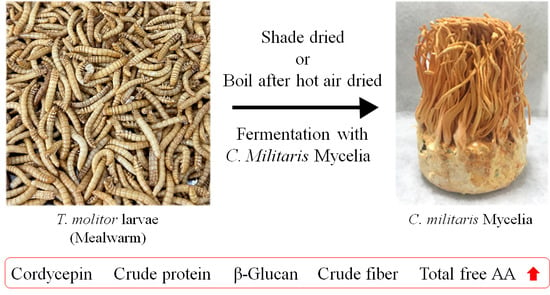
Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals and Standards
2.3. Fermentation
2.4. Proximate Composition Determination
2.5. β-Glucan Determination
2.6. Analysis of Free Amino Acids
2.7. Analysis of Cordycepin and Adenosine
2.8. Separation and Purification Cordycepin
2.9. Cell Culture and Viability Assay
2.10. Statistical analysis
3. Results
3.1. Proximate Composition and β-Glucan Contents
3.2. Free Amino Acids Content
3.3. Cordycepin and Adenosine Contents
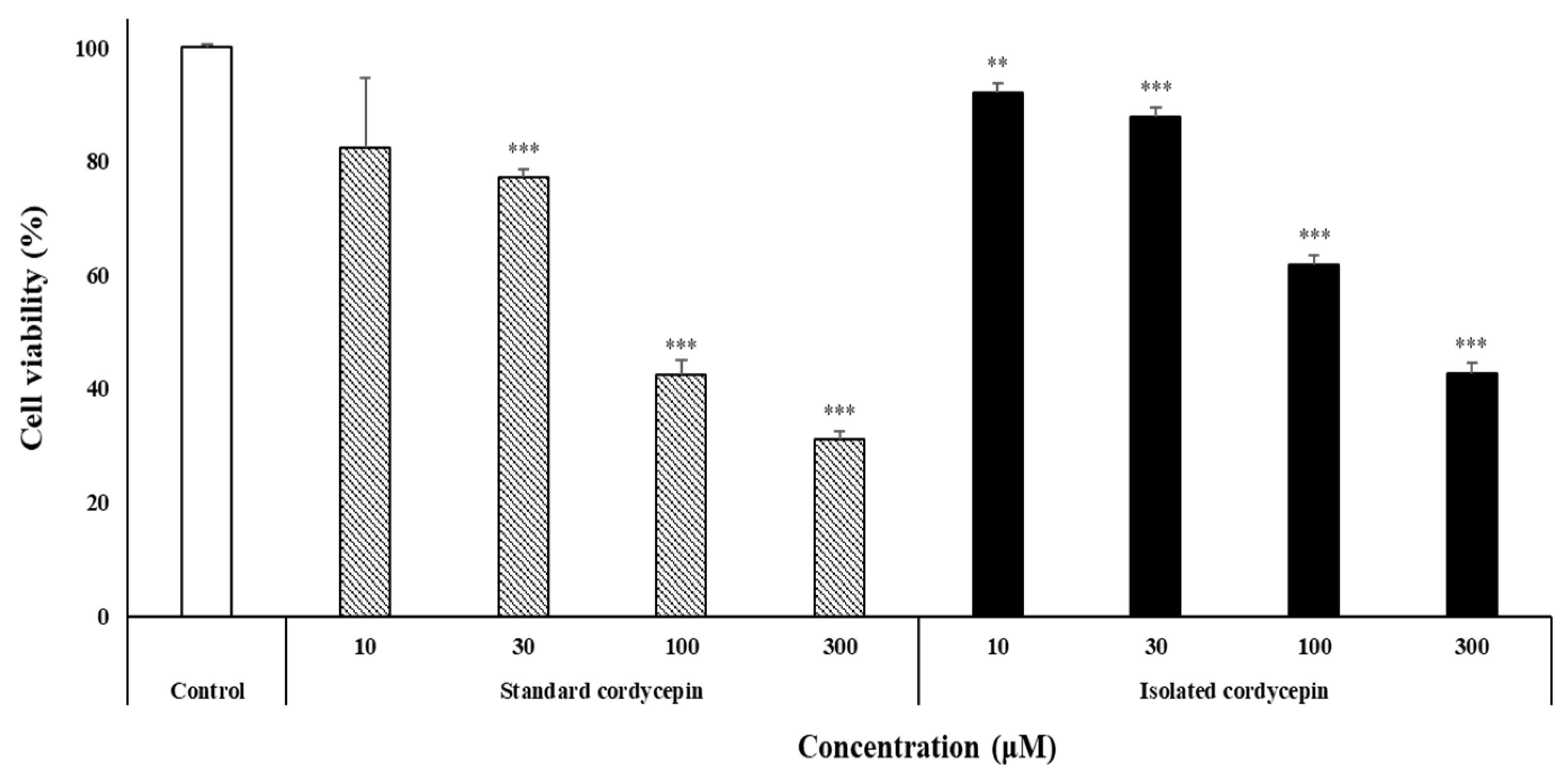
3.4. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Nat. C 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Itterbeeck, J.; Heetkamp, M.J.; Van Den Brand, H.; Van Loon, J.J.; Van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- McLeod, A. World Livestock 2011—Livestock in Food Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef]
- Dossey, A.T.; Morales-Ramos, J.A.; Rojas, M.G. Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient composition of selected whole invertebrates. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Hwang, J.; Choe, J.Y. How to enhance the image of edible insect restaurants: Focusing on perceived risk theory. Int. J. Hosp. Manag. 2020, 87, 102464. [Google Scholar] [CrossRef]
- Won, S.-Y.; Park, E.-H. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J. Ethnopharmacol. 2005, 96, 555–561. [Google Scholar] [CrossRef]
- Nam, K.S.; Jo, Y.S.; Kim, Y.H.; Hyun, J.W.; Kim, H.W. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 2001, 69, 229–237. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, H.-J.; Hwang, I.-H.; Kim, J.M.; Jo, E.; Lee, M.-G.; Jang, I.-S.; Joo, J.C. Cordyceps militaris extract inhibits the NF-κB pathway and induces apoptosis through MKK7-JNK signaling activation in TK-10 human renal cell carcinoma. Nat. Prod. Commun. 2018, 13, 465–470. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef]
- Cunningham, K.; Manson, W.; Spring, F.; Hutchinson, S. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Krishna, R.; Chouhan, G.K.; de Araujo Pereira, A.P.; Ade, A.B.; Prakash, S.; Verma, S.K.; Prasad, R.; Yadav, J.; Verma, J.P. Bio-fortification of minerals in crops: Current scenario and future prospects for sustainable agriculture and human health. Plant Growth Regul. 2022, 98, 5–22. [Google Scholar] [CrossRef]
- Marta, C.-K.; Beata, P. Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage. Molecules 2021, 26, 6063. [Google Scholar]
- Mrinal, S.; Rotimi, E.A.; Anil, K.P.; Tejpal, D. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar]
- Fox, P.F. Fermented Foods of the World. A Dictionary and Guide G. Campbell-Platt. Ir. J. Food Sci. Technol. 1987, 11, 199. [Google Scholar]
- An, B.; Sara, B.; Sorel, T.S.; Harshadrai, R.; Oliver, K.S.; Van, C.L. Effect of Blanching Plus Fermentation on Selected Functional Properties of Mealworm (Tenebrio molitor) Powders. Foods 2020, 9, 917. [Google Scholar]
- Gutarowska, B.; Żakowska, Z. Mathematical models of mycelium growth and ergosterol synthesis in stationary mould culture. Lett. Appl. Microbiol. 2009, 48, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Falanghe, H.; Smith, A.; Rackis, J. Production of fungal mycelial protein in submerged culture of soybean whey. Appl. Microbiol. 1964, 12, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Yabaya, A.; Ado, S. Mycelial protein production by Aspergillus niger using banana peels. Sci. World J. 2008, 3, 9–12. [Google Scholar] [CrossRef]
- Lu, R.; Conrad, P.; Yacine, H. Antitumor activity of mushroom polysaccharides. Food Funct. 2012, 3, 1118–1130. [Google Scholar]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Jang, H.W.; Choi, J.H.; Park, S.Y.; Park, B.R. Changes in Nutritional Composition of Gryllus bimaculatus Fermented by Bacillus sp. and Mycelium of Basidiomycetes. J. Korean Soc. Food Cult. 2019, 34, 785–792. [Google Scholar]
- Baek, L.-M.; Park, L.-Y.; Park, K.-S.; Lee, S.-H. Effect of Starter Cultures on the Fermentative. Korean J. Food Sci. Technol. 2008, 40, 400–405. [Google Scholar]
- Ionescu, R.E.; Fillit, C.; Jaffrezic-Renault, N.; Cosnier, S. Urease–gelatin interdigitated microelectrodes for the conductometric determination of protease activity. Biosens. Bioelectron. 2008, 24, 489–492. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007, 31, 266–287. [Google Scholar] [CrossRef]
- Wen, T.; Li, G.; Kang, J.; Kang, C.; Hyde, K.D. Optimization of solid-state fermentation for fruiting body growth and cordycepin production by Cordyceps militaris. Chiang Mai J. Sci. 2014, 41, 858–872. [Google Scholar]
- Li, J.F.; Hoang, V.A.; Ahn, J.C.; Yang, D.U.; Lee, D.W.; Yang, D.C. Isolation of New Strain of Cordyceps militaris HB8 and Optimal Condition for Production of Adenosine and Cordycepin in Fruit Body. Korean J. Plant Resour. 2020, 33, 696–706. [Google Scholar]
- Chiang, S.-S.; Liang, Z.-C.; Wang, Y.-C.; Liang, C.-H. Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps militaris. J. Food Compos. Anal. 2017, 60, 51–56. [Google Scholar] [CrossRef]
- Wei, C.; Yao, X.; Jiang, Z.; Wang, Y.; Zhang, D.; Chen, X.; Fan, X.; Xie, C.; Cheng, J.; Fu, J. Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol. Res. 2019, 144, 79–89. [Google Scholar] [CrossRef] [PubMed]
| Variables (%) | SD | 30BHAD | ||
|---|---|---|---|---|
| Unfermented | Fermented | Unfermented | Fermented | |
| Moisture | 3.5 ± 0.24 a1 | 9.15 ± 0.79 b | 2.25 ± 0.09 c | 8.87 ± 0.15 b |
| Crude protein | 44.01 ± 0.27 a | 59.34 ± 0.12 b | 47.49 ± 0.19 c | 64.24 ± 0.52 d |
| Crude fat | 32.02 ± 0.21 a | 18.54 ± 1.24 b | 36.9 ± 0.58 c | 32.02 ± 0.21 d |
| Crude ash | 3.21 ± 0.04 a | 3.84 ± 0.19 a | 2.63 ± 0.42 ab | 2.21 ± 0.15 b |
| Crude fiber | 5.54 ± 0.24 a | 9.48 ± 0.2 b | 12.97 ± 1.44 c | 15.62 ± 0.19 d |
| β-Glucan | 0.34 ± 0.17 a | 4.77 ± 0.26 b | 0.61 ± 0.29 a | 8.78 ± 0.33 c |
| Variables (mg/100 g) | SD | 30BHAD | ||
|---|---|---|---|---|
| Unfermented | Fermented | Unfermented | Fermented | |
| Aspartic acid | 91.22 ± 11.49 a1 | 84.8 ± 14.16 a | 28.72 ± 4.33 b | 149.61 ± 7.44 c |
| Glutamic acid | 224.22 ± 37.16 a | 292.31 ± 44.24 a | 143.68 ± 30.04 a | 588.89 ± 67.85 b |
| Serine | 81.47 ± 8.26 a | 59.53 ± 7.67 ab | 40.66 ± 7.24 b | 88.61 ± 11.54 a |
| Histidine | 165.14 ± 16.02 a | 81.7 ± 10.16 b | 52.18 ± 11.6 b | 128.87 ± 19.16 a |
| Glycine | 48.02 ± 3.56 a | 25.04 ± 2.94 b | 21.45 ± 2.95 b | 36.38 ± 5.56 c |
| Threonine | 77.35 ± 8.61 a | 52.28 ± 5.87 b | 38.44 ± 6.85 b | 112.77 ± 9.37 c |
| Arginine | 456.76 ± 42.2 a | 385.09 ± 35.44 a | 172.39 ± 32.27 b | 602.94 ± 85.19 c |
| Alanine | 239.22 ± 31.98 a | 56.68 ± 13.94 b | 122.08 ± 25.07 b | 98.04 ± 38 b |
| Tyrosine | 308.19 ± 42.63 a | 115.27 ± 12.17 b | 60.27 ± 11.24 b | 191.28 ± 24.32 c |
| Valine | 189.16 ± 30.24 a | 113.28 ± 11.29 b | 91.54 ± 16.9 b | 221.11 ± 18.24 a |
| Methionine | 63.82 ± 5.39 a | 33.36 ± 5.16 b | 18.48 ± 2.09 c | 26.16 ± 1.9 bc |
| Phenylalanine | 120.66 ± 12.59 a | 49.37 ± 5.59 b | 51.32 ± 9.62 b | 102.14 ± 11.98 a |
| Isoleucine | 124.7 ± 16.84 a | 48.77 ± 5.07 b | 59.51 ± 10.6 b | 70.32 ± 4.85 b |
| Leucine | 143.62 ± 16.6 a | 57.47 ± 5.58 b | 91.01 ± 17.92 bc | 117.56 ± 16.43 ac |
| Lysine | 254.18 ± 70.69 a | 171.1 ± 4.31 a | 88.41 ± 33.91 a | 483.57 ± 114.86 b |
| Total free AA | 2587.73 ± 330.84 a | 1626.05 ± 160.85 b | 1080.14 ± 219.62 b | 3018.25 ± 419.4 a |
| Total EAA | 1138.64 ± 162.42 a | 607.33 ± 41.08 b | 490.88 ± 107.59 b | 1262.5 ± 188.49 a |
| Samples | Variables (mg/g) | ||
|---|---|---|---|
| Cordycepin | Adenosine | ||
| SD | Unfermented | ND 1 | ND |
| Fermented | 13.75 ± 0.03 a3 | 0.85 ± 0.03 a | |
| 30BHAD | Unfermented | ND | ND |
| Fermented | 9.19 ± 0.02 b | 1.66 ± 0.05 b | |
| CCMBR1 2 | Fruiting body | 0.78 ± 0.02 c | 2.77 ± 0.03 c |
| Substrate | 0.26 ± 0.01 d | 0.4 ± 0.01 d | |
| CCMBR2 | Fruiting body | 1.16 ± 0.02 e | 3.24 ± 0.03 e |
| Substrate | 0.63 ± 0.01 f | 0.23 ± 0.01 f | |
| CCMP | Fruiting body | 1.06 ± 0.04 g | 4.1 ± 0.09 g |
| Substrate | 6.45 ± 0.02 h | 1.7 ± 0.12 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, N.-I.; Mun, S.-K.; Im, S.-B.; Jang, H.-Y.; Jeong, H.-G.; Kang, K.-Y.; Park, K.-W.; Seo, K.-S.; Ban, S.-E.; Kim, K.-J.; et al. Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia. Foods 2022, 11, 2477. https://doi.org/10.3390/foods11162477
Ha N-I, Mun S-K, Im S-B, Jang H-Y, Jeong H-G, Kang K-Y, Park K-W, Seo K-S, Ban S-E, Kim K-J, et al. Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia. Foods. 2022; 11(16):2477. https://doi.org/10.3390/foods11162477
Chicago/Turabian StyleHa, Neul-I, Seul-Ki Mun, Seung-Bin Im, Ho-Yeol Jang, Hee-Gyeong Jeong, Kyung-Yun Kang, Kyung-Wuk Park, Kyoung-Sun Seo, Seung-Eon Ban, Kyung-Je Kim, and et al. 2022. "Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia" Foods 11, no. 16: 2477. https://doi.org/10.3390/foods11162477
APA StyleHa, N.-I., Mun, S.-K., Im, S.-B., Jang, H.-Y., Jeong, H.-G., Kang, K.-Y., Park, K.-W., Seo, K.-S., Ban, S.-E., Kim, K.-J., & Yee, S.-T. (2022). Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia. Foods, 11(16), 2477. https://doi.org/10.3390/foods11162477