A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets and SPWL Rearing
2.2. Growth Performance
2.3. Proximate Composition and Mineral Profile
2.4. FA Composition
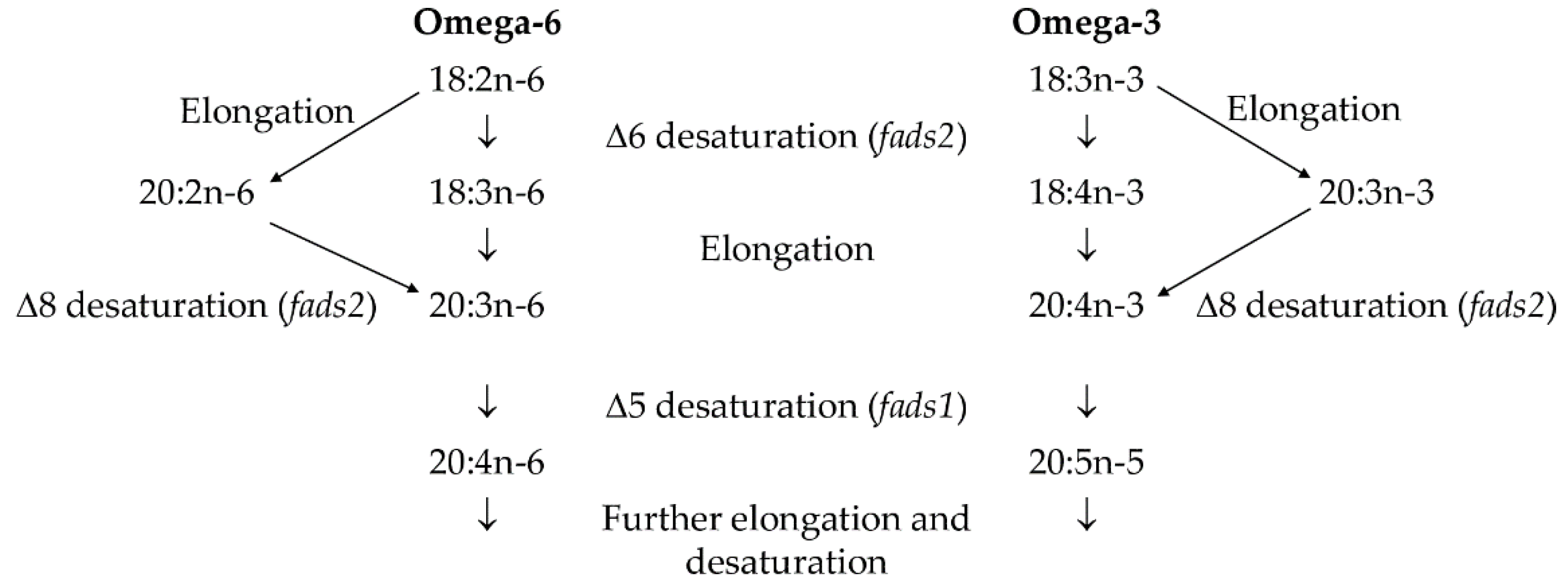
2.5. FA Metabolism Indices and fads2 Gene Expression of SPWL
2.6. Cholesterol Content
2.7. Amino Acid Profile
2.8. Statistical Analysis
3. Results and Discussion
3.1. Basic Composition and FA Profile of Diets
3.2. Growth Performance of SPWL
3.3. Proximate Composition, Cholesterol Content, and Mineral Profiles of SPWL
3.4. FA Composition and Health Promoting Indices of SPWL
3.5. FA Metabolic Indices and Δ6 Desaturase (fads2) Gene Expression
3.6. Amino Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Lucas, A.J.; de Oliveira, L.M.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of Mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Chinarak, K.; Chaijan, M.; Panpipat, W. Farm-raised sago palm weevil (Rhynchophorus ferrugineus) larvae: Potential and challenges for promising source of nutrients. J. Food Compos. Anal. 2020, 92, 103542. [Google Scholar] [CrossRef]
- Chinarak, K.; Panpipat, W.; Summpunn, P.; Panya, A.; Phonsatta, N.; Cheong, L.Z.; Chaijan, M. Insights into the effects of dietary supplements on the nutritional composition and growth performance of sago palm weevil (Rhynchophorus ferrugineus) larvae. Food Chem. 2021, 363, 130279. [Google Scholar] [CrossRef] [PubMed]
- Chinarak, K.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.Z.; Chaijan, M. Improved long-chain omega-3 polyunsaturated fatty acids in sago palm weevil (Rhynchophorus ferrugineus) larvae by dietary fish oil supplementation. Food Chem. 2022, 393, 133354. [Google Scholar] [CrossRef]
- Hussein, M.; Pillai, V.V.; Goddard, J.M.; Park, H.G.; Kothapalli, K.S.; Ross, D.A.; Selvaraj, V. Sustainable production of housefly (Musca domestica) larvae as a protein-rich feed ingredient by utilizing cattle manure. PLoS ONE 2017, 12, e0171708. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; Ma, T.; Wang, Z.; TanTai, W.; Nie, H.; Guo, Y.; Wang, F.; Fan, Y. Effects of perilla frutescens seed supplemented to diet on fatty acid composition and lipogenic gene expression in muscle and liver of Hu lambs. Livest. Sci. 2018, 211, 21–29. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gasco, L.; Brugiapaglia, A.; Gai, F. Effects of perilla (Perilla frutescens L.) seeds supplementation on performance, carcass characteristics, meat quality and fatty acid composition of rabbits. Livest. Sci. 2011, 138, 118–124. [Google Scholar] [CrossRef]
- Hadi, R.F.; Sudiyono; Jannah, S.N.; Indriyani, W. The effect of enriched perilla seed (Perilla frutescens L.) in the diets on percentage of carcass and non-carcass, chemical quality, and levels of α-linoleic acid (ALA) of meat ducks. AIP Conf. Proc. 2019, 2199, 050008. [Google Scholar]
- Sinclair, A.J.; Attar-Bashi, N.M.; Li, D. What is the role of α-linolenic acid for mammals? Lipids 2002, 37, 1113–1123. [Google Scholar] [CrossRef]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Sharma, S.; Baker, E.; Hawkins, W.; van der Merwe, M.; Puppa, M.J. Delta-6-desaturase (FADS2) inhibition and omega-3 fatty acids in skeletal muscle protein turnover. Biochem. Biophys. Rep. 2019, 18, 100622. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Kothapalli, K.S.; Reardon, H.T.; Lawrence, P.; Qian, S.B.; Brenna, J.T. A novel FADS1 isoform potentiates FADS2-mediated production of eicosanoid precursor fatty acids. J. Lipid Res. 2012, 53, 1502–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Kumar, F.; Tyagi, P.K.; Mir, N.A.; Dev, K.; Begum, J.; Biswas, A.; Sheikh, S.A.; Tyagi, P.K.; Sharma, D.; Sahu, B.; et al. Dietary flaxseed and turmeric is a novel strategy to enrich chicken meat with long chain ω-3 polyunsaturated fatty acids with better oxidative stability and functional properties. Food Chem. 2020, 305, 125458. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Li, Z.; Fan, Z.; Qin, M.; Yu, X.; Li, L. Effects of dietary addition of Perilla frutescens seeds on the content of polyunsaturated fatty acids in egg yolk of Gallus domesticus. Pak. J. Zool. 2021, 54, 161–166. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Dönmez, A.A. Composition of the essential oil of Perilla frutescens (L.) Britton from Turkey. Flavour Fragr. J. 2003, 18, 122–123. [Google Scholar] [CrossRef]
- Duarte, S.; Limão, J.; Barros, G.; Bandarra, N.M.; Roseiro, L.C.; Gonçalves, H.; Martins, L.L.; Mourato, M.P.; Carvalho, M.O. Nutritional and chemical composition of different life stages of Tribolium castaneum (Herbst). J. Stored Prod. Res. 2021, 93, 101826. [Google Scholar] [CrossRef]
- Mba, A.R.F.; Kansci, G.; Viau, M.; Rougerie, R.; Genot, C. Edible caterpillars of Imbrasia truncata and Imbrasia epimethea contain lipids and proteins of high potential for nutrition. J. Food Compos. Anal. 2019, 79, 70–79. [Google Scholar]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Araújo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Santos, E.M. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Batkowska, J.; Drabik, K.; Brodacki, A.; Czech, A.; Adamczuk, A. Fatty acids profile, cholesterol level and quality of table eggs from hens fed with the addition of linseed and soybean oil. Food Chem. 2021, 334, 127612. [Google Scholar] [CrossRef] [PubMed]
- Panpipat, W.; Xu, X.; Guo, Z. Towards a commercially potential process: Enzymatic recovery of phytosterols from plant oil deodoriser distillates mixture. Process Biochem. 2012, 47, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Panpipat, W.; Dong, M.; Xu, X.; Guo, Z. Thermal properties and nanodispersion behavior of synthesized β-sitosteryl acyl esters: A structure–activity relationship study. J. Colloid Interface Sci. 2013, 407, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zampelas, A.; Magriplis, E. New insights into cholesterol functions: A friend or an enemy? Nutrients 2019, 11, 1645. [Google Scholar] [CrossRef] [Green Version]
- Chaijan, M.; Panpipat, W. Removal of lipids, cholesterol, nucleic acids and haem pigments during production of protein isolates from broiler meat using pH-shift processes. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W. Chemical compositions of the roes from skipjack, tongol and bonito. Food Chem. 2011, 124, 1328–1334. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants—A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- World Health Organization (WHO). Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland; Food and Agricultural Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Chakravorty, J.; Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of Chondacris rosea and Brachytrupes orientalis: Two common insects used as food by tribes of Arunachal Pradesh, India. J. Asia Pac. Entomol. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- dos Santos Aguilar, J.G. An overview of lipids from insects. Biocatal. Agric. Biotechnol. 2021, 33, 101967. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Starčević, K.; Gavrilović, A.; Gottstein, Ž.; Mašek, T. Influence of substitution of sunflower oil by different oils on the growth, survival rate and fatty acid composition of Jamaican field cricket (Gryllus assimilis). Anim. Feed Sci. Technol. 2017, 228, 66–71. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.L.; Lognay, G.; Francis, F.; Megido, R.C. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- O’Hagan, D. Biosynthesis of fatty acid and polyketide metabolites. Nat. Prod. Rep. 1995, 12, 1–32. [Google Scholar] [CrossRef]
- Cui, J.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Δ6 fatty acid desaturases in polyunsaturated fatty acid biosynthesis: Insights into the evolution, function with substrate specificities and biotechnological use. Appl. Microbiol. Biotechnol. 2020, 104, 9947–9963. [Google Scholar] [CrossRef]
- Mattioli, S.; Paci, G.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Mancini, S. Former foodstuff in mealworm farming: Effects on fatty acids profile, lipid metabolism and antioxidant molecules. LWT 2021, 147, 111644. [Google Scholar] [CrossRef]
- Botton, V.; Chiarello, L.M.; Klunk, G.A.; Marin, D.; Curbani, L.; Gonçalves, M.J.; Vitorino, M.D. Evaluation of nutritional composition and ecotoxicity of the stick insect Cladomorphus phyllinum. Eur. Food Res. Technol. 2021, 247, 605–611. [Google Scholar] [CrossRef]
- Atowa, C.O.; Okoro, B.C.; Umego, E.C.; Atowa, A.O.; Emmanuel, O.; Ude, V.C.; Ugbogu, E.A. Nutritional values of Zonocerus variegatus, Macrotermes bellicosus and Cirina forda insects: Mineral composition, fatty acids and amino acid profiles. Sci. Afr. 2021, 12, e00798. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Homolková, D.; Božik, M.; Plachý, V.; Vrabec, V. Effect of developmental stage on the nutritional value of edible insects. A case study with Blaberus craniifer and Zophobas morio. J. Food Compos. Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Köhler, R.; Irias-Mata, A.; Ramandey, E.; Purwestri, R.; Biesalski, H.K. Nutrient composition of the Indonesian sago grub (Rhynchophorus bilineatus). Int. J. Trop. Insect Sci. 2020, 40, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Bunda, M.G.; Tumbokon, B.L.; Serrano Jr, A.E. Composition, chemical score (CS) and essential amino acid index (EAAI) of the crinkle grass Rhizoclonium sp. as ingredient for aquafeeds. Aquac. Aquar. Conserv. Legis. 2015, 8, 411–420. [Google Scholar]
- Food and Agriculture Organization of the United Nations (Rome). Dietary Protein Quality Evaluation in Human Nutrition. In Report of an FAO Expert Consultation; FAO Food and Nutrition Paper: Auckland, New Zealand, 31 March–2 April 2011.

| Ingredients | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| Ground sago palm trunk (g) | 1000 | 667 | 667 | 667 | 667 | 667 | 667 |
| Pig feed (g) | - | 333 | - | - | - | - | - |
| Rice bran (g) | - | - | 166.5 | 151.5 | 131.5 | 91.5 | 66.5 |
| Soybean (g) | - | - | 166.5 | 151.5 | 131.5 | 91.5 | 66.5 |
| Perilla seed (g) | - | - | 0 | 30 | 70 | 150 | 200 |
| Proximate Composition | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| Moisture (g/100 g) | 68.9 ± 0.8a | 51.9 ± 2.3e | 58.1 ± 0.1b | 57.1 ± 0.1b | 54.9 ± 0.3c | 52.9 ± 0.8de | 53.9 ± 0.9cd |
| Protein (g/100 g, dw) | 1.1 ± 0.1c | 10.8 ± 0.5b | 10.4 ± 0.5b | 10.7 ± 0.3b | 11.7 ± 0.3a | 10.9 ± 0.1b | 11.7 ± 0.1a |
| Lipid (g/100 g, dw) | 0.9 ± 0.2e | 2.0 ± 0.0e | 5.7 ± 1.2d | 6.8 ± 0.8d | 12.8 ± 0.4c | 18.3 ± 2.3b | 20.2 ± 0.5a |
| Ash (g/100 g, dw) | 4.1 ± 0.0e | 8.2 ± 0.2a | 5.9 ± 0.1c | 6.7 ± 0.9b | 5.3 ± 0.1cd | 4.7 ± 0.0de | 4.4 ± 0.1e |
| Carbohydrate (g/100 g, dw) | 93.9 ± 0.2a | 79.1 ± 0.5b | 78.0 ± 1.5bc | 75.8 ± 1.8c | 70.2 ± 0.8d | 66.2 ± 2.4e | 63.7 ± 0.4f |
| Fatty Acid (% Total Lipid) | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| C12:0 | 1.27 ± 0.27a | 0.35 ± 0.02b | 0.27 ± 0.06b | 0.31 ± 0.05b | 0.25 ± 0.06b | 0.24 ± 0.02b | 0.25 ± 0.03b |
| C14:0 | 0.86 ± 0.13b | 5.11 ± 0.17a | 0.12 ± 0.01c | 0.12 ± 0.01c | 0.14 ± 0.05c | 0.11 ± 0.04c | 0.11 ± 0.03c |
| C14:1 | 1.97 ± 0.17a | 1.86 ± 0.07a | 0.63 ± 0.02b | 0.53 ± 0.03bc | 0.43 ± 0.04c | 0.40 ± 0.06c | 0.39 ± 0.04c |
| C15:0 | 0.44 ± 0.02a | 0.11 ± 0.02b | 0.08 ± 0.02c | 0.08 ± 0.01c | 0.07 ± 0.01c | 0.07 ± 0.01c | 0.05 ± 0.02c |
| C15:1 (cis-10) | 0.36 ± 0.25a | 0.18 ± 0.04a | 0.13 ± 0.02a | 0.19 ± 0.05a | 0.16 ± 0.05a | 0.14 ± 0.06a | 0.12 ± 0.03a |
| C16:0 | 34.16 ± 0.8a | 21.92 ± 0.13c | 23.20 ± 0.16b | 19.19 ± 0.41d | 15.89 ± 0.48e | 13.17 ± 0.38f | 12.65 ± 0.85f |
| C16:1 (cis-9) | 0.82 ± 0.26a | 0.42 ± 0.14b | 0.28 ± 0.04bc | 0.21 ± 0.05bc | 0.20 ± 0.02bc | 0.14 ± 0.03c | 0.14 ± 0.03c |
| C17:0 | 0.50 ± 0.03a | 0.12 ± 0.01b | 0.09 ± 0.01c | 0.08 ± 0.00c | 0.07 ± 0.01c | 0.07 ± 0.00c | 0.08 ± 0.02c |
| C17:1 (cis-10) | 0.23 ± 0.04a | 0.02 ± 0.01b | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.04 ± 0.03b | 0.03 ± 0.03b |
| C18:0 | 9.66 ± 0.58a | 3.30 ± 0.08b | 3.58 ± 0.11b | 3.55 ± 0.08b | 3.70 ± 0.08b | 3.57 ± 0.01b | 3.52 ± 0.15b |
| C18:1 (cis-9) | 13.25 ± 0.63f | 24.70 ± 0.3c | 35.49 ± 0.38a | 29.82 ± 0.19b | 21.59 ± 0.46d | 15.10 ± 0.23e | 13.22 ± 0.21f |
| C18:2 (all-cis-9,12) | 5.38 ± 0.49g | 36.67 ± 0.36a | 31.59 ± 0.54b | 28.84 ± 0.44c | 25.07 ± 0.23d | 21.21 ± 0.23e | 19.64 ± 0.63f |
| C18:3 (all-cis-cis-6,9,12) | 0.21 ± 0.12a | 0.04 ± 0.04b | 0.04 ± 0.03b | 0.10 ± 0.00b | 0.05 ± 0.02b | 0.02 ± 0.03b | 0.05 ± 0.05b |
| C18:3 (all-cis-cis-9,12, 15) | 1.81 ± 0.18e | 1.38 ± 0.01e | 1.64 ± 0.05e | 14.59 ± 0.41d | 30.38 ± 0.33c | 43.83 ± 0.55b | 47.42 ± 0.83a |
| C20:0 | 0.36 ± 0.09b | 0.26 ± 0.04cd | 0.46 ± 0.02a | 0.37 ± 0.02b | 0.31 ± 0.01bc | 0.21 ± 0.04d | 0.22 ± 0.03d |
| C20:3 (all-cis-8,11,14) | 0.31 ±0.24a | 0.04 ± 0.03b | 0.04 ± 0.02b | 0.03 ± 0.02b | 0.04 ± 0.01b | 0.04 ± 0.02b | 0.02 ± 0.00b |
| C20:3 (all-cis-11,14,17) | 0.57 ± 0.09a | 0.08 ± 0.06bc | 0.02 ± 0.01c | 0.02 ± 0.01c | 0.04 ± 0.01c | 0.18 ± 0.14b | 0.10 ± 0.05bc |
| C20:4n6 | 0.17 ± 0.13a | 0.02 ± 0.01b | 0.04 ± 0.02b | 0.02 ± 0.03b | 0.01 ± 0.00b | 0.01 ± 0.01b | 0.02 ± 0.01b |
| C20:5 (all -cis-5,8,11,14,17) | 0.10 ± 0.01a | 0.05 ± 0.04b | 0.03 ± 0.02b | 0.04 ± 0.01b | 0.01 ± 0.01b | 0.02 ± 0.02b | 0.01 ± 0.01b |
| C22:2 (all-cis-13,16) | 0.48 ± 0.01a | 0.06 ± 0.04b | 0.01 ± 0.01b | 0.04 ± 0.03b | 0.10 ± 0.11b | 0.10 ± 0.10b | 0.08 ± 0.04b |
| C22:6 (all-cis-4,7,10,13,16,19) | 0.11 ± 0.07a | 0.07 ± 0.04ab | 0.02 ± 0.01b | 0.03 ± 0.00b | 0.04 ± 0.01b | 0.04 ± 0.01b | 0.05 ± 0.02b |
| Other | 26.96 ± 1.69a | 3.22 ± 0.07b | 2.22 ± 0.36bc | 1.80 ± 0.23c | 1.44 ± 0.24c | 1.29 ± 0.18c | 1.84 ± 0.47c |
| ∑SFA | 47.26 ± 1.13a | 31.18 ± 0.23b | 27.81 ± 0.15c | 23.69 ± 0.54d | 20.43 ± 0.40e | 17.44 ± 0.40f | 16.87 ± 0.98f |
| ∑MUFA | 16.64 ± 0.45e | 27.18 ± 0.19c | 36.55 ± 0.34a | 30.77 ± 0.30b | 22.40 ± 0.43d | 15.82 ± 0.25f | 13.90 ± 0.13g |
| ∑PUFA | 8.96 ± 0.34d | 38.50 ± 0.29c | 37.90 ± 8.18c | 48.95 ± 9.77b | 60.06 ± 7.82a | 66.90 ± 0.48a | 66.94 ± 1.20a |
| ∑n3-PUFA | 2.60 ± 0.20f | 1.58 ± 0.13e | 1.71 ± 0.08f | 14.69 ± 0.42d | 30.48 ± 0.29c | 44.08 ± 0.43b | 47.58 ± 0.86a |
| ∑n6-PUFA | 6.07 ± 0.22g | 36.78 ± 0.30a | 31.70 ± 0.57b | 29.00 ± 0.45c | 25.16 ± 0.25d | 21.27 ± 0.2e | 19.73 ± 0.66f |
| n6/n3 | 2.34 ± 0.19c | 23.35 ± 2.02a | 18.60 ± 0.79b | 1.98 ± 0.04cd | 0.83 ± 0.01de | 0.48 ± 0.01de | 0.41 ± 0.01e |
| Biometric Parameter | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| Survival rate (%) | 90.8 ± 1.2ab | 92.6 ± 2.3a | 84.6 ± 2.6c | 86.6 ± 0.2bc | 85.9 ± 0.6c | 85.8 ± 3.0c | 83.8 ± 1.8c |
| Live weight (g) | 2.6 ± 1.2c | 5.3 ± 0.4ab | 5.3 ± 0.6ab | 5.8 ± 0.6a | 5.5 ± 0.6a | 5.4 ± 0.4ab | 5.0 ± 0.3b |
| Dry weight (g) | 0.9 ± 0.1b | 1.7 ± 0.1a | 1.6 ± 0.2a | 1.9 ± 0.2a | 1.7 ± 0.2a | 1.9 ± 0.2a | 1.8 ± 0.2a |
| Dry matter content (%) | 26.3 ± 2.3c | 30.5 ± 2.9b | 35.5 ± 1.8a | 33.1 ± 2.3ab | 33.7 ± 1.8ab | 35.2 ± 1.4a | 37.0 ± 2.2a |
| Condition factor | 8.1 ± 2.8d | 9.7 ± 2.5c | 11.3 ± 2.8abc | 10.9 ± 1.7bc | 12.6 ± 2.2a | 10.9 ± 1.7bc | 11.6 ± 2.9ab |
| Growth rate (g/day) | 0.1 ± 0.1b | 0.2 ± 0.0a | 0.1 ± 0.0c | 0.1 ± 0.0c | 0.1 ± 0.0c | 0.1 ± 0.0c | 0.1 ± 0.0c |
| Appearance |  |  |  |  |  |  |  |
| Amount (mg/g Sample) | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| Proximate composition | |||||||
| Moisture (g/100 g) | 73.7 ± 2.3a | 69.5 ± 2.9b | 64.5 ± 1.8c | 66.9 ± 2.3bc | 66.3 ± 1.8bc | 64.8 ± 1.4c | 62.9 ± 2.2c |
| Protein (g/100 g, dw) | 22.3 ± 0.3c | 18.6 ± 0.2d | 24.5 ± 0.2b | 26.7 ± 0.7a | 22.6 ± 0.4c | 22.7 ± 0.6c | 22.1 ± 0.3c |
| Lipid (g/100 g, dw) | 34.9 ± 0.9d | 55.5 ± 2.6ab | 51.1 ± 3.3c | 51.0 ± 2.0c | 54.6 ± 2.4abc | 56.4 ± 3.0a | 51.8 ± 1.0bc |
| Ash (g/100 g, dw) | 4.2 ± 0.1a | 2.8 ± 0.4b | 3.8 ± 0.8ab | 2.9 ± 0.1b | 2.9 ± 0.1b | 2.8 ± 0.2b | 3.0 ± 1.2b |
| Carbohydrate (g/100 g, dw) | 38.7 ± 0.9a | 23.1 ± 2.2b | 20.6 ± 3.9b | 19.5 ± 2.4b | 19.9 ± 2.8b | 18.2 ± 3.3b | 23.1 ± 1.8b |
| Cholesterol (mg/100 g) | 246.2 ± 1.3e | 350.8 ± 13.2a | 288.7 ± 2.8c | 303.3 ± 3.1b | 261.0 ± 3.0d | 297.9 ± 2.5bc | 309.1 ± 12.3b |
| Mineral (mg/kg) | |||||||
| Potassium (K) | 9265 ± 286a | 6487 ± 45e | 7039 ± 110c | 7988 ± 100b | 6828 ± 137cd | 6421 ± 110e | 6673 ± 31de |
| Phosphorus (P) | 3563 ± 112bc | 2926 ± 55f | 3639 ± 24b | 4273 ± 40a | 3447 ± 44d | 3477 ± 64cd | 3292 ± 22e |
| Magnesium (Mg) | 1869 ± 49b | 132 1± 22f | 1669 ± 16c | 1985 ± 10a | 1631 ± 16c | 1428 ± 31d | 1372 ± 6e |
| Sodium (Na) | 1351 ± 37a | 986 ± 37e | 1010 ± 17cd | 1182 ± 11b | 1035 ± 16c | 1025 ± 11cd | 915 ± 29d |
| Calcium (Ca) | 699 ± 21a | 441 ± 18f | 625 ± 12b | 674 ± 4a | 549 ± 5c | 514 ± 8d | 478 ± 7e |
| Zinc (Zn) | 99.4 ± 2.2b | 101.9 ± 1.3ab | 85.3 ± 1.8c | 104.6 ± 2.0a | 88.3 ± 1.5c | 81.8 ± 2.3d | 87.2 ± 0.4c |
| Manganese (Mn) | 49.9 ± 1.5a | 13.6 ± 0.4c | 12.5 ± 0.4cd | 16.7 ± 0.3b | 10.4 ± 0.2f | 11.0 ± 0.6f | 11.7 ± 0.7de |
| Iron (Fe) | 14.2 ± 0.2d | 15.4 ± 0.4c | 18.8 ± 0.3a | 19.0 ± 1.1a | 16.8 ± 1.0b | 16.2 ± 0.6bc | 15.8 ± 0.1bc |
| Copper (Cu) | 10.2 ± 0.6d | 26.1 ± 0.6a | 10.8 ± 0.6cd | 12.3 ± 0.5b | 11.5 ± 0.4bc | 11.6 ± 0.4bc | 12.2 ± 0.2b |
| Fatty Acid (% Total Lipid) | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.16 ± 0.03a | 0.15 ± 0.04a | 0.18 ± 0.04a | 0.14 ± 0.02a | 0.14 ± 0.03a | 0.14 ± 0.00a | 0.13 ± 0.01a |
| C14:0 | 1.42 ± 0.15c | 1.69 ± 0.10ab | 1.76 ± 0.11ab | 1.68 ± 0.08ab | 1.88 ± 0.12a | 1.76 ± 0.11ab | 1.61 ± 0.06b |
| C14:1 | 0.06 ± 0.03a | 0.03 ± 0.00a | 0.11 ± 0.05a | 0.05 ± 0.05a | 0.12 ± 0.08a | 0.09 ± 0.07a | 0.05 ± 0.03a |
| C15:0 | 0.06 ± 0.02a | 0.07 ± 0.01a | 0.09 ± 0.02a | 0.07 ± 0.00a | 0.08 ± 0.03a | 0.09 ± 0.02a | 0.08 ± 0.00a |
| C15:1 (cis-10) | 0.23 ± 0.18a | 0.26 ± 0.08a | 0.14 ± 0.10a | 0.42 ± 0.10a | 0.27 ± 0.19a | 0.47 ± 0.06a | 0.25 ± 0.04a |
| C16:0 | 45.05 ± 0.27a | 43.99 ± 0.40b | 35.47 ± 0.60f | 41.77 ± 0.16c | 38.23 ± 0.34d | 37.24 ± 0.33e | 38.99 ± 0.82d |
| C16:1 (cis-9) | 7.70 ± 0.40d | 10.21 ± 0.40b | 8.34 ± 0.36c | 5.72 ± 0.15e | 10.77 ± 0.36a | 5.82 ± 0.17e | 5.46 ± 0.15e |
| C17:0 | 0.04 ± 0.01b | 0.07 ± 0.01a | 0.07 ± 0.01a | 0.09 ± 0.01a | 0.07 ± 0.01a | 0.09 ± 0.01a | 0.08 ± 0.01a |
| C17:1 (cis-10) | 0.09 ± 0.11a | 0.04 ± 0.02a | 0.05 ± 0.02a | 0.04 ± 0.04a | 0.04 ± 0.05a | 0.03 ± 0.02a | 0.03 ± 0.00a |
| C18:0 | 1.64 ± 0.28c | 1.28 ± 0.06d | 1.89 ± 0.23bc | 2.37 ± 0.05a | 1.80 ± 0.04c | 2.12 ± 0.04ab | 2.31 ± 0.17a |
| C18:1 (cis-9) | 40.28 ± 1.49a | 38.59 ± 0.54a | 40.40 ± 1.63a | 39.18 ± 0.39a | 38.75 ± 1.08a | 38.47 ± 0.50a | 34.92 ± 0.52b |
| C18:2 (all-cis-9,12) | 0.22 ± 0.06g | 11.64 ± 0.08f | 8.11 ± 0.29a | 5.65 ± 0.14d | 3.92 ± 0.08e | 6.28 ± 0.08b | 5.93 ± 0.14c |
| C18:3 (all-cis-cis -6,9,12) | 0.22 ± 0.07a | 0.10 ± 0.03a | 0.13 ± 0.13a | 0.06 ± 0.06a | 0.07 ± 0.08a | 0.04 ± 0.02a | 0.01 ± 0.01a |
| C18:3 (all-cis-cis -9,12, 15) | 0.08 ± 0.01e | 0.17 ± 0.02e | 0.83 ± 0.07d | 0.99 ± 0.05d | 1.90 ± 0.05c | 4.97 ± 0.06b | 8.40 ± 0.45a |
| C20:0 | 0.09 ± 0.05a | 0.08 ± 0.04a | 0.07 ± 0.04a | 0.08 ± 0.02a | 0.09 ± 0.02a | 0.06 ± 0.01a | 0.09 ± 0.00a |
| C20:3 (all-cis-8,11,14) | 0.22 ± 0.05a | 0.03 ± 0.02b | 0.12 ± 0.13ab | 0.03 ± 0.04b | 0.06 ± 0.04b | 0.04 ± 0.03b | 0.04 ± 0.01b |
| C20:3 (all-cis-11,14,17) | 0.07 ± 0.03a | 0.04 ± 0.02a | 0.06 ± 0.02a | 0.06 ± 0.02a | 0.06 ± 0.05a | 0.05 ± 0.04a | 0.03 ± 0.03a |
| C20:4n6 | 0.06 ± 0.01abc | 0.06 ± 0.02ab | 0.04 ± 0.02bc | 0.04 ± 0.01c | 0.07 ± 0.00a | 0.01 ± 0.00d | 0.01 ± 0.01d |
| C20:5 (all -cis-5,8,11,14,17) | 0.03 ± 0.02b | 0.02 ± 0.01b | 0.04 ± 0.00b | 0.02 ± 0.01b | 0.06 ± 0.00a | 0.03 ± 0.00b | 0.02 ± 0.01b |
| C22:2 (all-cis-13,16) | 0.04 ± 0.02a | 0.06 ± 0.04a | 0.06 ± 0.06a | 0.03 ± 0.01a | 0.05 ± 0.05a | 0.04 ± 0.04a | 0.04 ± 0.01a |
| C22:6 (all-cis-4,7,10,13,16,19) | 0.04 ± 0.01b | 0.05 ± 0.01b | 0.08 ± 0.02a | 0.06 ± 0.00b | 0.05 ± 0.02b | 0.03 ± 0.00b | 0.04 ± 0.00b |
| Other | 2.17 ± 0.60a | 1.39 ± 0.33a | 1.97 ± 0.54a | 1.44 ± 0.04a | 1.51 ± 0.19a | 2.15 ± 0.18a | 1.48 ± 0.56a |
| ∑SFA | 48.47 ± 0.41a | 47.33 ± 0.23b | 39.53 ± 0.60f | 46.20 ± 0.19c | 42.29 ± 0.39e | 41.51 ± 0.43e | 43.29 ± 0.82d |
| ∑MUFA | 48.37 ± 1.04b | 49.12 ± 0.42ab | 49.04 ± 1.36ab | 45.42 ± 0.38c | 49.96 ± 0.61a | 44.87 ± 0.49c | 40.71 ± 0.30d |
| ∑PUFA | 1.00 ± 0.12d | 2.41 ± 0.42d | 9.50 ± 0.51b | 7.23 ± 0.61c | 7.25 ± 1.73c | 12.81 ± 2.19a | 14.32 ± 0.10a |
| ∑n3-PUFA | 0.23 ± 0.04e | 0.27 ± 0.02e | 1.00 ± 0.08d | 1.12 ± 0.04d | 2.06 ± 0.11c | 5.08 ± 0.04b | 8.50 ± 0.45a |
| ∑n6-PUFA | 0.72 ± 0.08f | 1.83 ± 0.05e | 8.41 ± 0.50a | 5.79 ± 0.17c | 4.13 ± 0.03d | 6.37 ± 0.11b | 5.99 ± 0.16c |
| n6/n3 | 3.21 ± 0.73d | 6.71 ± 0.52b | 8.46 ± 0.23a | 5.15 ± 0.04c | 2.01 ± 0.13e | 1.25 ± 0.03f | 0.1 ± 0.02f |
| PUFA/SFA | 0.02 ± 0.00d | 0.05 ± 0.01d | 0.24 ± 0.01b | 0.16 ± 0.01c | 0.17 ± 0.04c | 0.31 ± 0.05a | 0.33 ± 0.01a |
| Atherogenicity index (IA) | 0.41 ± 0.14ab | 0.54 ± 0.12a | 0.32 ± 0.09b | 0.53 ± 0.04a | 0.38 ± 0.03ab | 0.38 ± 0.01ab | 0.46 ± 0.08ab |
| Thrombogenicity index (IT) | 0.75 ± 0.25ab | 0.97 ± 0.21a | 0.54 ± 0.15b | 0.90 ± 0.07a | 0.58 ± 0.06b | 0.48 ± 0.02b | 0.47 ± 0.10b |
| Amount (mg/g Sample) | C | PF | P0 | P3 | P7 | P15 | P20 |
|---|---|---|---|---|---|---|---|
| Essential amino acid (EAA) | |||||||
| Valine | 9.2 ± 0.2c | 10.5 ± 1.9bc | 10.7 ± 0.6b | 12.5 ± 0.3a | 11.4 ± 0.2ab | 10.1 ± 0.2bc | 10.7 ± 0.3b |
| Leucine | 13.0 ± 0.4d | 12.8 ± 0.6d | 15.3 ± 1.3bc | 17.9 ± 0.6a | 16.2 ± 0.1b | 14.2 ± 0.5c | 14.8 ± 0.4c |
| Isoleucine | 7.5 ± 0.2e | 8.0 ± 0.4de | 8.7 ± 0.5c | 10.0 ± 0.4a | 9.4 ± 0.2b | 8.2 ± 0.1cd | 8.6 ± 0.3c |
| Threonine | 4.8 ± 0.1a | 6.1 ± 2.5a | 5.6 ± 0.2a | 6.4 ± 0.2a | 5.9 ± 0.1a | 5.3 ± 0.1a | 5.3 ± 0.1a |
| Lysine | 44.4 ± 1.5ab | 28.4 ± 23.5b | 54.6 ± 4.8a | 59.9 ± 7.8a | 57.4 ± 0.9a | 48.6 ± 2.0a | 50.0 ± 1.6a |
| Histidine | 6.0 ± 0.1d | 6.4 ± 0.4cd | 6.9 ± 0.4bc | 7.8 ± 0.6a | 7.3 ± 0.1ab | 6.5 ± 0.2cd | 6.9 ± 0.2bc |
| Tryptophan | 0.1 ± 0.0d | 0.2 ± 0.0c | 0.2 ± 0.0b | 0.2 ± 0.0a | 0.2 ± 0.0b | 0.2 ± 0.0c | 0.2 ± 0.0b |
| Phenylalanine | 3.7 ± 0.1d | 4.0 ± 0.4cd | 4.5 ± 0.3b | 5.0 ± 0.1a | 4.6 ± 0.1b | 4.1 ± 0.1cd | 4.3 ± 0.1bc |
| Methionine | 1.5 ± 0.0de | 1.4 ± 0.1e | 1.8 ± 0.1abc | 2.0 ± 0.2a | 1.9 ± 0.0ab | 1.7 ± 0.0cd | 1.7 ± 0.0bc |
| Total EAA | 90.2 ± 2.6cd | 77.8 ± 18.5d | 108.2 ± 8.2ab | 121.7 ± 9.2a | 114.4 ± 1.3ab | 98.9 ± 2.9bc | 102.6 ± 3.0bc |
| Essential amino acid index (EAAI) | 44.5 ± 1.0c | 50.3 ± 5.3ab | 47.8 ± 2.8bc | 49.5 ± 2.1ab | 54.2 ± 0.9a | 47.9 ± 0.9bc | 51.4 ± 1.2ab |
| Biological value (BV) | 36.8 ± 1.1c | 43.2 ± 5.8ab | 40.4 ± 3.0bc | 42.3 ± 2.3ab | 47.4 ± 0.9a | 40.5 ± 0.9bc | 44.4 ± 1.3ab |
| Non-essential amino acid (non-EAA) | |||||||
| Tyrosine | 3.8 ± 0.1a | 5.7 ± 3.1a | 4.5 ± 0.1a | 5.0 ± 0.2a | 4.6 ± 0.2a | 4.2 ± 0.0a | 4.3 ± 0.0a |
| Alanine | 9.4 ± 0.2a | 11.1 ± 3.3a | 10.7 ± 0.6a | 12.3 ± 0.5a | 11.3 ± 0.4a | 10.1 ± 0.2a | 10.3 ± 0.2a |
| Glycine | 17.5 ± 0.4b | 15.9 ± 3.4b | 21.2 ± 1.4a | 23.6 ± 2.3a | 22.5 ± 0.5a | 20.5 ± 0.4a | 21.1 ± 0.5a |
| Serine | 3.2 ± 0.1a | 3.9 ± 1.1a | 3.6 ± 0.2a | 4.2 ± 0.1a | 3.8 ± 0.1a | 3.5 ± 0.1a | 3.5 ± 0.0a |
| Aspartic acid | 5.0 ± 0.2a | 8.6 ± 6.1a | 6.0 ± 0.2a | 6.7 ± 0.3a | 6.2 ± 0.3a | 5.6 ± 0.1a | 5.5 ± 0.1a |
| Hydroxyproline | 0.4 ± 0.0a | 0.3 ± 0.0a | 0.4 ± 0.0a | 0.4 ± 0.1a | 0.4 ± 0.0a | 0.4 ± 0.0a | 0.4 ± 0.0a |
| Proline | 13.5 ± 0.3bc | 11.1 ± 5.8c | 18.3 ± 1.0ab | 20.0 ± 4.7a | 19.7 ± 0.4a | 17.3 ± 0.2ab | 19.1 ± 0.4a |
| Glutamic acid | 9.7 ± 0.2a | 14.4 ± 8.2a | 10.9 ± 0.6a | 12.6 ± 0.7a | 11.7 ± 0.4a | 10.6 ± 0.2a | 10.6 ± 0.1a |
| Arginine | 10.0 ± 0.5c | 9.6 ± 0.9c | 11.3 ± 0.3b | 12.5 ± 0.9a | 10.6 ± 0.2bc | 9.6 ± 0.3c | 10.1 ± 0.1c |
| Ornithine | 24.0 ± 1.1b | 23.5 ± 4.3b | 29.5 ± 3.4a | 34.4 ± 4.4a | 34.7 ± 0.4a | 30.0 ± 1.6a | 31.5 ± 1.1a |
| Glutathione | 0.6 ± 0.0c | 0.7 ± 0.1b | 0.7 ± 0.0b | 0.8 ± 0.0a | 0.7 ± 0.0b | 0.6 ± 0.0c | 0.7 ± 0.0b |
| Cystine | 0.4 ± 0.0c | 0.4 ± 0.0bc | 0.5 ± 0.0a | 0.5 ± 0.0a | 0.5 ± 0.0ab | 0.4 ± 0.0c | 0.5 ± 0.0ab |
| Total non-EAA | 97.6 ± 2.8d | 105.1 ± 9.2cd | 117.6 ± 7.7bc | 132.9 ± 13.0a | 126.7 ± 2.4ab | 112.7 ± 2.7c | 117.5 ± 2.5bc |
| Total amino acid | 187.9 ± 5.4d | 183.0 ± 9.4d | 225.9 ± 15.8bc | 254.6 ± 22.0a | 241.1 ± 3.6ab | 211.6 ± 5.7c | 220.0 ± 5.3bc |
| Essential Amino Acid | C | PF | P0 | P3 | P7 | P15 | P20 | Reference Protein # |
|---|---|---|---|---|---|---|---|---|
| Valine | 41.1 ± 0.9c (1.03) * | 56.7 ± 10.1a (1.42) | 43.5 ± 2.6bc (1.09) | 47.0 ± 1.1bc (1.18) | 50.5 ± 1.1ab (1.26) | 44.6 ± 0.8bc (1.11) | 48.5 ± 1.2bc (1.21) | 40 |
| Leucine | 58.1 ± 1.9c (0.95) | 68.8 ± 3.0a (1.13) | 62.3 ± 5.3bc (1.02) | 67.2 ± 2.4ab (1.10) | 71.6 ± 0.6a (1.17) | 62.7 ± 2.1bc (1.03) | 66.9 ± 1.9ab (1.10) | 61 |
| Isoleucine | 33.4 ± 0.9d (1.11) | 42.8 ± 2.2a (1.43) | 35.3 ± 2.0cd (1.18) | 37.4 ± 1.3bc (1.25) | 41.5 ± 1.0a (1.38) | 36.2 ± 0.4c (1.21) | 38.8 ± 1.4b (1.29) | 30 |
| Threonine | 21.7 ± 0.4a (0.87) | 33.1 ± 13.5a (1.32) | 22.9 ± 1.0a (0.92) | 24.0 ± 0.7a (0.96) | 26.1 ± 0.6a (1.04) | 23.2 ± 0.5a (0.93) | 24.2 ± 0.4a (0.97) | 25 |
| Lysine | 199.3 ± 6.7a (4.15) | 225.7 ± 7.1a (4.70) | 222.7 ± 19.6a (4.64) | 224.7 ± 29.4a (4.68) | 253.5 ± 3.9a (5.28) | 214.3 ± 8.7a (4.46) | 226.5 ± 4.0a (4.72) | 48 |
| Histidine | 26.8 ± 0.3d (1.67) | 34.3 ± 2.4a (2.15) | 28.2 ± 1.7d (1.76) | 29.2 ± 2.1cd (1.82) | 32.4 ± 0.6ab (2.02) | 28.6 ± 0.8cd (1.79) | 31.2 ± 0.8bc (1.95) | 16 |
| Tryptophan | 0.6 ± 0.0d (0.10) | 0.8 ± 0.1a (0.13) | 0.7 ± 0.0c (0.11) | 0.7 ± 0.0c (0.11) | 0.7 ± 0.0bc (0.11) | 0.7 ± 0.0c (0.10) | 0.8 ± 0.0b (0.12) | 6.6 |
| Phenylalanine + Tyrosine | 33.9 ± 0.9a (0.83) | 52.2 ± 19.0a (1.27) | 36.9 ± 1.6a (0.90) | 37.8 ± 1.4a (0.92) | 40.5 ± 1.4a (0.99) | 36.4 ± 0.4a (0.89) | 38.6 ± 0.8a (0.94) | 41 |
| Methionine + Cysteine | 6.9 ± 0.2c (0.30) | 7.6 ± 0.3b (0.33) | 7.4 ± 0.6bc (0.32) | 7.4 ± 0.8bc (0.32) | 8.4 ± 0.1a (0.37) | 7.4 ± 0.1bc (0.32) | 7.9 ± 0.2ab (0.34) | 23 |
| Total EAA | 421.8 ± 11.9a (1.45) | 522.1 ± 50.3a (1.55) | 459.8 ± 33.8a (1.58) | 475.5 ± 35.0a (1.64) | 525.3 ± 6.6a (1.81) | 454.1 ± 13.1a (1.56) | 483.4 ± 13.6a (1.66) | 290.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinarak, K.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Foods 2022, 11, 2036. https://doi.org/10.3390/foods11142036
Chinarak K, Panpipat W, Panya A, Phonsatta N, Cheong L-Z, Chaijan M. A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Foods. 2022; 11(14):2036. https://doi.org/10.3390/foods11142036
Chicago/Turabian StyleChinarak, Khanittha, Worawan Panpipat, Atikorn Panya, Natthaporn Phonsatta, Ling-Zhi Cheong, and Manat Chaijan. 2022. "A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae" Foods 11, no. 14: 2036. https://doi.org/10.3390/foods11142036
APA StyleChinarak, K., Panpipat, W., Panya, A., Phonsatta, N., Cheong, L.-Z., & Chaijan, M. (2022). A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Foods, 11(14), 2036. https://doi.org/10.3390/foods11142036








