The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Fish, Storage on Ice, and Canning
2.2. Chemical Analyses
2.3. Sensory Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Determination of Moisture and Lipid Contents
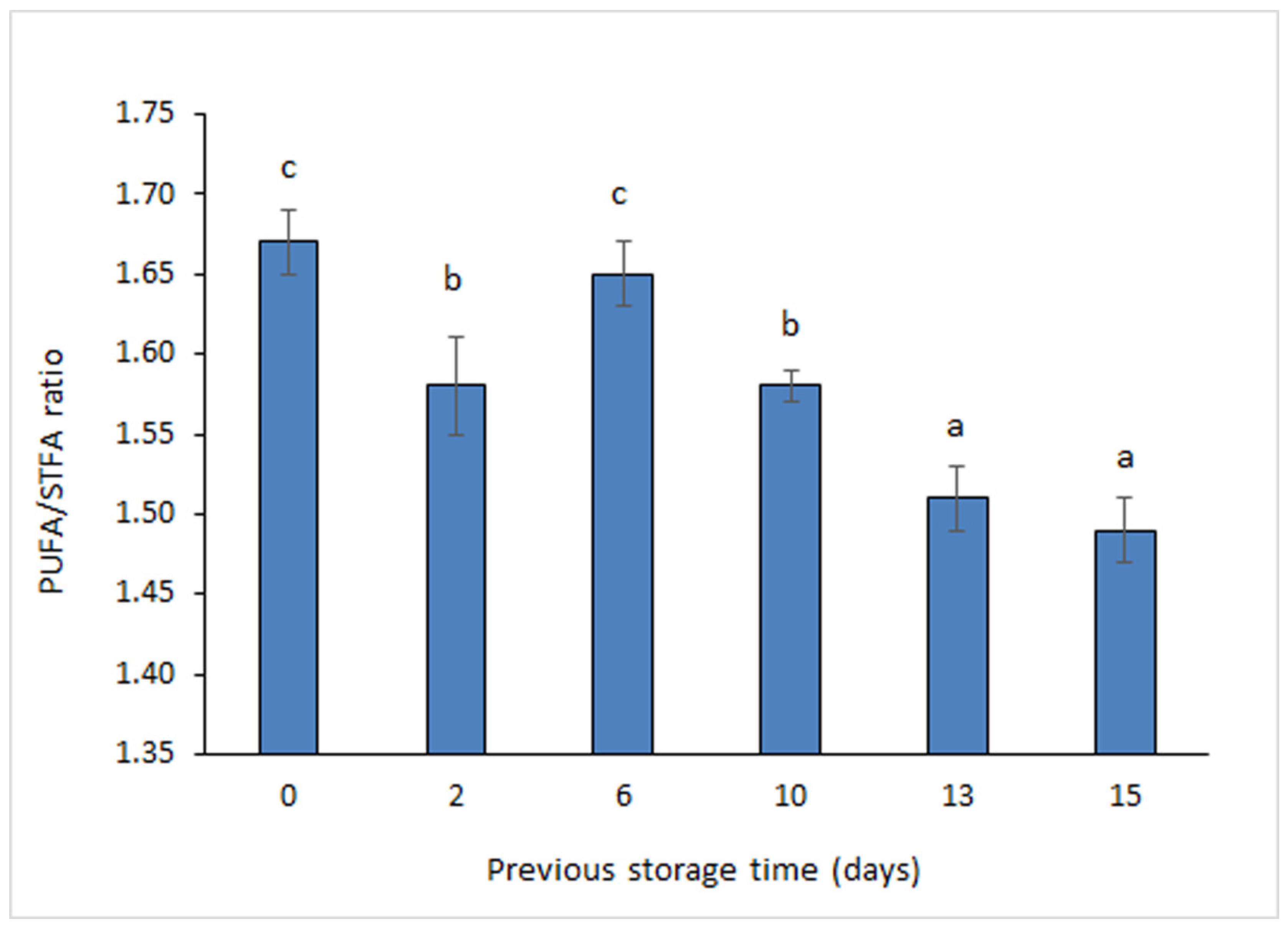
3.2. Fatty Acid (FA) Analysis
3.3. Assessment of Triacylglycerol (TAG) and Diacylglycerol (DAG) Values
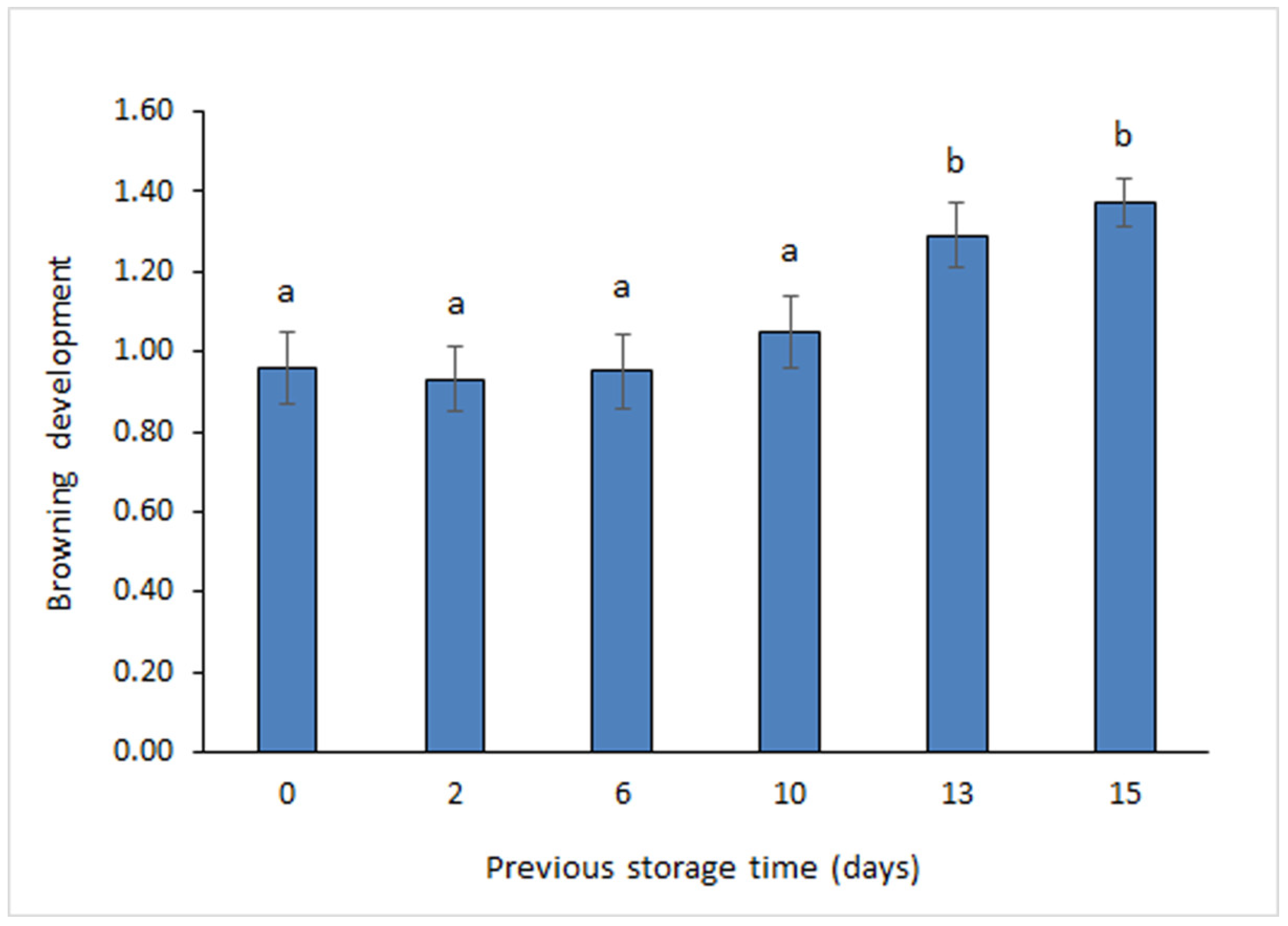
3.4. Determination of Browning Development
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horner, W. Canning fish and fish products. In Fish Processing Technology, 2nd ed.; Hall, G., Ed.; Blackie Academic and Professional: New York, NY, USA; Chapman and Hall: London, UK, 1997; pp. 119–159. [Google Scholar]
- Banga, J.; Alonso, A.; Gallardo, J.M.; Pérez-Martín, R. Mathematical modelling and simulation of the thermal processing of anisotropic and non-homogeneous conduction-heated canned foods: Application to canned tuna. J. Food Eng. 1993, 18, 369–387. [Google Scholar] [CrossRef] [Green Version]
- FAO. El Estado Mundial de la Pesca y la Acuicultura; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2018; pp. 52–58. [Google Scholar]
- Lukoshkina, M.; Odoeva, G. Kinetics of chemical reactions for prediction of quality of canned fish during storage. App. Biochem. Microb. 2003, 39, 321–327. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M. Lipid changes during long-term storage of canned sprat. J. Aquat. Food Prod. Technol. 2012, 21, 48–58. [Google Scholar] [CrossRef]
- Aitken, A.; Connell, J. Fish. In Effects of Heating on Food Stuffs; Priestley, R., Ed.; Applied Science Publishers Ltd.: London, UK, 1979; pp. 219–254. [Google Scholar]
- Aubourg, S.P. Review: Loss of quality during the manufacture of canned fish products. Food Sci. Technol. Int. 2001, 7, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Aubourg, S.P. Practices and processing from catching or harvesting till packaging: Effect on canned product quality. In Quality Parameters in Canned Seafoods; Cabado, A.G., Vieites, J.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2008; pp. 1–24. [Google Scholar]
- Gómez-Limia, L.; Cobas, N.; Franco, I.; Martínez-Suárez, S. Fatty acid profiles and lipid quality indices in canned European eels: Effects of processing steps, filling medium and storage. Food Res. Int. 2020, 136, 109601. [Google Scholar] [CrossRef]
- Sikorski, Z.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes; Haard, N., Simpson, B., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Ghali, A.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation: Review. Amer. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Gliemmo, M.; Aubourg, S.P.; Barros-Velázquez, J. Novel technologies for the preservation of chilled aquatic food products. In Novel Technologies in Food Science; McElhatton, A., Amaral Sobral, P., Eds.; Springer: New York, NY, USA, 2012; Chapter 13; pp. 299–323. [Google Scholar]
- Jeyasekaran, G.; Saralaya, K.V. Studies on the canning of White sardine, Escualosa thoracata. Asian Fish. Sci. 1991, 4, 147–154. [Google Scholar]
- Aubourg, S.P.; Trigo, M.; Martínez, B.; Rodríguez, A. Effect of prior chilling period and alga-extract packaging on the quality of a canned underutilised fish species. Foods 2020, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Medina, I. Quality differences assessment in canned sardine (Sardina pilchardus) by fluorescence detection. J. Agric. Food Chem. 1997, 45, 3617–3621. [Google Scholar] [CrossRef] [Green Version]
- Losada, V.; Rodríguez, A.; Ortiz, J.; Aubourg, S.P. Quality enhancement of canned sardine (Sardina pilchardus) by a preliminary chilling treatment. Eur. J. Lipid Sci. Technol. 2006, 108, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Carriles, N.; Aubourg, S.P. Effect of chill storage under different icing conditions on sensory and physical properties of canned farmed salmon (Oncorhynchus kisutch). Int. J. Food Sci. Technol. 2010, 45, 295–304. [Google Scholar] [CrossRef]
- Pérez-Martín, R.; Banga, J.; Sotelo, C.; Aubourg, S.P.; Gallardo, J.M. Prediction of pre-cooking times for albacore (Thunnus alalunga) by computer simulation. J. Food Eng. 1989, 10, 83–95. [Google Scholar] [CrossRef]
- AOAC. Official Methods for Analysis of the Association of Analytical Chemistry, 15th ed.; Association of Official Chemists, Inc.: Arlington, VA, USA, 1990; pp. 931–937. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Herbes, S.E.; Allen, C.P. Lipid quantification of freshwater invertebrates: Method modification for microquantitation. Can. J. Fish. Aquat. Sci. 1983, 40, 1315–1317. [Google Scholar] [CrossRef]
- Vaclavik, L.; Belková, B.; Reblová, Z.; Riddellová, K.; Hajslová, J. Rapid monitoring of heat-accelerated reactions in vegetable oils using direct analysis in real time ionization coupled with high resolution mass spectrometry. Food Chem. 2013, 138, 2312–2320. [Google Scholar] [CrossRef]
- Labuza, T.; Massaro, S. Browning and amino acid loss in model total parenteral nutrition solutions. J. Food Sci. 1990, 55, 821–826. [Google Scholar] [CrossRef]
- Howgate, P. Codex review on inspection procedures for the sensory evaluation of fish and shellfish. CX/FFP 1992, 92, 14. [Google Scholar]
- Romero, N.; Robert, P.; Masson, L.; Luck, C.; Buschmann, L. Composición en ácidos grasos y aporte de colesterol de conservas de jurel, sardine, salmon y atún al natural. Arch. Latinoamer. Nutr. 1996, 46, 75–77. [Google Scholar]
- Tarley, C.R.T.; Visentainer, J.V.; Matsushita, M.; de Souza, N.E. Proximate composition, cholesterol and fatty acids profile of canned sardines (Sardinella brasiliensis) in soybean oil and tomato sauce. Food Chem. 2004, 88, 1–6. [Google Scholar] [CrossRef]
- Piclet, G. Le poisson aliment. Composition-Intérêt nutritionnel. Cah. Nutr. Diét. 1987, 22, 317–335. [Google Scholar]
- Echarte, M.; Conchillo, A.; Ansorena, D.; Astiasarán, I. Evaluation of the nutritional aspects and cholesterol oxidation products of pork liver and fish patés. Food Chem. 2004, 86, 47–53. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Carriles, N.; Gallardo, J.M.; Aubourg, S.P. Chemical changes during farmed Coho salmon (Oncorhynchus kisutch) canning: Effect of a preliminary chilled storage. Food Chem. 2009, 112, 362–368. [Google Scholar] [CrossRef]
- Medina, I.; Gallardo, J.M.; Aubourg, S.P. Quality preservation in chilled and frozen fish products by employment of slurry ice and natural antioxidants. Int. J. Food Sci. Technol. 2009, 44, 1467–1479. [Google Scholar] [CrossRef]
- Uauy, R.; Valenzuela, A. Marine oils: The health benefits of n-3 fatty acids. Nutrition 2000, 16, 680–684. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Labuza, T. Kinetics of lipid oxidation in foods. CRC Crit. Rev. Food Technol. 1971, 2, 355–405. [Google Scholar] [CrossRef]
- Refsgaard, H.; Brockhoff, P.; Jensen, B. Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. J. Agric. Food Chem. 2000, 48, 3280–3285. [Google Scholar] [CrossRef]
- Rustad, T. Lipid oxidation. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Francis and Taylor Group: Boca Raton, FL, USA, 2010; Chapter 7; pp. 87–95. [Google Scholar]
- Kolakowska, A. Lipid oxidation in food systems. In Chemical and Functional Properties of Food Lipids; Sikorski, Z., Kolakowska, A., Eds.; CRC Press: London, UK, 2003; pp. 133–165. [Google Scholar]
- Pokorný, J. Browning from lipid-protein interactions. Prog. Food Nutr. Sci. 1981, 5, 421–428. [Google Scholar]
- Howell, N. Interaction of proteins with small molecules. In Ingredient Interactions-Effects on Food Quality; Gaonkar, A., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 269–289. [Google Scholar]
- Tironi, V.; Tomás, M.; Añón, M.C. Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). J. Food Sci. 2002, 67, 930–935. [Google Scholar] [CrossRef]
- Jeya Shakila, R.; Jeyasekaran, G.; Aunto Princy Vyla, S.; Saravana Kumar, R. Effect of delayed processing on changes in histamine and other quality characteristics of three commercially canned fishes. J. Food Sci. 2005, 70, M24–M29. [Google Scholar] [CrossRef]

| Chemical Determination | Previous Storage Time on Ice (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 10 | 13 | 15 | |
| Moisture (g·kg−1 muscle) | 714.6 ± 8.5 c,d | 711.1 ± 8.4 b,c,d | 698.8 ± 5.6 a,b | 687.8 ± 8.1 a | 701.6 ± 12.0 a,b,c | 725.2 ± 3.7 d |
| Lipids (g·kg−1 muscle) | 48.8 ± 2.8 a | 53.9 ± 9.6 a,b | 60.1 ± 4.2 a,b | 68.4 ± 7.2 b | 62.4 ± 10.2 a,b | 61.7 ± 14.3 a,b |
| Triacylglycerols (g·kg−1 lipids) | 642.4 ± 12.5 a,b | 654.7 ± 24.6 a,b,c | 661.7 ± 15.4 b,c | 678.1 ± 4.7 c | 647.0 ± 4.7 a,b | 618.8 ± 35.7 a |
| Diacylglycerols (g·kg−1 lipids) | 13.3 ± 1.0 a | 12.4 ± 6.7 a | 12.4 ± 5.3 a | 11.9 ± 7.9 a | 21.9 ± 7.5 a | 43.4 ± 7.0 b |
| FA or FA Group | Previous Storage Time on Ice (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 10 | 13 | 15 | |
| 14:0 | 1.0 ± 0.3 | 1.2 ± 0.2 | 0.9 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.3 | 1.8 ± 0.5 |
| 15:0 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 |
| 16:0 | 27.1 ± 1.5 | 27.5 ± 1.1 | 27.2 ± 1.2 | 27.6 ± 1.9 | 28.3 ± 2.1 | 28.4 ± 1.4 |
| 17:0 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 |
| 18:0 | 3.6 ± 0.8 | 4.0 ± 0.5 | 3.6 ± 1.0 | 4.1 ± 1.1 | 4.1 ± 0.8 | 4.6 ± 0.9 |
| 24:0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.0 |
| 16:1 ω7 | 2.3 ± 0.4 | 2.4 ± 0.7 | 2.5 ± 0.7 | 2.2 ± 0.6 | 2.2 ± 0.6 | 2.4 ± 0.5 |
| 18:1 ω9 | 5.6 ± 0.7 | 5.7 ± 0.9 | 5.9 ± 0.8 | 5.5 ± 0.8 | 5.6 ± 0.3 | 5.8 ± 0.7 |
| 18:1 ω7 | 2.0 ± 0.3 | 1.8 ± 0.3 | 2.1 ± 0.6 | 1.8 ± 0.7 | 2.0 ± 0.2 | 2.0 ± 0.8 |
| 20:1 ω9 | 1.7 ± 0.7 | 1.5 ± 0.9 | 1.8 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.7 | 1.8 ± 0.8 |
| 22:1 ω11 | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 |
| 18:2 ω6 | 1.0 ± 0.4 | 1.6 ± 0.3 | 1.2 ± 0.7 | 1.4 ± 0.6 | 1.3 ± 0.6 | 1.5 ± 0.5 |
| 20:4 ω6 | 4.7 ± 0.7 | 4.5 ± 0.9 | 4.6 ± 0.8 | 4.8 ± 0.8 | 4.3 ± 0.6 | 5.2 ± 0.7 |
| 18:3 ω3 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.4 | 0.7 ± 0.1 | 0.5 ± 0.2 |
| 18:4 ω3 | 0.9 ± 0.1 | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.2 |
| 20:5 ω3 (EPA) | 12.8 ± 1.3 | 11.9 ± 1.1 | 12.4 ± 1.1 | 11.7 ± 1.7 | 11.5 ± 2.1 | 11.3 ± 1.9 |
| 22:5 ω3 | 0.8 ± 0.1 | 0.8 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.4 |
| 22:6 ω3 (DHA) | 33.7 ± 3.1 | 33.0 ± 4.1 | 33.3 ± 3.7 | 33.1 ± 3.7 | 32.8 ± 5.1 | 33.2 ± 3.0 |
| Total STFA | 32.7 ± 3.2 b | 33.8 ± 3.0 b | 32.6 ± 2.7 b | 34.0 ± 3.9 b | 34.7 ± 3.7 b | 35.1 ± 3.0 b |
| Total MUFA | 11.9 ± 2.2 a | 11.6 ± 2.0 a | 12.6 ± 2.3 a | 11.5 ± 1.6 a | 11.8 ± 1.7 a | 10.4 ± 2.2 a |
| Total PUFA | 54.5 ± 5.2 c | 53.4 ± 4.2 c | 53.9 ± 5.3 c | 53.6 ± 5.5 c | 52.4 ± 4.9 c | 52.3 ± 4.7 c |
| Descriptor | Previous Storage Time on Ice (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 10 | 13 | 15 | |
| Appearance | 43.0 ± 9.0 a | 39.7 ± 6.0 a | 34.0 ± 6.1 a | 35.0 ± 2.0 a | 44.0 ± 11.4 a | 72.7 ± 14.6 b |
| Odour | 35.0 ± 2.7 b | 31.0 ± 5.3 a,b | 26.3 ± 5.9 a,b | 26.3 ± 3.8 a | 35.7 ± 7.4 a,b | 44.0 ± 6.2 b |
| Texture | 43.1 ± 1.3 a | 40.9 ± 2.7 a | 38.4 ± 4.8 a | 40.5 ± 1.6 a | 52.4 ± 3.8 b | 59.1 ± 6.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reblová, Z.; Aubourg, S.P.; Pokorný, J. The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods 2022, 11, 1987. https://doi.org/10.3390/foods11131987
Reblová Z, Aubourg SP, Pokorný J. The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods. 2022; 11(13):1987. https://doi.org/10.3390/foods11131987
Chicago/Turabian StyleReblová, Zuzana, Santiago P. Aubourg, and Jan Pokorný. 2022. "The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines" Foods 11, no. 13: 1987. https://doi.org/10.3390/foods11131987
APA StyleReblová, Z., Aubourg, S. P., & Pokorný, J. (2022). The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods, 11(13), 1987. https://doi.org/10.3390/foods11131987






