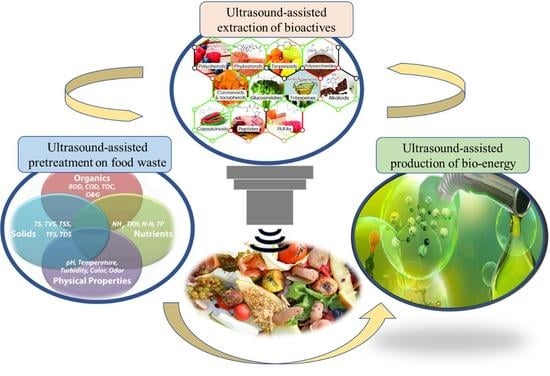
Ultrasonic Processing of Food Waste to Generate Value-Added Products
Abstract
:1. Introduction
2. The Influence of Ultrasound Pre-Treatment on Food Waste Materials
2.1. The Impact of Ultrasound on Organic Substance Solubilisation
2.2. The Influence of Ultrasound on Particle Size
2.3. The Effects of Ultrasound on Typical Polysaccharides
3. Ultrasound-Assisted Extraction of Bioactives from Food Waste
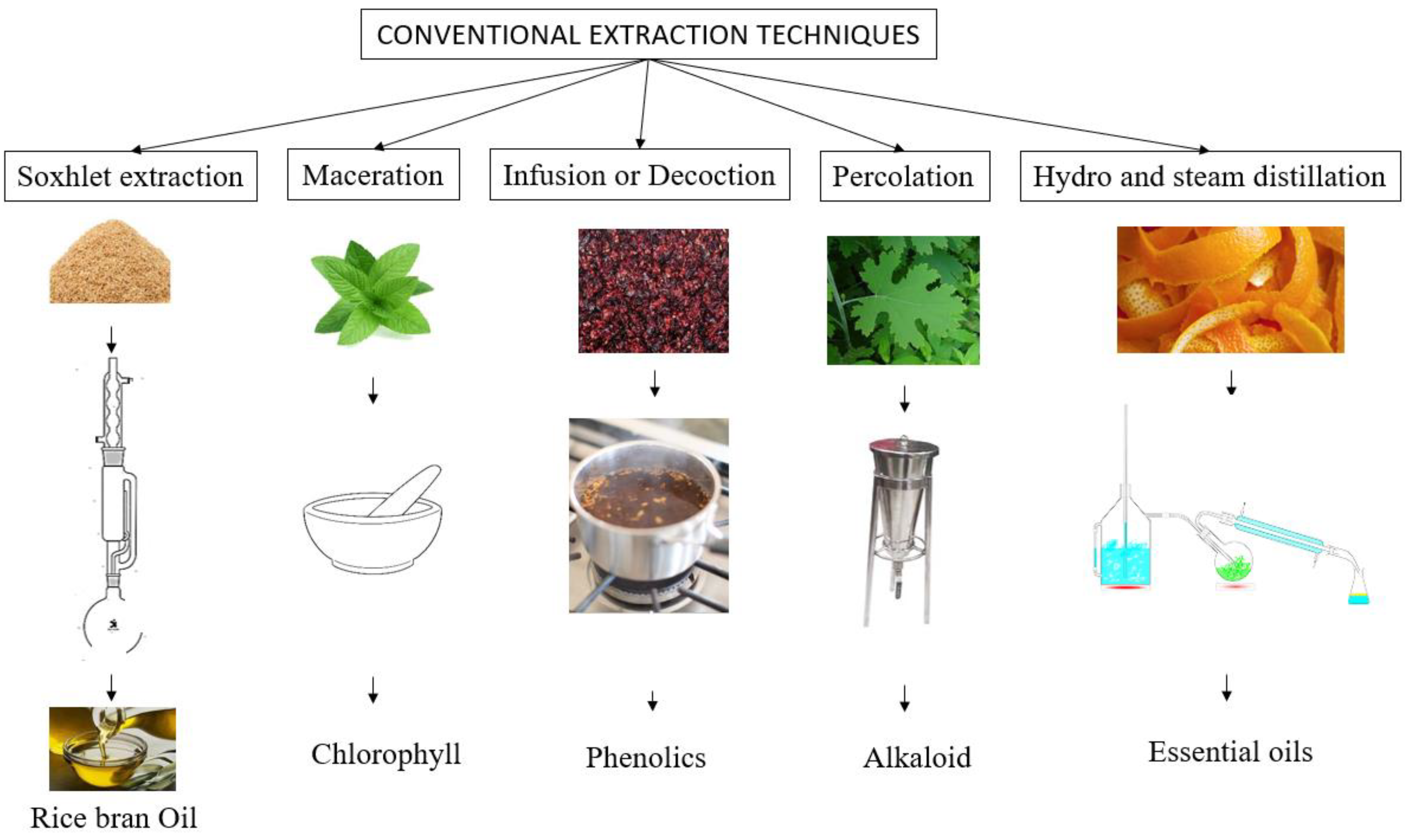
3.1. Conventional Methods of Extraction
3.2. Extraction Using Ultrasound
3.3. Ultrasonic Extraction of Useful Materials from Cereal Processing Waste
3.4. Ultrasonic Extraction of Useful Materials from Fruit and Vegetable Processing Waste
3.5. Ultrasonic Extraction of Useful Materials from Oilseeds and Nuts, Pulses and Legume Processing Waste
3.6. Ultrasonic Extraction Design and Optimization Methods
3.7. Alterations after Exposure to Ultrasound and the Possible Effects and Consequences for Product Quality
4. Ultrasound-Assisted Production of Bio-Energy Products from Food Waste
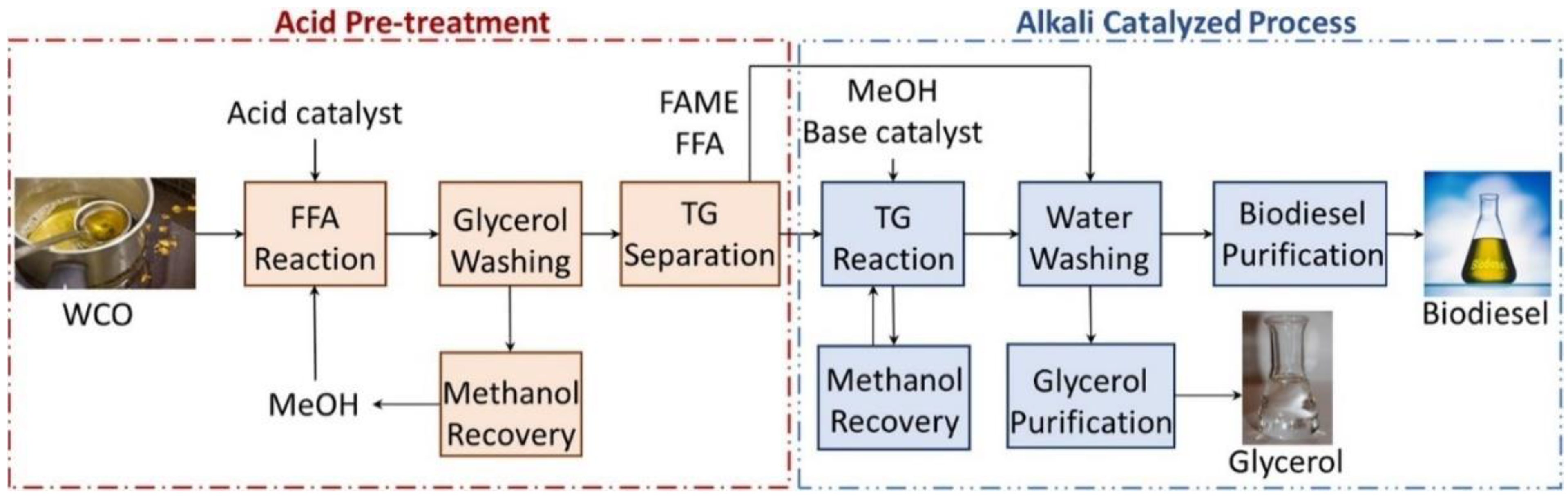
4.1. Biodiesel Production
4.2. Bio-Methane Production
4.3. Bio-Hydrogen Production
4.4. Bio-Ethanol Production
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Xu, J.; Nyambura, S.M.; Wang, J.; Li, C.; Zhu, X.; Feng, X.; Wang, Y. Food waste pyrolysis by traditional heating and microwave heating: A review. Fuel 2022, 324, 124574. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; FAO: Rome, Italy, 2011. [Google Scholar]
- Amicarelli, V.; Lagioia, G.; Bux, C. Global warming potential of food waste through the life cycle assessment: An analytical review. Environ. Impact Assess. Rev. 2021, 91, 106677. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, H.; Yao, D.; Zhang, J.; Zhu, Z.; Wang, Y.; Cui, P. Modeling and comprehensive analysis of food waste gasification process for hydrogen production. Energy Convers. Manag. 2022, 258, 115509. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S.; Bharadwaj, N.; Verma, R. Low temperature steam gasification to produce hydrogen rich gas from kitchen food waste: Influence of steam flow rate and temperature. Int. J. Hydrog. Energy 2020, 45, 20843–20850. [Google Scholar] [CrossRef]
- Xiong, S.; He, J.; Yang, Z.; Guo, M.; Yan, Y.; Ran, J. Thermodynamic analysis of CaO enhanced steam gasification process of food waste with high moisture and low moisture. Energy 2019, 194, 116831. [Google Scholar] [CrossRef]
- Grassino, A.N.; Ostoji, J.; Mileti, V.; Djakovi, S.; Bosiljkov, T.; Zori, Z.; Jeek, D.; Brni, S.R.; Brni, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste —ScienceDirect. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass-Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Liu, B.; Cai, J.; Huai, X.; Li, F. Cavitation Bubble Collapse Near a Heated Wall and Its Effect on the Heat Transfer. J. Heat Transf. 2013, 136, 022901. [Google Scholar] [CrossRef]
- Ozdemir, D.S.; Baspinar, N.; Akalin, P.P. Effects of ultrasound homogenisation on the activities of superoxide dismutase, glutathione peroxidase, catalase and levels of lipid peroxide in liver homogenates. Eurasian J. Vet. Sci. 2015, 31, 16. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124991–124994. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Mettu, S.; Law, S.Q.; Ashokkumar, M.; Martin, G.J. The effect of high-intensity ultrasound on cell disruption and lipid extraction from high-solids viscous slurries of Nannochloropsis sp. biomass. Algal Res. 2018, 35, 341–348. [Google Scholar] [CrossRef]
- Silva, F.V. Evelyn Resistant moulds as pasteurization target for cold distributed high pressure and heat assisted high pressure processed fruit products. J. Food Eng. 2020, 282, 109998. [Google Scholar] [CrossRef]
- Dhar, B.R.; Nakhla, G.; Ray, M.B. Techno-economic evaluation of ultrasound and thermal pretreatments for enhanced anaerobic digestion of municipal waste activated sludge. Waste Manag. 2012, 32, 542–549. [Google Scholar] [CrossRef]
- Yue, L.; Cheng, J.; Tang, S.; An, X.; Hua, J.; Dong, H.; Zhou, J. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy 2021, 228, 120525. [Google Scholar] [CrossRef]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Maes, J.; Campagnone, D.; Blasi, F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhao, B.; Li, H.; Miao, S.; Zheng, B. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.). Innov. Food Sci. Emerg. Technol. 2019, 54, 51–63. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, C.; Li, W. Ultrasound-assisted enzyme catalyzed hydrolysis of olive waste and recovery of antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2017, 44, 224–234. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Li, M.; Fakayode, O.A.; Yan, D.; Zhou, C.; Chen, L. Ultrasound-Ionic Liquid Pretreatment Enhanced Conversion of the Sugary Food Waste to 5-Hydroxymethylfurfural in Ionic Liquid/Solid Acid Catalyst System. Catal. Lett. 2019, 150, 1373–1388. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.-Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-assisted dilute acid hydrolysis for production of essential oils, pectin and bacterial cellulose via a citrus processing waste biorefinery. Biresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef] [PubMed]
- Germec, M.; Tarhan, K.; Yatmaz, E.; Tetik, N.; Karhan, M.; Demirci, A.; Turhan, I. Ultrasound-assisted dilute acid hydrolysis of tea processing waste for production of fermentable sugar. Biotechnol. Prog. 2016, 32, 393–403. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean. Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Qiu-Ying, W.U.; Chen, F.S.; Shi, D.M.; Gong, B.W.; Zhang, P.L. Optimization of ultrasound-assisted alkali extraction of xylan from peanut shell according by response surface methodology. Sci. Technol. Food Ind. 2012. [Google Scholar]
- Li, Y.Y.; Noike, T. Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment. Water Sci. Technol. 1992, 26, 857–866. [Google Scholar] [CrossRef]
- Lafitte-Trouqué, S.; Forster, C. The use of ultrasound and γ-irradiation as pre-treatments for the anaerobic digestion of waste activated sludge at mesophilic and thermophilic temperatures. Bioresour. Technol. 2002, 84, 113–118. [Google Scholar] [CrossRef]
- Dewil, B. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
- Li, W.; Ashokkumar, M. Introduction to Ultrasound and Sonochemistry. Electrochem. Soc. Interface 2018, 27, 43–46. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, P.; Dai, Y.; Luo, X.; Manickam, S.; Li, D.; Han, Y.; Show, P.L. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chem. Eng. J. 2022, 436, 135158. [Google Scholar] [CrossRef]
- Quiroga, G.; Castrillón, L.; Fernández-Nava, Y.; Marañón, E.; Negral, L.; Rodríguez-Iglesias, J.; Ormaecha, P. Effect of ultrasound pre-treatment in the anaerobic co-digestion of cattle manure with food waste and sludge. Bioresour. Technol. 2014, 154, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Show, K.-Y.; Mao, T.; Lee, D.-J. Optimisation of sludge disruption by sonication. Water Res. 2007, 41, 4741–4747. [Google Scholar] [CrossRef] [PubMed]
- Hua, I.; Hoffmann, M.R. Optimization of Ultrasonic Irradiation as an Advanced Oxidation Technology. Environ. Sci. Technol. 1997, 31, 2237–2243. [Google Scholar] [CrossRef]
- Chang, T.-C.; You, S.-J.; Damodar, R.A.; Chen, Y.-Y. Ultrasound pre-treatment step for performance enhancement in an aerobic sludge digestion process. J. Taiwan Inst. Chem. Eng. 2011, 42, 801–808. [Google Scholar] [CrossRef]
- Kosseva, M.R. Sources, Characterization, and Composition of Food Industry Wastes; Academic Press: Cambridge, MA, USA, 2013; pp. 37–60. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrog. Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, C.; Wang, J.; Tian, S.; Zhang, Y. Effects of ultrasound pre-treatment on the amount of dissolved organic matter extracted from food waste. Bioresour. Technol. 2014, 155, 266–271. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R. Intensifying the biogas production from food waste using ultrasound: Understanding into effect of operating parameters. Ultrason. Sonochem. 2019, 59, 104755. [Google Scholar] [CrossRef]
- Grönroos, A.; Kyllönen, H.; Korpijärvi, K.; Pirkonen, P.; Paavola, T.; Jokela, J.; Rintala, J. Ultrasound assisted method to increase soluble chemical oxygen demand (SCOD) of sewage sludge for digestion. Ultrason. Sonochem. 2005, 12, 115–120. [Google Scholar] [CrossRef]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M. Improved biohydrogen production and treatment of pulp and paper mill effluent through ultrasonication pretreatment of wastewater. Energy Convers. Manag. 2015, 106, 576–583. [Google Scholar] [CrossRef]
- Riau, V.; De la Rubia, M.A.; Pérez, M. Upgrading the temperature-phased anaerobic digestion of waste activated sludge by ultrasonic pretreatment. Chem. Eng. J. 2015, 259, 672–681. [Google Scholar] [CrossRef]
- Zorba, G.T.; Sanin, F.D. Disintegration of Sludge by Sonication and Improvement of Methane Production Rates in Batch Anaerobic Digesters. CLEAN—Soil Air Water 2012, 41, 396–402. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrog. Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Tian, X.; Ng, W.J.; Trzcinski, A.P. Optimizing the synergistic effect of sodium hydroxide/ultrasound pre-treatment of sludge. Ultrason. Sonochem. 2018, 48, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Anupoju, G.R.; Eshtiaghi, N. Anaerobic co-digestion of food waste and cardboard in different mixing ratios: Impact of ultrasound pre-treatment on soluble organic matter and biogas generation potential at varying food to inoculum ratios. Biochem. Eng. J. 2020, 166, 107853. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Enhanced production of biohydrogen from dairy waste activated sludge pre-treated using multi hydrolytic garbage enzyme complex and ultrasound-optimization. Energy Convers. Manag. 2018, 164, 277–287. [Google Scholar] [CrossRef]
- Snehya, A.; Sundaramahalingam, M.; Rajeshbanu, J.; Anandan, S.; Sivashanmugam, P. Studies on evaluation of surfactant coupled sonication pretreatment on Ulva fasciata (marine macroalgae) for enhanced biohydrogen production. Ultrason. Sonochem. 2021, 81, 105853. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Nakhla, G. Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Bioresour. Technol. 2011, 102, 6449–6457. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Q.; Yang, S.; Luo, H.; Peng, S.; Ren, N. Optimization of ultrasonic pretreatment and substrate/inoculum ratio to enhance hydrolysis and volatile fatty acid production from food waste. RSC Adv. 2014, 4, 53321–53326. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; He, Y.; Jia, F.; Wang, S.; Guo, S. Applying low frequency ultrasound on different biological nitrogen activated sludge types: An analysis of particle size reduction, soluble chemical oxygen demand (SCOD) and ammonia release. Int. Biodeterior. Biodegrad. 2016, 112, 42–50. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Yan, F.; Gao, Y.; Meng, Y.; Aihemaiti, A.; Ju, T. Enhancement of volatile fatty acid production and biogas yield from food waste following sonication pretreatment. J. Environ. Manag. 2018, 217, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.; Song, K.; Gu, Y.; Gao, X. Improving methane production from algal sludge based anaerobic digestion by co-pretreatment with ultrasound and zero-valent iron. J. Clean. Prod. 2020, 255, 120214. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Viability of ultrasonication of food waste for hydrogen production. Int. J. Hydrog. Energy 2011, 37, 2960–2964. [Google Scholar] [CrossRef]
- Azman, S.; Milh, H.; Somers, M.H.; Zhang, H.; Huybrechts, I.; Meers, E.; Meesschaert, B.; Dewil, R.; Appels, L. Ultrasound-assisted digestate treatment of manure digestate for increased biogas production in small pilot scale anaerobic digesters. Renew. Energy 2020, 152, 664–673. [Google Scholar] [CrossRef]
- Boni, M.R.; D’Amato, E.; Polettini, A.; Pomi, R.; Rossi, A. Effect of ultrasonication on anaerobic degradability of solid waste digestate. Waste Manag. 2016, 48, 209–217. [Google Scholar] [CrossRef]
- Somers, M.H.; Azman, S.; Sigurnjak, I.; Ghyselbrecht, K.; Meers, E.; Meesschaert, B.; Appels, L. Effect of digestate disintegration on anaerobic digestion of organic waste. Bioresour. Technol. 2018, 268, 568–576. [Google Scholar] [CrossRef]
- Bundhoo, Z.M. Effects of microwave and ultrasound irradiations on dark fermentative bio-hydrogen production from food and yard wastes. Int. J. Hydrog. Energy 2017, 42, 4040–4050. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason. Sonochem. 2013, 20, 931–936. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochem. 2015, 22, 565–572. [Google Scholar] [CrossRef]
- Cesaro, A.; Naddeo, V.; Amodio, V.; Belgiorno, V. Enhanced biogas production from anaerobic codigestion of solid waste by sonolysis. Ultrason. Sonochem. 2012, 19, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Oz, N.A.; Yarimtepe, C.C. Ultrasound assisted biogas production from landfill leachate. Waste Manag. 2014, 34, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Z.; Wang, M.; Shi, X.; Zhou, Y.; Zhou, Y.; Kong, Y. Inactivation of harmful Anabaena flos-aquae by ultrasound irradiation: Cell disruption mechanism and enhanced coagulation. Ultrason. Sonochem. 2020, 69, 105254. [Google Scholar] [CrossRef] [PubMed]
- Krzendrfer, A. Vibrations and ultrasound in food processing—Sources of vibrations, adverse effects, and beneficial applications—An overview. J. Food. Eng. 2021, 324, 110875. [Google Scholar] [CrossRef]
- Martín, J.F.G.; Guillemet, L.; Feng, C.-H.; Sun, D.-W. Cell viability and proteins release during ultrasound-assisted yeast lysis of light lees in model wine. Food Chem. 2013, 141, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Eskicioglu, C.; Marin, J. Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour. Technol. 2011, 102, 7815–7826. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, K.; Dahiya, R.; Bera, T. Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Academic Press: Cambridge, MA, USA, 2015; pp. 121–126. [Google Scholar] [CrossRef]
- Li, X.; Mettu, S.; Martin, G.J.; Ashokkumar, M.; Lin, C.S.K. Ultrasonic pretreatment of food waste to accelerate enzymatic hydrolysis for glucose production. Ultrason. Sonochem. 2018, 53, 77–82. [Google Scholar] [CrossRef]
- Romero-Pareja, P.; Aragon, C.A.; Quiroga, J.M.; Coello, M.D. Evaluation of a biological wastewater treatment system combining an osa process with ultrasound for sludge reduction. Ultrason. Sonochem. 2017, 36, 336–342. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Trakselyte-Rupsiene, K.; Gasauskaite, K.; Bartkiene, E.; Lele, V.; Viskelis, P.; Bernatoniene, J.; Ivanauskas, L.; Jakstas, V. Functionalisation of flaxseed proteins assisted by ultrasonication to produce coatings enriched with raspberries phytochemicals. LWT 2020, 124, 109180. [Google Scholar] [CrossRef]
- Maskooki, A.; Mortazavi, S.A.; Maskooki, A. Cleaning of spiralwound ultrafiltration membranes using ultrasound and alkaline solution of EDTA. Desalination 2010, 264, 63–69. [Google Scholar] [CrossRef]
- Pejin, D.J.; Mojović, L.V.; Pejin, J.D.; Grujić, O.S.; Markov, S.L.; Nikolić, S.B.; Marković, M.N. Increase in bioethanol production yield from triticale by simultaneous saccharification and fermentation with application of ultrasound. J. Chem. Technol. Biotechnol. 2011, 87, 170–176. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Rakshit, S.K.; Grewell, D.; van Leeuwen, J.; Khanal, S.K. Ultrasound pretreatment of cassava chip slurry to enhance sugar release for subsequent ethanol production. Biotechnol. Bioeng. 2008, 101, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Montalbo, M.; van Leeuwen, J.; Srinivasan, G.; Grewell, D. Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnol. Bioeng. 2007, 98, 978–985. [Google Scholar] [CrossRef]
- Walton, J.D. Deconstructing the Cell Wall. Plant Physiol. 1994, 104, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; Gogate, P.R. Intensified Synthesis of Bioethanol from Sustainable Biomass. In Waste Biomass Management—A Holistic Approach; Springer: Cham, Switzerland, 2017; Volume 58, pp. 251–287. [Google Scholar] [CrossRef]
- García, A.; Erdocia, X.; Alriols, M.G.; Labidi, J. Effect of ultrasound treatment on the physicochemical properties of alkaline lignin. Chem. Eng. Process. Process Intensif. 2012, 62, 150–158. [Google Scholar] [CrossRef]
- Leite, A.L.M.P.; Zanon, C.D.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr. Polym. 2017, 157, 962–970. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crop. Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Perrone, O.M.; Colombari, F.M.; Rossi, J.S.; Moretti, M.M.S.; Bordignon, S.E.; Nunes, C.d.C.C.; Gomes, E.; Boscolo, M.; Da-Silva, R. Ozonolysis combined with ultrasound as a pretreatment of sugarcane bagasse: Effect on the enzymatic saccharification and the physical and chemical characteristics of the substrate. Bioresour. Technol. 2016, 218, 69–76. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Sono-assisted enzymatic saccharification of sugarcane bagasse for bioethanol production. Biochem. Eng. J. 2012, 63, 1–9. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: Optimization through response surface methodology. Bioresour. Technol. 2012, 112, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Shen, Y.; Wang, L.; Zhang, H.; Qian, H.; Qi, X. Extrusion followed by ultrasound as a chemical-free pretreatment method to enhance enzymatic hydrolysis of rice hull for fermentable sugars production. Ind. Crop. Prod. 2020, 149, 112356. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Jahim, J.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y.; Lee, C.B.T.L.; Cheong, N.W.R.; Shak, K.P.Y. Sequential ultrasonication and deep eutectic solvent pretreatment to remove lignin and recover xylose from oil palm fronds. Ultrason. Sonochem. 2019, 58, 104598. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Jashina, L.; Telysheva, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus. Chim. 2018, 21, 563–571. [Google Scholar] [CrossRef]
- Mohapatra, S.; Dandapat, S.J.; Thatoi, H. Physicochemical characterization, modelling and optimization of ultrasono-assisted acid pretreatment of two Pennisetum sp. using Taguchi and artificial neural networking for enhanced delignification. J. Environ. Manag. 2017, 187, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Sakdaronnarong, C.; Srimarut, N.; Lucknakhul, N.; Na-Songkla, N.; Jonglertjunya, W. Two-step acid and alkaline ethanolysis/alkaline peroxide fractionation of sugarcane bagasse and rice straw for production of polylactic acid precursor. Biochem. Eng. J. 2014, 85, 49–62. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Evaluation of ultrasound assisted potassium permanganate pre-treatment of spent coffee waste. Bioresour. Technol. 2017, 224, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Yusof, N.S.M.; Babgi, B.; Alghamdi, Y.; Aksu, M.; Madhavan, J.; Ashokkumar, M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016, 29, 568–576. [Google Scholar] [CrossRef]
- New, E.K.; Wu, T.Y.; Lee, C.B.T.L.; Poon, Z.Y.; Loow, Y.-L.; Foo, L.Y.W.; Procentese, A.; Siow, L.F.; Teoh, W.H.; Daud, N.N.N.; et al. Potential use of pure and diluted choline chloride-based deep eutectic solvent in delignification of oil palm fronds. Process Saf. Environ. Prot. 2018, 123, 190–198. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Utilization of sugarcane bagasse for bioethanol production: Sono-assisted acid hydrolysis approach. Bioresour. Technol. 2011, 102, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Pandey, A. A novel sono-assisted acid pretreatment of chili post harvest residue for bioethanol production. Bioresour. Technol. 2016, 213, 58–63. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Jin, J.; Lin, H.; Yagoub, A.E.A.; Xiong, S.; Xu, L.; Udenigwe, C. Effects of high power ultrasound on the enzymolysis and structures of sweet potato starch. J. Sci. Food Agric. 2020, 100, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Ohta, A.; Omote, S.; Ogino, C.; Takahashi, K.; Shimizu, N. Combined use of completely bio-derived cholinium ionic liquids and ultrasound irradiation for the pretreatment of lignocellulosic material to enhance enzymatic saccharification. Chem. Eng. J. 2012, 215, 811–818. [Google Scholar] [CrossRef]
- Galanakis, C.M.J.F.; Processing, B. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Wood, I.P.; Cook, N.M.; Wilson, D.R.; Ryden, P.; Robertson, J.A.; Waldron, K.W. Ethanol from a biorefinery waste stream: Saccharification of amylase, protease and xylanase treated wheat bran. Food Chem. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, M.; Liu, X.; Zhong, K.; Tong, L.; Zhou, X.; Zhou, S. Effect of steam explosion-assisted extraction on phenolic acid profiles and antioxidant properties of wheat bran. J. Sci. Food Agric. 2016, 96, 3484–3491. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods 2021, 10, 279. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Golisch, B.; Lei, Z.; Tamura, K.; Brumer, H. Configured for the Human Gut Microbiota: Molecular Mechanisms of Dietary β-Glucan Utilization. ACS Chem. Biol. 2021, 16, 2087–2102. [Google Scholar] [CrossRef]
- Hromádková, Z.; Košt’álová, Z.; Ebringerová, A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason. Sonochem. 2008, 15, 1062–1068. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Babacan, Ü.; Akinci, E.; Kesci, S.T.; Kaba, A. Extraction of phenolic acids from ancient wheat bran samples by ultrasound application. J. Chem. Technol. Biotechnol. 2020, 96, 134–141. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Ind. Crop. Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2016, 94, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Teslić, N.; Bojanić, N.; Čolović, D.; Fišteš, A.; Rakić, D.; Solarov, M.B.; Zeković, Z.; Pavlić, B. Technology, Conventional versus novel extraction techniques for wheat germ oil recovery: Multi-response optimization of supercritical fluid extraction. Sep. Sci. Technol. 2021, 56, 1546–1561. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2022, 60, 1388–1416. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- De Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2018, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. fruit peel. Int. J. Biol. Macromol. 2014, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Kan, J. Polysaccharides from ginger stems and leaves: Effects of dual and triple frequency ultrasound assisted extraction on structural characteristics and biological activities. Food Biosci. 2021, 42, 101166. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, Y.; Zhu, C.; Mason, T. Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrason. Sonochem. 2002, 9, 231–236. [Google Scholar] [CrossRef]
- De Souza, M.A.; Vilas-Boas, I.T.; Leite-da-Silva, J.M.; Abrahão, P.d.N.; Teixeira-Costa, B.E.; Veiga-Junior, V.F.J.P. Polysaccharides in Agro-Industrial Biomass Residues. Polysaccharides 2022, 3, 95–120. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Chen, K.; Yan, W.; Zhang, X.; Tang, X.; Han, X.; Li, J. Ultrasound-assisted extraction of dietary fibre from Citrus Changshan-huyou peels. J. Chem. Pharm. Res. 2014, 6, 312–318. [Google Scholar]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and Functional Properties of Water-Soluble Dietary Fiber from Apple Pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Effect of processing on structural, thermal, and physicochemical properties of dietary fiber of culinary banana bracts. J. Food Process. Preserv. 2019, 43, e14256. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Ezzati, S.; Ayaseh, A.; Ghanbarzadeh, B.; Heshmati, M.K. Pectin from sunflower by-product: Optimization of ultrasound-assisted extraction, characterization, and functional analysis. Int. J. Biol. Macromol. 2020, 165, 776–786. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2019, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.N.; Mushtaq, Z.; Ahmad, N.; Khan, M.K.; Ahmad, M.H.; Hussain, A.I.; Imran, M. Optimal Ultrasound-Assisted Process Extraction, Characterization, and Functional Product Development from Flaxseed Meal Derived Polysaccharide Gum. Processes 2019, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, Z.; Xiao, F.; Wei, Q.; Jing, Z. Ultrasound-assisted alkali extraction of insoluble dietary fiber from soybean residues. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 052005. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Jalili, F.; Jafari, S.M.; Emam-Djomeh, Z.; Malekjani, N.; Farzaneh, V. Optimization of Ultrasound-Assisted Extraction of Oil from Canola Seeds with the Use of Response Surface Methodology. Food Anal. Methods 2017, 11, 598–612. [Google Scholar] [CrossRef]
- Amigh, S.; Dinani, S.T. Combination of ultrasound-assisted aqueous enzymatic extraction and cooking pretreatment for date seed oil recovery. Heat Mass Transf. 2020, 56, 2345–2354. [Google Scholar] [CrossRef]
- Shen, M.; Weihao, W.; Cao, L. Soluble dietary fibers from black soybean hulls: Physical and enzymatic modification, structure, physical properties, and cholesterol binding capacity. J. Food Sci. 2020, 85, 1668–1674. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolics compounds from wheat using response surface methodology. Food. Chem. 2005, 93, 45–46. [Google Scholar]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Fei, S.; Gu, H.; Yang, L. Optimization of ultrasonic circulating extraction of samara oil from Acer saccharum using combination of Plackett–Burman design and Box–Behnken design. Ultrason. Sonochem. 2017, 35, 161–175. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Cravotto, G.; Bicchi, C.; Mantegna, S.; Binello, A.; Tomao, V.; Chemat, F. Extraction of kiwi seed oil: Soxhlet versus four different non-conventional techniques. Nat. Prod. Res. 2011, 25, 974–981. [Google Scholar] [CrossRef]
- Metherel, A.H.; Taha, A.Y.; Izadi, H.; Stark, K.D. The application of ultrasound energy to increase lipid extraction throughput of solid matrix samples (flaxseed). Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour. Fragr. J. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojević, P.; Palić, R. Volatiles of the grape hybrid cultivar Othello (Vitis vinifera x (Vitis labrusca x Vitis riparia)) cultivated in Serbia. J. Essent. Oil. Res. 2010, 22, 616–619. [Google Scholar] [CrossRef]
- Živković, S.; Veljković, M. Environmental impacts the of production and use of biodiesel. Environ. Sci. Pollut. Res. 2017, 25, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.-H.; Tian, B.; Ashokkumar, V.; Lim, S.; Seljak, T.; Józsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2011, 16, 1193–1209. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D.; Hernandez, R. Base-Catalyzed Fast Transesterification of Soybean Oil Using Ultrasonication. In Proceedings of the 2006 ASAE Annual Meeting, American Society of Agricultural and Biological Engineers, St. Joseph, MI, USA, 9–12 July 2006. [Google Scholar]
- Kalva, A.; Sivasankar, T.; Moholkar, V. Physical Mechanism of Ultrasound-Assisted Synthesis of Biodiesel. Ind. Eng. Chem. Res. 2009, 48, 534–544. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Martin, G.J.; Ashokkumar, M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 130057. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Sáez-Bastante, J.; Pinzi, S.; Dorado, M. Optimization of solid food waste oil biodiesel by ultrasound-assisted transesterification. Fuel 2019, 255, 115817. [Google Scholar] [CrossRef]
- Oza, S.; Prajapati, N.; Kodgire, P.; Kachhwaha, S.S. An ultrasound-assisted process for the optimization of biodiesel production from waste cottonseed cooking oil using response surface methodology. Water-Energy Nexus 2021, 4, 187–198. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Investigation of ultrasound-assisted KOH and CaO catalyzed transesterification for biodiesel production from waste cotton-seed cooking oil: Process optimization and conversion rate evaluation. J. Clean. Prod. 2020, 259, 120982. [Google Scholar] [CrossRef]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K.; Murugesan, T.; Arunagiri, A. Production of biodiesel from waste bio-oil through ultrasound assisted transesterification using immobilized lipase. Environ. Technol. Innov. 2020, 21, 101199. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, L.; Jiao, Y. Ultrasound strengthened biodiesel production from waste cooking oil using modified coal fly ash as catalyst. J. Environ. Chem. Eng. 2016, 4, 818–824. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Ultrasound-assisted biodiesel production from waste cooking oil using hydrotalcite prepared by combustion method as catalyst. Appl. Catal. A Gen. 2016, 514, 214–223. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Rahman, K.M.; Woodard, R.; Manzanares, E.; Harder, M.K. An assessment of anaerobic digestion capacity in Bangladesh. Renew. Sustain. Energy Rev. 2014, 32, 762–769. [Google Scholar] [CrossRef]
- Erden, G.; Filibeli, A. Biotechnology, Ultrasonic pre-treatment of biological sludge: Consequences for disintegration, anaerobic biodegradability, and filterability. J. Chem. Technol. Biotechnol. 2010, 85, 145–150. [Google Scholar] [CrossRef]
- Mirko, C.; Daniela, P.; Chiara, T.; Giovanni, G. Pretreatments for enhanced biomethane production from buckwheat hull: Effects on organic matter degradation and process sustainability. J. Environ. Manag. 2021, 285, 112098. [Google Scholar] [CrossRef]
- Shanthi, M.; Banu, J.R.; Sivashanmugam, P. Effect of surfactant assisted sonic pretreatment on liquefaction of fruits and vegetable residue: Characterization, acidogenesis, biomethane yield and energy ratio. Bioresour. Technol. 2018, 264, 35–41. [Google Scholar] [CrossRef]
- Ormaechea, P.; Castrillón, L.; Marañón, E.; Fernández-Nava, Y.; Negral, L.; Megido, L. Influence of the ultrasound pretreatment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017, 38, 682–686. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrog. Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Enhancement of biohydrogen producing using ultrasonication. Int. J. Hydrog. Energy 2010, 35, 6184–6193. [Google Scholar] [CrossRef]
- Leaño, E.P.; Anceno, A.J.; Babel, S. Ultrasonic pretreatment of palm oil mill effluent: Impact on biohydrogen production, bioelectricity generation, and underlying microbial communities. Int. J. Hydrog. Energy 2012, 37, 12241–12249. [Google Scholar] [CrossRef]
- Suresh, T.; Sivarajasekar, N.; Balasubramani, K.; Ahamad, T.; Alam, M.; Naushad, M. Process intensification and comparison of bioethanol production from food industry waste (potatoes) by ultrasonic assisted acid hydrolysis and enzymatic hydrolysis: Statistical modelling and optimization. Biomass-Bioenergy 2020, 142, 105752. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Mathew, A.K.; Abraham, A.; Gnansounou, E.; Ummalyma, S.B.; Thomas, L.; Pandey, A. Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Bioresour. Technol. 2017, 242, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Mancini, G.; Ruggeri, B.; Fino, D. Selection of the best pretreatment for hydrogen and bioethanol production from olive oil waste products. Renew. Energy 2016, 88, 401–407. [Google Scholar] [CrossRef]
- John, I.; Pola, J.; Appusamy, A. Optimization of Ultrasonic Assisted Saccharification of Sweet Lime Peel for Bioethanol Production Using Box–Behnken Method. Waste Biomass Valoriz. 2017, 10, 441–453. [Google Scholar] [CrossRef]
- Mozhiarasi, V. Overview of pretreatment technologies on vegetable, fruit and flower market wastes disintegration and bioenergy potential: Indian scenario. Chemosphere 2021, 288, 132604. [Google Scholar] [CrossRef] [PubMed]

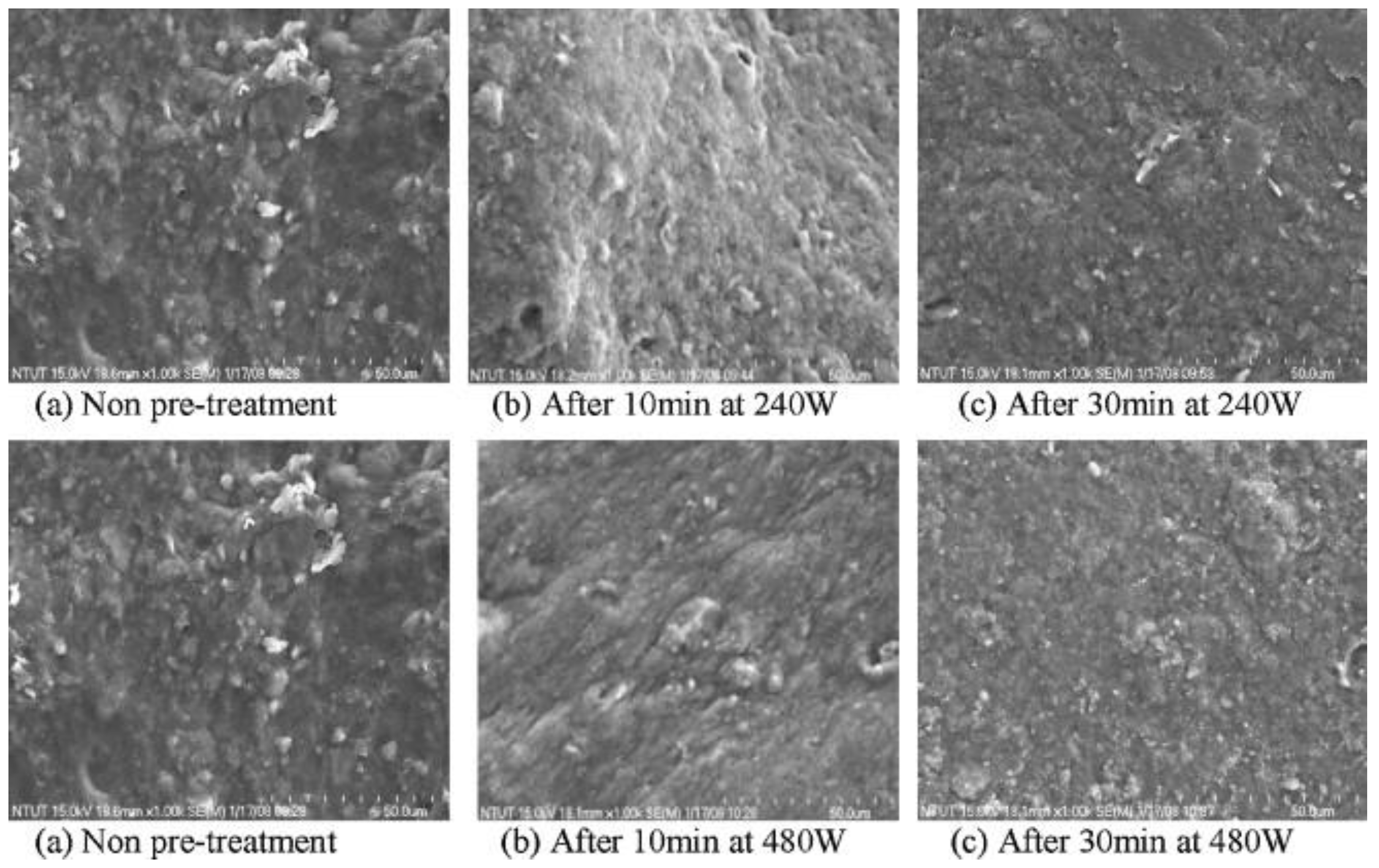
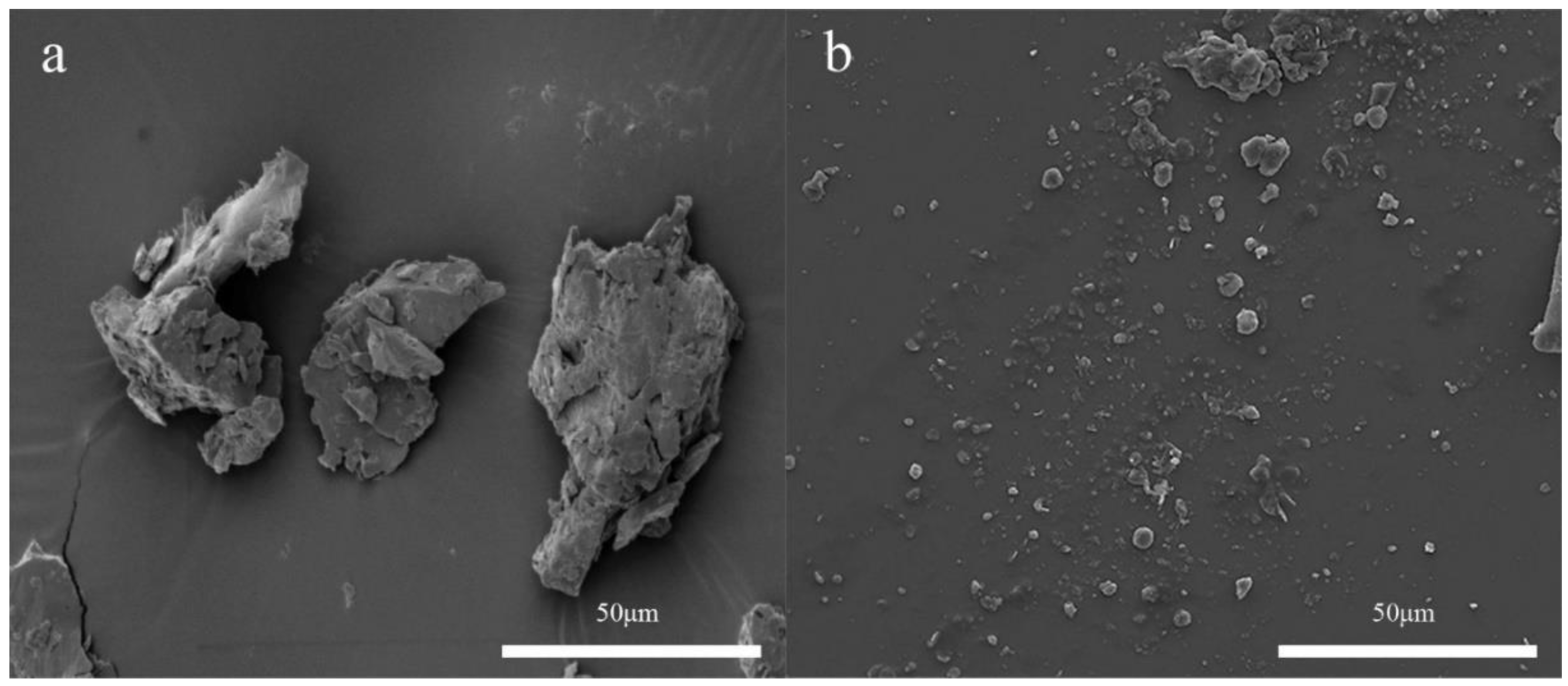
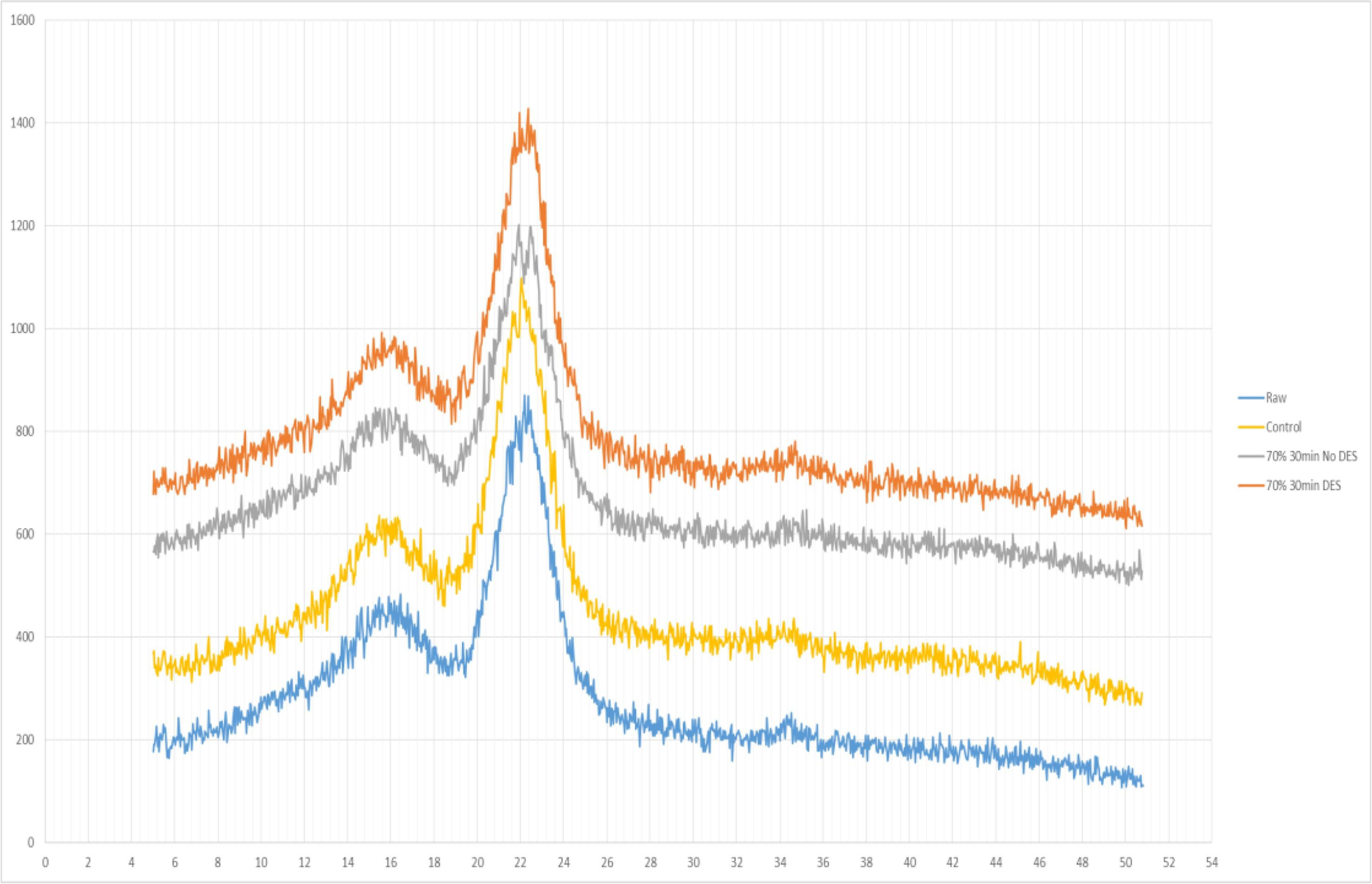
| Source/Composition of Food Waste | Ultrasonic Conditions | Outcomes | Reference |
|---|---|---|---|
| A mixture of rice, cabbage, pork, and tofu waste with different total solid (TS) content | 20 kHz, 480 W/L, 15 min | TS = 40 g/L, ΔSCOD: +157% TS = 100 g/L, ΔSCOD: +172% | [39] |
| Sewage sludge | 27 kHz, 200 W/L | 2.5 min, ΔSCOD: +239% 10 min, ΔSCOD: +577% | [41] |
| Activated sludge | 20 kHz, 21 kJ/g TS, 9 min | ΔSCOD: +246% | [47] |
| A mixture of wheat, gram flour, rice, fruit peel, and vegetable waste | 20 kHz, 0.4 W/mL, 10 min | ΔSCOD: +61.5% | [40] |
| Food waste obtained from the Dufferin Organics Processing Facility | 20 kHz, 79 kJ/g TS | ΔSCOD: +25% | [46] |
| Food waste from the Aurora treatment plant | 20 kHz, 10,384, 15,577, 20,769 kJ/kg TS for 30, 45, 60 min US sample | 30 min, ΔSCOD: +10.3% 45 min, ΔSCOD: +29.4% 60 min, ΔSCOD: +37% | [48] |
| Dairy waste | 20 kHz, 0.6 W/mL, 25 min | ΔSCOD: +28.4% | [49] |
| Complex food waste | 20 kHz, 16,875 kJ/kg TS, 15 min | ΔSCOD: +56.5% | [38] |
| Algae | 20 kHz, 30 min, 36,000 KJ/Kg TS | Maximum ΔSCOD: +1950% was observed at 200 W US power | [50] |
| Pulp mill | 20 kHz, 3.1 W/mL | 15 min, ΔSCOD: +14.9% 60 min, ΔSCOD: +44.3% | [42] |
| Food waste | 20 kHz, 5000 kJ/kg | ΔSCOD: +9.0% | [51] |
| Rice, noodles, vegetables, and meat waste obtained from a cafeteria at Harbin Institute | 20 kHz, 1.25 W/mL, 30 min | ΔSCOD: +115% | [52] |
| Activated sludge | 24 kHz, 1690 and 3380 kJ/kg TS for 5- and 10 min sonication sample | 5 min, ΔSCOD: +17% 10 min, ΔSCOD: +21% | [43] |
| Activated sludge | 20 kHz, 1.04 W/mL, 2.5 min | ΔSCOD: +12.6% | [53] |
| Food waste | 20 kHz, 2 W/mL, 15 min | ΔSCOD: +71.5% | [54] |
| Algae sludge | 40 kHz, 30 min, the power density was not mentioned | ΔSCOD: +520% | [55] |
| Food waste | 20 kHz, 23 kJ/g TS, 30 min | ΔSCOD: +22.1% | [56] |
| Digestate | 20 kHz, 3000 kJ/kg TS | ΔSCOD: +21% | [57] |
| Organic waste from the food industry | 20 kHz, 50,000 kJ/kg TS | ΔSCOD: +20% | [58] |
| Dairy digestate waste | 20 kHz, 15,000 kJ/kg TS | ΔSCOD: +15% | [59] |
| Food waste | 20 kHz, 6946 kJ/kg TS, 30 min | ΔSCOD: +159% | [60] |
| Solid organic waste | 20 kHz, 15,000 kJ/kg TS | ΔSCOD: +9.0% | [61] |
| Olive mill wastewater | 20 kHz, 0.4 W/mL, 10 min | ΔSCOD: +23% | [62] |
| Solid waste | 20 kHz, 0.2 W/mL, 60 min | ΔSCOD: +18.5% | [63] |
| Fermentation residues | 20 kHz pulsed US (4 s on, 6 s off), 2 W/mL, 30 min | ΔSCOD: +39.5% | [25] |
| Seed sludge from food factory | 20 kHz, 200 W/L, 45 min | ΔSCOD: +11% | [64] |
| Source/Composition of Food Waste | Ultrasonic Conditions | Outcomes | Reference |
|---|---|---|---|
| Wheat waste | 20 kHz, 30 min, NaOH (2% w/v) combination | Δcellulose: +13.2%; Δlignin: −10.1% | [88] |
| Vegetable waste | 20 kHz, 20 min | Δcellulose: +23.1%; Δhemicellulose: −9.0%; Δlignin: −10.2% | [82] |
| Oil palm fronds | 20 kHz, 50 min | Δlignin: −5.8% | [89] |
| Wheat straw | 20 kHz, 120 min at 50 °C | Δlignin: −6.2% | [90] |
| Rice straw | 30 kHz, water bath 90 °C, 4 h | Δlignin: −4.6% | [92] |
| Rice hull | 40 kHz, 500 W, 1.5 h | Δcellulose: −2.8%; Δhemicellulose: −3.7%; Δlignin: +1.6% | [86] |
| Sugarcane bagasse | 22 kHz, 50 W, 25 min, ozone/alkaline assisted | Δcellulose: +10.5%; Δhemicellulose: −8.1%; Δlignin: −15.6% | [83] |
| Sugarcane bagasse | 24 kHz, 500 W, water bath 40 °C | Δcellulose: +21.4%; Δhemicellulose: −18.6%; Δlignin: −8.4% | [84] |
| Spent coffee waste | 47 kHz, 310 W, 20 min | Δlignin: −8.8% | [93] |
| Materials | Ultrasonic Frequency | Ultrasonic Power | Temperature | Biodiesel Yield | Reference |
|---|---|---|---|---|---|
| Solid food waste oil | 20 kHz | 50% amplitude | 52.5 °C | 93.23% | [161] |
| Waste cottonseed cooking oil | 20 kHz | 500 W | 50 °C | 98% | [162] |
| Waste cottonseed cooking oil | 20 kHz | 500 W | 40–60 °C | 70.21~97.76% | [163] |
| Waste bio-oil | 20 kHz | 20~100% amplitude | 45 °C | 98.7% | [164] |
| Waste cooking oil | 20 kHz | 108 W | 70 °C | 95.57% | [165] |
| Waste cooking oil | 22 kHz | 120 W | 60 °C | 93.5% | [166] |
| Waste cooking oil | 20 kHz | 55% amplitude | 57 °C | 76.45% | [167] |
| Materials | Ultrasonic Frequency | Ultrasonic Power | Temperature | Bio-Methane Yield | Reference |
|---|---|---|---|---|---|
| Buckwheat hull | 40 kHz | 110 W | 25 °C | 141.9 NL Kg VS-1 | [171] |
| Restaurant waste | 20 kHz | 0–500 W | Room temperature | 647.49 mL/g TVS~927.97 mL/g | [17] |
| Fruit and vegetable residue | 25 kHz | 90 W | - | 0.61 g/g COD | [172] |
| Mixture of food waste, cattle manure, and sludge | 24 kHz | 400 W | - | 0.85 L/L day | [33] |
| Mixture of food waste, cattle manure, and crude glycerine | 24 kHz | 400 W | 55 °C | 520 L/kg VS | [173] |
| Food waste | 20 kHz and 25 kHz | 120 W and 200 W | 35 °C | ~0.26 L/day | [40] |
| Materials | Ultrasonic Frequency | Ultrasonic Power | Temperature | Bio-Hydrogen Yield | Reference |
|---|---|---|---|---|---|
| Mixture of food and yard waste | 20 kHz | 0~500 W | - | 7.87 mL/g VS, decreased by 11% | [60] |
| Food waste | 20 kHz | 500 W | <30 °C | 1.55 mol/mol VS, increased by 120% | [175] |
| Food waste | 20 kHz | 500 W | <30 °C | 97 mL/g VS, increased by 131% | [46] |
| Food waste | 20 kHz | 500 W | <30 °C | 80 mL/g VS, increased by 88% | [56] |
| Food waste | 20 kHz | 1200 W | <30 °C | 0.62~5.23 mL/h, increased by 75% | [38] |
| Food waste | 20 kHz | 130 W | - | 0.7 mmol/g COD, increased by 38% | [176] |
| Dairy waste | 20 kHz | 0.3~1.1 W/mL | 25 °C | 15.51 mL/g VS, increased by 3 times | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yao, S.; Narale, B.A.; Shanmugam, A.; Mettu, S.; Ashokkumar, M. Ultrasonic Processing of Food Waste to Generate Value-Added Products. Foods 2022, 11, 2035. https://doi.org/10.3390/foods11142035
Wu Y, Yao S, Narale BA, Shanmugam A, Mettu S, Ashokkumar M. Ultrasonic Processing of Food Waste to Generate Value-Added Products. Foods. 2022; 11(14):2035. https://doi.org/10.3390/foods11142035
Chicago/Turabian StyleWu, Yue, Shunyu Yao, Bhakti Anand Narale, Akalya Shanmugam, Srinivas Mettu, and Muthupandian Ashokkumar. 2022. "Ultrasonic Processing of Food Waste to Generate Value-Added Products" Foods 11, no. 14: 2035. https://doi.org/10.3390/foods11142035
APA StyleWu, Y., Yao, S., Narale, B. A., Shanmugam, A., Mettu, S., & Ashokkumar, M. (2022). Ultrasonic Processing of Food Waste to Generate Value-Added Products. Foods, 11(14), 2035. https://doi.org/10.3390/foods11142035