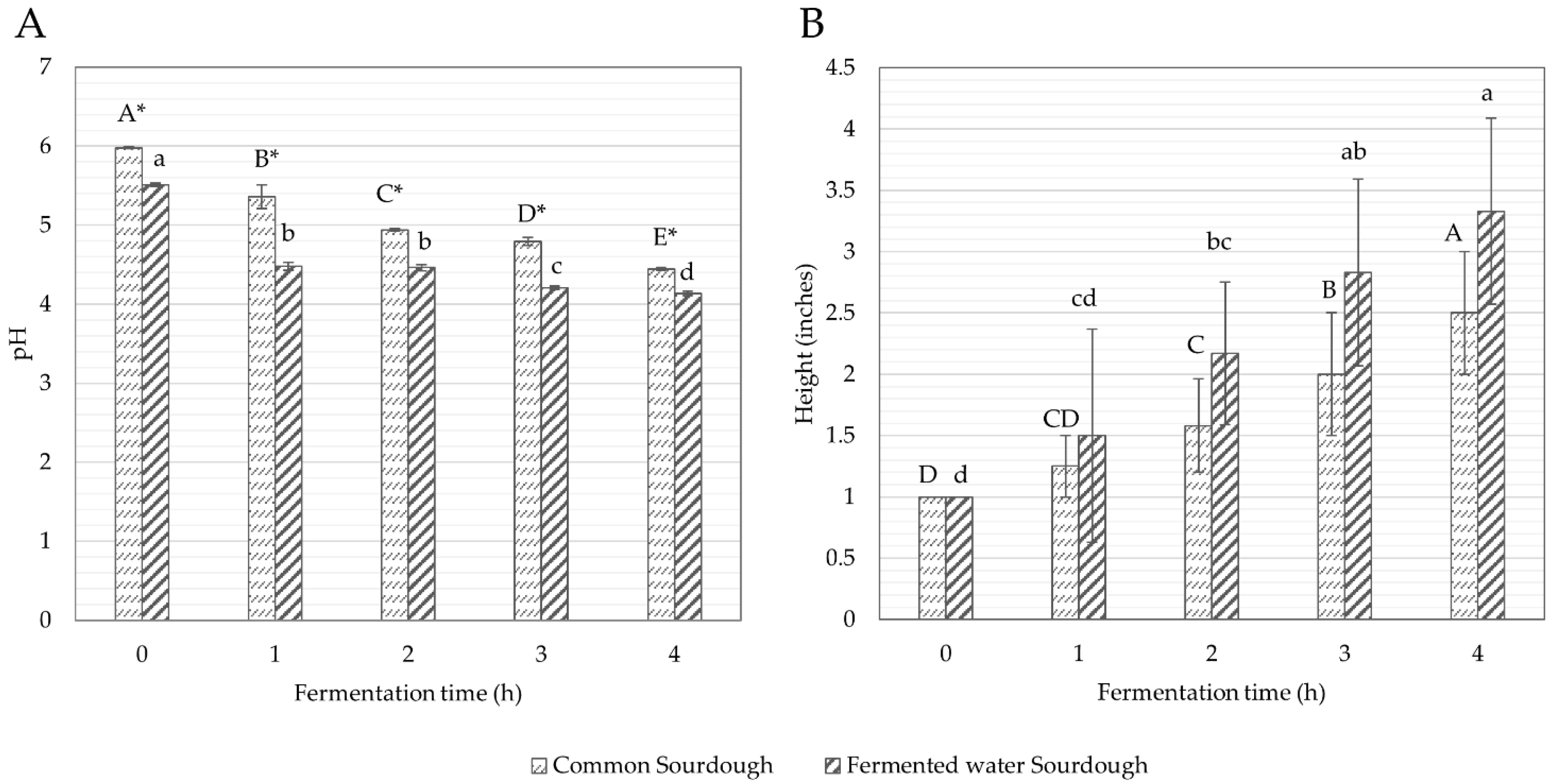
3.1.2. pH and Height
3.1.3. Total Phenolic Contents and Antioxidant Activities
3.2. Effect of Fermented Water on Bread Quality
3.2.1. Chemical and Physical Quality
3.2.2. Nutritional Quality
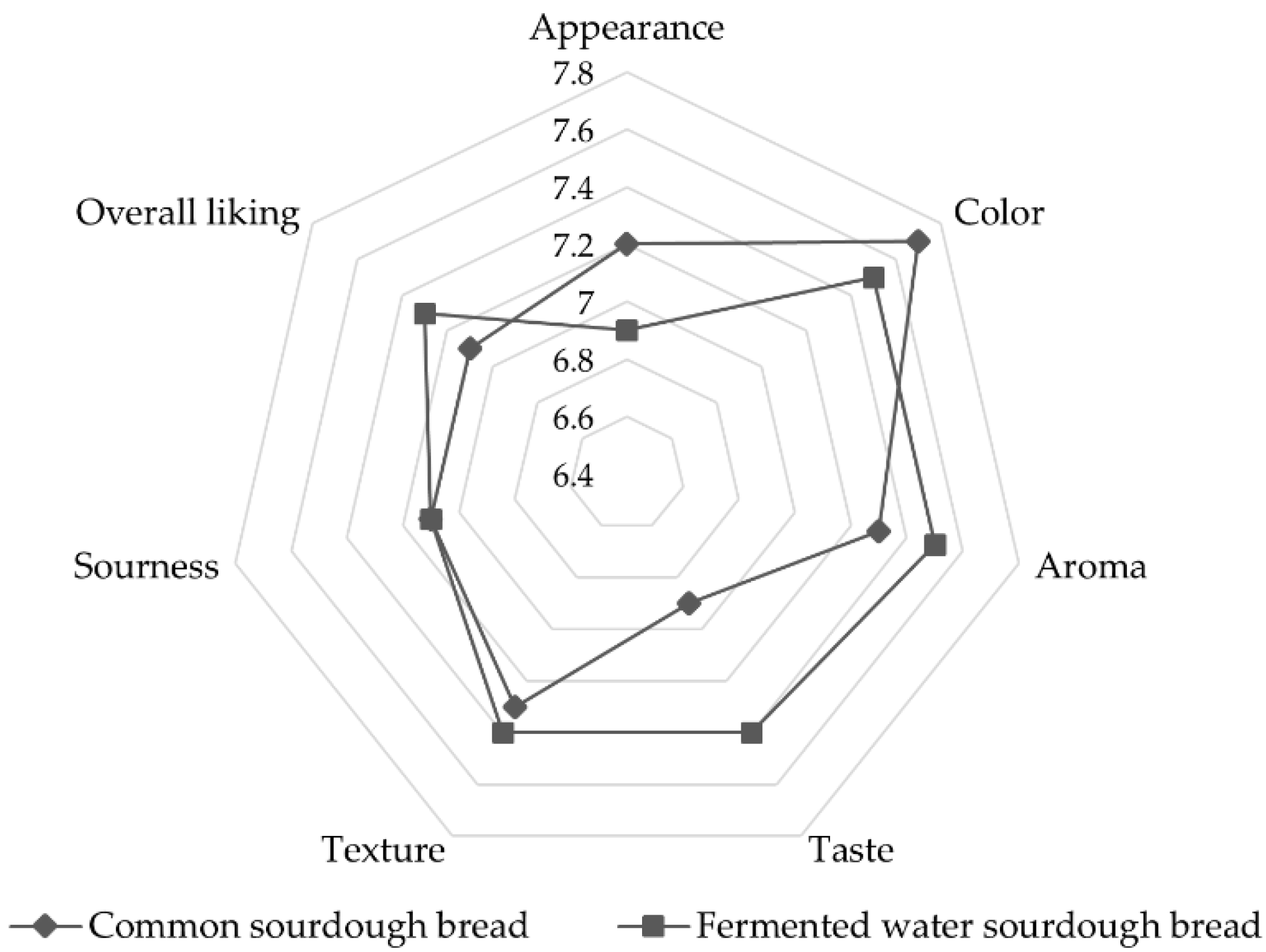
3.2.3. Sensory Evaluation
3.3. Shelf-Life Stability
3.3.1. Microbiological Quality
3.3.2. Total Phenolic Contents and Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development—Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Singh, S.; Sethi, S.; Gupta, S.; Kaur, C.; Wood, E. Fermentation of Multigrain Dough—An Approach to Reduce Glycemic Index for Healthy Bread. EUREKA Life Sci. 2019, 5, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and L. helveticus for sourdough bread making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- do Amaral Santos, C.C.A.; da Silva Libeck, B.; Schwan, R.F. Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int. J. Food Microbiol. 2014, 186, 32–41. [Google Scholar] [CrossRef]
- Reis, V.R.; Bassi, A.P.G.; da Silva, J.C.G.; Ceccato-Antonini, S.R. Characteristics of Saccharomyces cerevisiae yeasts exhibiting rough colonies and pseudohyphal morphology with respect to alcoholic fermentation. Braz. J. Microbiol. 2014, 44, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalcanton, F.; Carrasco, E.; Pérez-Rodríguez, F.; Posada-Izquierdo, G.D.; Falcão de Aragão, G.M.; García-Gimeno, R.M. Modeling the Combined Effects of Temperature, pH, and Sodium Chloride and Sodium Lactate Concentrations on the Growth Rate of Lactobacillus plantarum ATCC 8014. J. Food Qual. 2018, 2018, 1726761. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, J.; Wang, S.; Liu, Y.; Li, L.; Gao, M. Lactic acid bacteria synergistic fermentation affects the flavor and texture of bread. J. Food Sci. 2022, 87, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Karimi, N.; Nikoo, M.; Ahmadi Gavlighi, H.; Piri Gheshlaghi, S.; Regenstein, J.M.; Xu, X. Effect of pacific white shrimp (Litopenaeus vannamei) protein hydrolysates (SPH) and (−)-epigallocatechin gallate (EGCG) on sourdough and bread quality. LWT 2020, 131, 109800. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Qi, X. Contribution of spontaneously-fermented sourdoughs with pear and navel orange for the bread-making. LWT 2018, 89, 336–343. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The Effect of Steaming and Fermentation on Nutritive Values, Antioxidant Activities, and Inhibitory Properties of Tea Leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Ungsongtham, P.; Puengpan, V.; Aksornchoo, P.; Supanuwat, J. Nutritional Values in Fruits, 1st ed.; Agricultural Cooperative Federation of Thailand: Nonthaburi, Thailand, 2010. [Google Scholar]
- Jiang, G.; Ameer, K.; Eun, J.-B. Physicochemical, antioxidant, microstructural, and sensory properties of sesame bars sweetened with pear juice concentrate. J. Food Sci. Technol. 2020, 57, 4551–4561. [Google Scholar] [CrossRef]
- Reiland, H.; Slavin, J. Systematic Review of Pears and Health. Nutr. Today 2015, 50, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V. Assessment of Phytochemical and Antioxidant Potential of Underutilized Pear (Pyrus pyrifolia) and Plum (Prunus domestica) from Indigenous Himalayan Region of Himachal Pradesh. Int. J. Pharm. Sci. Res. 2017, 8, 1000–1006. [Google Scholar]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef]
- Supasil, R.; Thangsiri, S.; Whanmek, K.; Khemthong, C.; Tangsuphoom, N.; Santivarangkna, C.; Suttisansanee, U.; On-nom, N. The improvement of total phenolic content and antioxidant activity in fermented water from Asian pears (Pyrus pyrifolia) and Assam tea (Camellia sinensis var. assamica) leaves with co-cultures between Lactobacillus plantarum and Saccharomyces cerevisiae. In Proceedings of the 23rd Food Innovation Asia Conference 2021 (FIAC 2021), Bangkok, Thailand, 17–19 June 2021. [Google Scholar]
- Hammes, W.P.; Brandt, M.J.; Francis, K.L.; Rosenheim, J.; Seitter, M.F.H.; Vogelmann, S.A. Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 2005, 16, 4–11. [Google Scholar] [CrossRef]
- Mohd Roby, B.H.; Muhialdin, B.J.; Abadl, M.M.T.; Mat Nor, N.A.; Marzlan, A.A.; Lim, S.A.H.; Mustapha, N.A.; Meor Hussin, A.S. Physical properties, storage stability, and consumer acceptability for sourdough bread produced using encapsulated kombucha sourdough starter culture. J. Food Sci. 2020, 85, 2286–2295. [Google Scholar] [CrossRef]
- Bender, D.; Fraberger, V.; Szepasvári, P.; D’Amico, S.; Tömösközi, S.; Cavazzi, G.; Jäger, H.; Domig, K.J.; Schoenlechner, R. Effects of selected lactobacilli on the functional properties and stability of gluten-free sourdough bread. Eur. Food Res. Technol. Z. Lebensm.-Unters. Forschung. A 2018, 244, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujalance, C.; Jiménez-Valera, M.; Moreno, E.; Ruiz-Bravo, A. A selective differential medium for Lactobacillus plantarum. J. Microbiol. Methods 2006, 66, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of Mixed Cultures of Yeast and Lactobacilli on the Quality of Wheat Sourdough Bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- FDA. Bacteriological Analytical Manual (BAM); US Food and Drug Administration: Silver Spring, MD, USA, 2001. [Google Scholar]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Li, Y.; Wu, G.; Qi, X.; Xu, M.; Rao, Z. Effect of selected strains on physical and organoleptic properties of breads. Food Chem. 2019, 276, 547–553. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2001. [Google Scholar]
- Sripum, C.; Kukreja, R.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- AOAC. Official Method of Analysis of AOAC Internation, 21st ed.; AOAC International: Arlington, VA, USA, 2019. [Google Scholar]
- Wannasaksri, W.; Temviriyanukul, P.; Aursalung, A.; Sahasakul, Y.; Thangsiri, S.; Inthachat, W.; On-Nom, N.; Chupeerach, C.; Pruesapan, K.; Charoenkiatkul, S.; et al. Influence of Plant Origins and Seasonal Variations on Nutritive Values, Phenolics and Antioxidant Activities of Adenia viridiflora Craib., an Endangered Species from Thailand. Foods 2021, 10, 2799. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Fleet, G.H.; Heard, G.M. Yeast-Growth during Fermentation; Harwood Academic Publishers: Chur, Switzerland, 1992. [Google Scholar]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Shetty, K.; Khanongnuch, C. Nutritional biotransformation in traditional fermented tea (Miang) from north Thailand and its impact on antioxidant and antimicrobial activities. J. Food Sci. Technol. 2019, 56, 2687–2699. [Google Scholar] [CrossRef]
- Ketwal, S.; Chueamchaitrakun, P.; Theppakorn, T.; Wongsakul, S. Contents of total polyphenol, microorganisms and antioxidant capacities of pickled tea (miang) commercially available in Chiang Rai, Thailand. In Proceedings of the 16th Food Innovation Asia Conference (FIAC 2014), Bangkok, Thailand, 12–13 June 2014. [Google Scholar]
- Somsong, P.; Santivarangkna, C.; Tiyayon, P.; Hsieh, C.-M.; Srichamnong, W. Assessing Polyphenol Components and Antioxidant Activity during Fermented Assam Tea Ball Processing. Sustainability 2020, 12, 5853. [Google Scholar] [CrossRef]
- López de Felipe, F.; Curiel, J.A.; Muñoz, R. Improvement of the fermentation performance of Lactobacillus plantarum by the flavanol catechin is uncoupled from its degradation. J. Appl. Microbiol. 2010, 109, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Kayikci, Ö.; Nielsen, J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov068. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules 2019, 24, 4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.; Zhang, Y.X.; Lu, T.T.; Cao, D.X.; Chen, J.D. Shrimp Waste Fermentation Using Symbiotic Lactic Acid Bacteria. Adv. Mater. Res. 2011, 194–196, 2156–2163. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Pattananandecha, T.; Saenjum, C.; Shetty, K.; Khanongnuch, C. Microbial Community Dynamics During the Non-filamentous Fungi Growth-Based Fermentation Process of Miang, a Traditional Fermented Tea of North Thailand and Their Product Characterizations. Front. Microbiol. 2020, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Cho, J.-Y.; Jeong, H.Y.; Jeong, D.E.; Kim, D.; Cho, S.-Y.; Kim, W.-S.; Moon, J.-H. Comparison of bioactive compound contents and in vitro and ex vivo antioxidative activities between peel and flesh of pear (Pyrus pyrifolia Nakai). Food Sci. Biotechnol. 2015, 24, 207–216. [Google Scholar] [CrossRef]
- Winters, M.; Panayotides, D.; Bayrak, M.; Rémont, G.; Viejo, C.G.; Liu, D.; Le, B.; Liu, Y.; Luo, J.; Zhang, P.; et al. Defined co-cultures of yeast and bacteria modify the aroma, crumb and sensory properties of bread. J. Appl. Microbiol. 2019, 127, 778–793. [Google Scholar] [CrossRef]
- Złotek, U.; Świeca, M.; Reguła, J.; Jakubczyk, A.; Sikora, M.; Gawlik-Dziki, U.; Kapusta, I. Effects of probiotic L. plantarum 299v on consumer quality, accumulation of phenolics, antioxidant capacity and biochemical changes in legume sprouts. Int. J. Food Sci. 2019, 54, 2437–2446. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L.). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Aman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.; Głowacki, A.; Piórecki, N. Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads. Biomolecules 2021, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Mayrhofer, S.; Domig, K. The contribution of P. acidilactici, L. plantarum, and L. curvatus starters and L-(+)-lactic acid to the acrylamide content and quality parameters of mixed rye—Wheat bread. LWT 2017, 80, 43–50. [Google Scholar] [CrossRef]
- Katina, K.; Heiniö, R.L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT—Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Burton, P.; Lightowler, H.J. Influence of bread volume on glycaemic response and satiety. Br. J. Nutr. 2006, 96, 877–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingsbury, J.M.; Goldstein, A.L.; McCusker, J.H. Role of nitrogen and carbon transport, regulation, and metabolism genes for Saccharomyces cerevisiae survival in vivo. Eukaryot. Cell 2006, 5, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.H.; Mukerjea, R.; Robyt, J.F. Specificity of yeast (Saccharomyces cerevisiae) in removing carbohydrates by fermentation. Carbohydr. Res. 2003, 338, 1127–1132. [Google Scholar] [CrossRef]
- Gámbaro, A.; Giménez, A.; Ares, G.; Gilardi, V. Influence of Enzymes on the Texture of Brown Pan Bread. J. Texture Stud. 2006, 37, 300–314. [Google Scholar] [CrossRef]
- Giménez, A.; Ares, G.; Gámbaro, A. Survival Analysis to Estimate Sensory Shelf Life Using Acceptability Scores. J. Sens. Stud. 2008, 23, 571–582. [Google Scholar] [CrossRef]
- Giménez, A.; Varela, P.; Salvador, A.; Ares, G.; Fiszman, S.; Garitta, L. Shelf life estimation of brown pan bread: A consumer approach. Food Qual. Prefer. 2007, 18, 196–204. [Google Scholar] [CrossRef]
- TISI. Thai Community Product Standard, Bread; Thai Industrial Standards Institute: Bangkok, Thailand, 2012. [Google Scholar]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and Antimicrobial Activities of (−)-Epigallocatechin-3-gallate (EGCG) and its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sae-Lee, N.; Kerdchoechuen, O.; Laohakunjit, N. Composition of epigallocatechin gallate (EGCG) and catechins derivatives in Chinese and assam tea. J. Agric. Sci. 2009, 40, 9–12. [Google Scholar]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
| Common Sourdough | ||||
|---|---|---|---|---|
| Time (h) | TPCs (mg GAE/g DW) | Antioxidant Activities | ||
| ORAC Assay (μmol TE/g DW) | FRAP Assay (μmol TE/g DW) | DPPH Radical Scavenging Assay (μmol TE/100 g DW) | ||
| 0 | 0.24 ± 0.02 b | 9.88 ± 1.16 b | 2.51 ± 0.07 b | 0.16 ± 0.01 b |
| 4 | 0.50 ± 0.05 a | 17.64 ± 1.22 a | 4.20 ± 0.29 a | 0.50 ± 0.04 a |
| Fermented Water Sourdough | ||||
| Time (h) | TPCs (mg GAE/g DW) | Antioxidant Activities | ||
| ORAC assay (μmol TE/g DW) | FRAP assay (μmol TE/g DW) | DPPH radical scavenging assay (μmol TE/100 g DW) | ||
| 0 | 1.76 ± 0.03 b,* | 91.36 ± 4.31 b,* | 19.40 ± 0.27 a,* | 0.81 ± 0.08 b,* |
| 4 | 2.52 ± 0.24 a,* | 121.55 ± 6.93 a,* | 17.74 ± 0.74 b,* | 1.19 ± 0.08 a,* |
| Properties | Common Sourdough Bread | Fermented Water Sourdough Bread | Significance |
|---|---|---|---|
| pH | 4.12 ± 0.01 * | 4.02 ± 0.01 | t = 22.627; sig. = 0.000 |
| Specific volume (mL/g) | 5.22 ± 0.05 | 5.28 ± 0.11 | t = −0.873; sig. = 0.432 |
| Water activity | 0.82 ± 0.00 | 0.82 ± 0.00 | t = 0.000; sig. = 1.000 |
| Texture profile | |||
| Hardness (g) | 268.06 ± 17.81 | 248.54 ± 30.49 | t = 0.958; sig. = 0.392 |
| Chewiness | 205.69 ± 1.78 | 192.62 ± 17.87 | t = 1.261; sig. = 0.332 |
| Springiness (cm) | 0.92 ± 0.03 | 0.92 ± 0.02 | t = −0.085; sig. = 0.936 |
| Cohesiveness (g) | 0.84 ± 0.02 | 0.85 ± 0.02 | t = −0.387; sig. = 0.719 |
| Nutrients (per 100 g FW) | Common Sourdough Bread | Fermented Water Sourdough Bread | Significance |
|---|---|---|---|
| Energy (kcal) | 271.24 ± 0.66 | 273.75 ± 1.07 | t = −2.831; sig. = 0.105 |
| Moisture (%) | 33.76 ± 0.28 | 33.16 ± 0.45 | t = 1.620; sig. = 0.247 |
| Protein (N × 6.25) (g) | 9.73 ± 0.07 | 9.79 ± 0.10 | t = −0.697; sig. = 0.558 |
| Total fat (g) | 2.80 ± 0.11 | 2.83 ± 0.15 | t = −0.232; sig. = 0.838 |
| Total carbohydrate (g) | 51.78 ± 0.33 | 52.28 ± 0.70 | t = -0.912; sig. = 0.458 |
| Total dietary fiber (g) | 1.54 ± 0.00 | 1.73 ± 0.04 * | t = −6.333; sig. = 0.024 |
| Total sugar (g) | 8.94 ± 0.07 * | 8.09 ± 0.01 | t = 16.866; sig. = 0.003 |
| Ash (g) | 1.93 ± 0.02 | 1.94 ± 0.01 | t = −0.632; sig. = 0.592 |
| Storage Days | Common Sourdough Bread | Fermented Water Sourdough Bread | ||
|---|---|---|---|---|
| Total Aerobic Bacteria (Log CFU/g) | Yeast and Mold (Log CFU/g) | Total Aerobic Bacteria (Log CFU/g) | Yeast and Mold (Log CFU/g) | |
| 0 | 0.00 ± 0.00 d | Less than 1 | 2.57 ± 0.01 b,* | Less than 1 |
| 1 | 0.00 ± 0.00 d | Less than 1 | 2.37 ± 0.13 bc,* | Less than 1 |
| 3 | 2.98 ± 0.03 c,* | Less than 1 | 2.30 ± 0.14 c | Less than 1 |
| 5 | 3.23 ± 0.01 b | Less than 1 | 3.21 ± 0.02 a | Less than 1 |
| 7 | 4.26 ± 0.06 a,* | Less than 1 | 3.38 ± 0.02 a | Less than 1 |
| Common Sourdough Bread | ||||
|---|---|---|---|---|
| Storage Days | TPCs (mg GAE/g DW) | Antioxidant Activities | ||
| ORAC Assay (μmol TE/g DW) | FRAP Assay (μmol TE/g DW) | DPPH Radical Scavenging Assay (μmol TE/100 g DW) | ||
| 0 | 0.28 ± 0.02 a | 6.04 ± 0.60 ab | 0.98 ± 0.07 a | 0.16 ± 0.01 a |
| 1 | 0.27 ± 0.03 a | 6.44 ± 0.60 a | 0.83 ± 0.03 b | 0.12 ± 0.01 c |
| 3 | 0.24 ± 0.01 b | 6.10 ± 0.57 ab | 0.74 ± 0.04 c | 0.13 ± 0.01 b |
| 5 | 0.25 ± 0.01 b | 5.88 ± 0.29 ab | 0.70 ± 0.02 d | 0.14 ± 0.01 b |
| 7 | 0.23 ± 0.02 b | 5.79 ± 0.33 b | 0.75 ± 0.03 c | 0.12 ± 0.01 c |
| Fermented Water Sourdough Bread | ||||
| Storage Days | TPCs (mg GAE/g DW) | Antioxidant Activities | ||
| ORAC assay (μmol TE/g DW) | FRAP assay (μmol TE/g DW) | DPPH radical scavenging assay (μmol TE/100 g DW) | ||
| 0 | 0.68 ± 0.02 a* | 14.89 ± 1.44 c,* | 3.12 ± 0.22 a,* | 0.33 ± 0.03 a,* |
| 1 | 0.66 ± 0.03 ab,* | 26.73 ± 0.65 a,* | 3.08 ± 0.29 a,* | 0.29 ± 0.01 b,* |
| 3 | 0.64 ± 0.03 b* | 26.54 ± 2.63 a,* | 2.77 ± 0.18 bc,* | 0.29 ± 0.03 b,* |
| 5 | 0.61 ± 0.02 c,* | 23.45 ± 0.93 b,* | 2.66 ± 0.08 c,* | 0.29 ± 0.02 b,* |
| 7 | 0.57 ± 0.05 d,* | 15.26 ± 1.47 c,* | 2.90 ± 0.11 b,* | 0.27 ± 0.02 c,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supasil, R.; Suttisansanee, U.; Santivarangkna, C.; Tangsuphoom, N.; Khemthong, C.; Chupeerach, C.; On-nom, N. Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Foods 2022, 11, 2071. https://doi.org/10.3390/foods11142071
Supasil R, Suttisansanee U, Santivarangkna C, Tangsuphoom N, Khemthong C, Chupeerach C, On-nom N. Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Foods. 2022; 11(14):2071. https://doi.org/10.3390/foods11142071
Chicago/Turabian StyleSupasil, Ramita, Uthaiwan Suttisansanee, Chalat Santivarangkna, Nattapol Tangsuphoom, Chanakan Khemthong, Chaowanee Chupeerach, and Nattira On-nom. 2022. "Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae" Foods 11, no. 14: 2071. https://doi.org/10.3390/foods11142071
APA StyleSupasil, R., Suttisansanee, U., Santivarangkna, C., Tangsuphoom, N., Khemthong, C., Chupeerach, C., & On-nom, N. (2022). Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Foods, 11(14), 2071. https://doi.org/10.3390/foods11142071